Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates
Abstract
1. Introduction
2. The Role of EGCG on Metabolic Syndrome
2.1. Effects of EGCG on Insulin Resistance and High Blood Pressure
2.2. Effects of EGCG on Adipose Mass, Blood Cholesterol, and Triglycerides
2.3. Effects of EGCG on Hyperuricemia and Uric Acid Metabolism
3. Effect of EGCG on Metabolic Diseases
3.1. Obesity
3.2. Type 2 Diabetes Mellitus
3.3. Cardiovascular Diseases
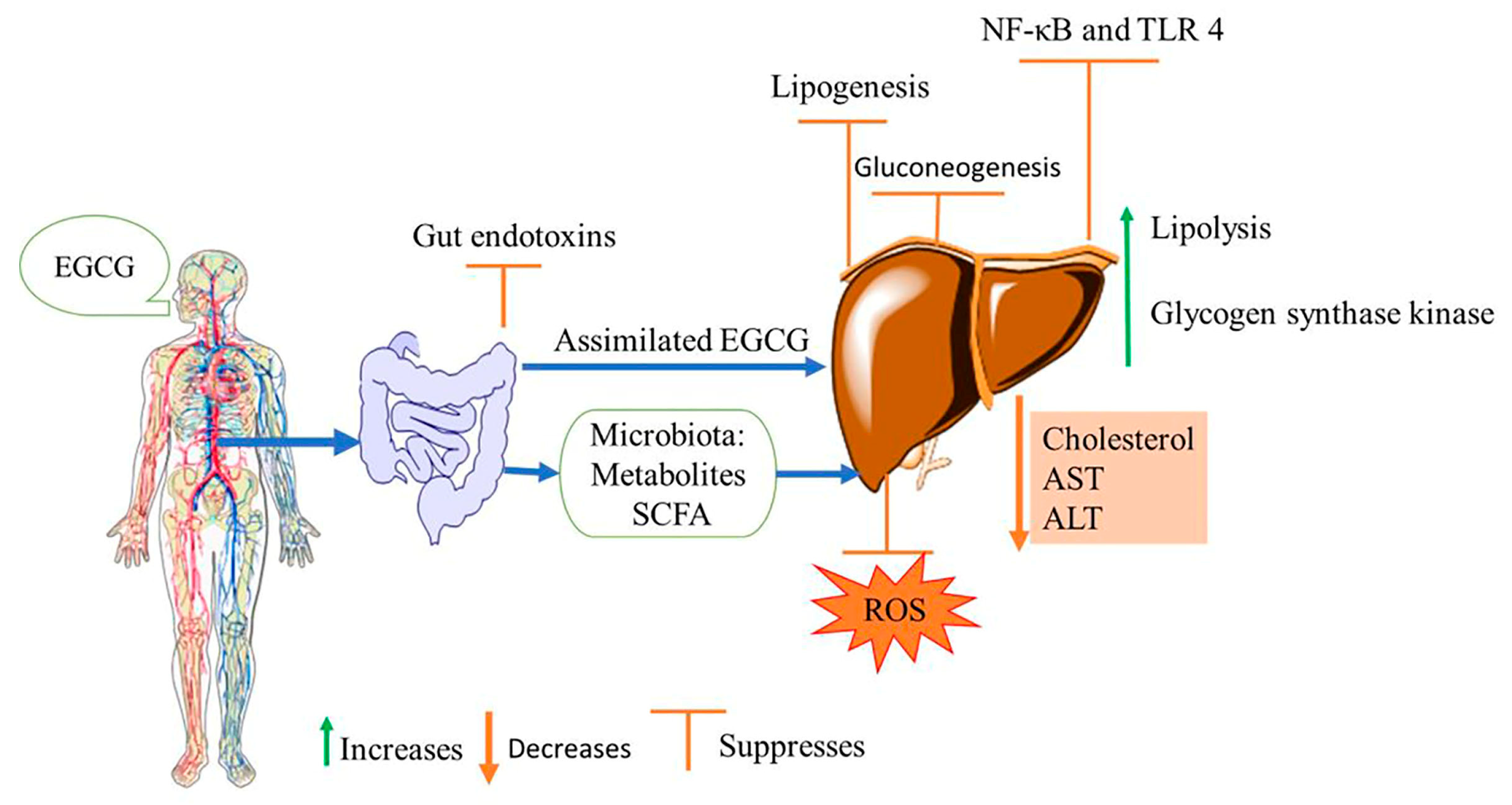
4. Effects of EGCG on Non-Alcoholic Fatty Liver Disease
5. Safety Implications of EGCG
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narotzki, B.; Reznick, A.Z.; Aizenbud, D.; Levy, Y. Green tea: A promising natural product in oral health. Arch. Oral Biol. 2012, 57, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liang, L.; Li, Y.; Yang, T.; Fan, Y.; Mao, X.; Wang, Y. Studies of quality development and major chemical composition of green tea processed from tea with different shoot maturity. LWT 2021, 142, 111055. [Google Scholar] [CrossRef]
- Xu, R.; Bai, Y.; Yang, K.; Chen, G. Effects of green tea consumption on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria—A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Liu, Y.; Wu, Z.; Weng, P. The Intestinal Microbiota Links Tea Polyphenols with the Regulation of Mood and Sleep to Improve Immunity. Food Rev. Int. 2021, 39, 1485–1498. [Google Scholar] [CrossRef]
- Fernández, V.A.; Toledano, L.A.; Lozano, N.P.; Tapia, E.N.; Roig, M.D.G.; Fornell, R.D.L.T.; Algar, Ó.G. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Van Amelsvoort, J.M.M.; Van Het Hof, K.H.; Mathot, J.N.J.J.; Mulder, T.P.J.; Wiersma, A.; Tijburg, L.B.M. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica 2008, 31, 891–901. [Google Scholar] [CrossRef]
- Raya-Cano, E.; Vaquero-Abellán, M.; Molina-Luque, R.; De Pedro-Jiménez, D.; Molina-Recio, G.; Romero-Saldaña, M. Association between metabolic syndrome and uric acid: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 18412. [Google Scholar] [CrossRef]
- Diniz, M.D.; Beleigoli, A.M.; Galvão, A.I.; Telles, R.W.; Schmidt, M.I.; Duncan, B.B.; Benseñor, I.M.; Ribeiro, A.L.; Vidigal, P.G.; Barreto, S.M. Serum uric acid is a predictive biomarker of incident metabolic syndrome at the Brazilian longitudinal study of adult Health (ELSA—Brasil). Diabetes Res. Clin. Pract. 2022, 191, 110046. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; Rosticci, M.; D’Addato, S.; Borghi, C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018, 8, 11529. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Trisha, A.; Das Hasan, A.; Islam, F. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Xie, Y.; Liu, Z.; Zou, G. Epigallocatechin gallate reduces uric acid levels by regulating xanthine oxidase activity and uric acid excretion in vitro and in vivo. Ann. Palliat. Med. 2020, 9, 331–338. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, C.N.; Li, B.Y.; Tang, G.Y.; Shang, A.; Gan, R.Y.; Feng, Y.; Li, H.B. Effects and mechanisms of tea on obesity. Crit. Rev. Food Sci. Nutr. 2021, 63, 3716–3733. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. In Proceedings of the Lancet; Elsevier Limited: Amsterdam, The Netherlands, 2005; Volume 365, pp. 1415–1428. [Google Scholar]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- James, A.; Ke, H.; Yao, T.; Wang, Y. The Role of Probiotics in Purine Metabolism, Hyperuricemia and Gout: Mechanisms and Interventions. Food Rev. Int. 2023, 39, 261–277. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Lee, S.J.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R.; Robinson, L.E. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism 2007, 56, 332–338. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Gerdes, N.; Liu, C.; Liao, M.; Liu, J.; Shi, M.A.; He, A.; Zhou, Y.; Sukhova, G.K.; et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat. Med. 2015, 21, 820–826. [Google Scholar] [CrossRef]
- Costa, F.F.; Rosário, W.R.; Ribeiro Farias, A.C.; de Souza, R.G.; Duarte Gondim, R.S.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Hang, L.; Basil, A.H.; Lim, K.L. Nutraceuticals in Parkinson’s Disease. NeuroMolecular Med. 2016, 18, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Bosze, S.; Horvath, R. Biophysical characteristics of proteins and living cells exposed to the green tea polyphenol epigallocatechin-3-gallate (EGCg): Review of recent advances from molecular mechanisms to nanomedicine and clinical trials. Eur. Biophys. J. 2017, 46, 1–24. [Google Scholar] [CrossRef]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Skora, N.C.; Whelan, J.; Villa, B.P.; Yuan, X.; Mannam, V.; Howard, S.; et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox Biol. 2020, 37, 101731. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, M.; Liao, Y.; Gong, D.; Hu, X. Action mechanisms of two key xanthine oxidase inhibitors in tea polyphenols and their combined effect with allopurinol. J. Sci. Food Agric. 2022, 102, 7195–7208. [Google Scholar] [CrossRef]
- Yuan, D.; Lin, L.; Peng, Y.; Zhou, Y.; Li, L.; Xiao, W.; Gong, Z. Effects of black tea and black brick tea with fungal growth on lowering uric acid levels in hyperuricemic mice. J. Food Biochem. 2022, 46, e14140. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Aghamohammadi, V.; Choghakhori, R.; Abbasnezhad, A. Effect of Green Tea on Anthropometric Indices and Body Composition in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Complement. Med. Res. 2021, 28, 244–251. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 2021, 46, 101090. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lin, J.-K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol. Nutr. Food Res. 2008, 52, 930–939. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, R.; Miao, S.; Gao, B.; Lu, Y.; Hui, S.; Li, L.; Shi, X.-P.; Wen, A.-D. Epigallocatechin-3-gallate ameliorates insulin resistance in hepatocytes. Mol. Med. Rep. 2017, 15, 3803–3809. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Huang, J.; Liang, Q.; Yan, Y.; Lin, H.; Xiao, W.; Lin, Y.; Zhang, S.; Tan, B.; et al. Proteomic analysis of the inhibitory effect of epigallocatechin gallate on lipid accumulation in human HepG2 cells. Proteome Sci. 2013, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Huang, C.J.; Huang, L.H.; Chen, I.J.; Chiu, J.P.; Hsu, C.H. Effects of Green Tea Extract on Insulin Resistance and Glucagon-Like Peptide 1 in Patients with Type 2 Diabetes and Lipid Abnormalities: A Randomized, Double-Blinded, and Placebo-Controlled Trial. PLoS ONE 2014, 9, e91163. [Google Scholar] [CrossRef] [PubMed]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Hodges, J.K.; Sasaki, G.Y.; Bruno, R.S. Anti-inflammatory activities of green tea catechins along the gut–liver axis in nonalcoholic fatty liver disease: Lessons learned from preclinical and human studies. J. Nutr. Biochem. 2020, 85, 108478. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Zhu, J.; Zhang, M.; Ho, C.T.; Huang, Q.; Cao, J. Metagenomics Analysis of Gut Microbiota in a High Fat Diet–Induced Obesity Mouse Model Fed with (−)-Epigallocatechin 3-O-(3-O-Methyl) Gallate (EGCG3″Me). Mol. Nutr. Food Res. 2018, 62, 1800274. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Zhang, B.; Shao, Z.; Roura, E.; Wang, S. Tea polyphenol—Gut microbiota interactions: Hints on improving the metabolic syndrome in a multi-element and multi-target manner. Food Sci. Hum. Wellness 2022, 11, 11–21. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.-X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.-J.Y. Obesity treatment by epigallocatechin-3-gallate−regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, 6371–6384. [Google Scholar] [CrossRef]
- Courtney, C.H.; Olefsky, J.M. Insulin Resistance. In Mechanisms of Insulin Action; Medical Intelligence Unit; Springer: New York, NY, USA, 2021; pp. 185–209. [Google Scholar] [CrossRef]
- Reaven, G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol. Metab. Clin. 2004, 33, 283–303. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Raje, V.; Ahern, K.W.; Martinez, B.A.; Howell, N.L.; Oenarto, V.; Granade, M.E.; Kim, J.W.; Tundup, S.; Bottermann, K.; Gödecke, A.; et al. Adipocyte lipolysis drives acute stress-induced insulin resistance. Sci. Rep. 2020, 10, 18166. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and insulin resistance in the obese may develop as part of a homeostatic response to elevated free fatty acids: A mechanistic case-control and a population-based cohort study. eBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Biol. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef]
- Choi, C.; Song, H.D.; Son, Y.; Cho, Y.K.; Ahn, S.Y.; Jung, Y.S.; Yoon, Y.C.; Kwon, S.W.; Lee, Y.H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients 2020, 12, 3072. [Google Scholar] [CrossRef]
- Lee, Y.E.; Yoo, S.H.; Chung, J.O.; Park, M.Y.; Hong, Y.D.; Park, S.H.; Park, T.S.; Shim, S.M. Hypoglycemic effect of soluble polysaccharide and catechins from green tea on inhibiting intestinal transport of glucose. J. Sci. Food Agric. 2020, 100, 3979–3986. [Google Scholar] [CrossRef]
- Bakhtiyari, S.; Zaherara, M.; Haghani, K.; Khatami, M.; Rashidinejad, A. The Phosphorylation of IRS1S307 and AktS473 Molecules in Insulin-Resistant C2C12 Cells Induced with Palmitate Is Influenced by Epigallocatechin Gallate from Green Tea. Lipids 2019, 54, 141–148. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R.C. Can EGCG Reduce Abdominal Fat in Obese Subjects? J. Am. Coll. Nutr. 2013, 26, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Fouladvand, F.; Moradi, S.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 293–301. [Google Scholar] [CrossRef]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Takechi, R.; Alfonso, H.; Hiramatsu, N.; Ishisaka, A.; Tanaka, A.; Tan, L.; Lee, A.H. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr. Res. 2016, 36, 220–226. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Zhang, T.; Cai, L.; Liu, H.; Yu, X.; Wu, J. Hyperuricemia is associated with metabolic syndrome in the community very elderly in Chengdu. Sci. Rep. 2020, 10, 8678. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Perkins, B.A.; Cherney, D.Z.I. Uric Acid as a Biomarker and a Therapeutic Target in Diabetes. Can. J. Diabetes 2015, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.; Wortmann, R. Gout therapeutics: New drugs for an old disease. Lancet 2011, 377, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Strilchuk, L.; Fogacci, F.; Cicero, A.F. Safety and tolerability of available urate-lowering drugs: A critical review. Expert Opin. Drug Saf. 2019, 18, 261–271. [Google Scholar] [CrossRef]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Chen, Y.; You, R.; Wang, K.; Wang, Y. Recent Updates of Natural and Synthetic URAT1 Inhibitors and Novel Screening Methods. Evid. Based Complement. Altern. Med. 2021, 2021, 5738900. [Google Scholar] [CrossRef]
- Sang, S.; Wang, L.; Liang, T.; Su, M.; Li, H. Potential role of tea drinking in preventing hyperuricaemia in rats: Biochemical and molecular evidence. Chin. Med. 2022, 17, 108. [Google Scholar] [CrossRef]
- Wu, D.; Chen, R.; Zhang, W.; Lai, X.; Sun, L.; Li, Q.; Zhang, Z.; Cao, J.; Wen, S.; Lai, Z.; et al. Tea and its components reduce the production of uric acid by inhibiting xanthine oxidase. Food Nutr. Res. 2022, 66, 8239. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yasuda, A.; Hayashi, M.; Akiyama, M.; Asai, T.; Hosaka, T.; Arai, H. Acute effect of green tea catechins on uric acid metabolism after alcohol ingestion in Japanese men. Clin. Rheumatol. 2021, 40, 2881–2888. [Google Scholar] [CrossRef]
- Jatuworapruk, K.; Srichairatanakool, S.; Ounjaijean, S.; Kasitanon, N.; Wangkaew, S.; Louthrenoo, W. Effects of green tea extract on serum uric acid and urate clearance in healthy individuals. J. Clin. Rheumatol. 2014, 20, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Jain, S.; Kamimoto, L.; Bramley, A.M.; Schmitz, A.M.; Benoit, S.R.; Louie, J.; Sugerman, D.E.; Druckenmiller, J.K.; Ritger, K.A.; Chugh, R.; et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April–June 2009. N. Engl. J. Med. 2009, 361, 1935–1944. [Google Scholar] [CrossRef]
- Cui, W.; Zhao, H.; Lu, X.; Wen, Y.; Zhou, Y.; Deng, B.; Wang, Y.; Wang, W.; Kang, J.; Liu, P. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect. Dis. 2010, 10, 145. [Google Scholar] [CrossRef]
- Farrell, E.; Hollmann, E.; Le Roux, C.; Nadglowski, J.; McGillicuddy, D. At home and at risk: The experiences of Irish adults living with obesity during the COVID-19 pandemic. eClinicalMedicine 2022, 51, 101568. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Wiltz, J.L.; Shrestha, S.S.; Grosse, S.D.; Boehmer, T.; Blanck, H.M. Inpatient care cost, duration, and acute complications associated with BMI in children and adults hospitalized for COVID-19. Obesity 2022, 30, 2055–2063. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.A.; Sedanur Macit, M.; Borges do Nascimento, I.J.; Guimaraes, N.S. The effect of green tea supplementation on obesity: A systematic review and dose–response meta-analysis of randomized controlled trials. Phyther. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, H.; Xu, P.; Yao, L.; Xie, Q.; Mao, L.; Wang, Y. Matcha green tea prevents obesity-induced hypothalamic inflammation via suppressing the JAK2/STAT3 signaling pathway. Food Funct. 2020, 11, 8987–8995. [Google Scholar] [CrossRef]
- Rocha, A.; Bolin, A.P.; Cardoso, C.A.L.; Otton, R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur. J. Nutr. 2015, 55, 2231–2244. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef]
- Wan, C.; Ouyang, J.; Li, M.; Rengasamy, K.R.R.; Liu, Z. Effects of green tea polyphenol extract and epigallocatechin-3-O-gallate on diabetes mellitus and diabetic complications: Recent advances. Crit. Rev. Food Sci. Nutr. 2022, 29, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Li, F.; Jiang, Y.; Guan, H.; Wang, D.; Sun-Waterhouse, D.; Wu, M.; Li, D. The synergistic protection of EGCG and quercetin against streptozotocin (STZ)-induced NIT-1 pancreatic β cell damage via upregulation of BCL-2 expression by miR-16-5p. J. Nutr. Biochem. 2021, 96, 108748. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Boarescu, P.M.; Porfire, A.S.; Dogaru, G.; Barbalata, C.; Valeanu, M.; Munteanu, C.; Râjnoveanu, R.M.; Nicula, C.A.; Stanescu, I.C. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants 2020, 9, 172. [Google Scholar] [CrossRef]
- Kumazoe, M.; Fujimura, Y.; Tachibana, H. 67-kDa Laminin Receptor Mediates the Beneficial Effects of Green Tea Polyphenol EGCG. Curr. Pharmacol. Rep. 2020, 6, 280–285. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, M.; He, Y.; Wang, F.; Kong, Y.; Ling, T.J.; Zhang, J. EGCG-derived polymeric oxidation products enhance insulin sensitivity in db/db mice. Redox Biol. 2022, 51, 102259. [Google Scholar] [CrossRef]
- Alfke, J.; Esselen, M. Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants 2022, 11, 1746. [Google Scholar] [CrossRef]
- Hadi, S.; Alipour, M.; Aghamohammadi, V.; Shahemi, S.; Ghafouri-Taleghani, F.; Pourjavidi, N.; Foroughi, M.; Chraqipoor, M. Improvement in fasting blood sugar, anthropometric measurement and hs-CRP after consumption of epigallocatechin-3-gallate (EGCG) in patients with type 2 diabetes mellitus. Nutr. Food Sci. 2020, 50, 348–359. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Sehgal, A.; Behl, T.; Kaur, I.; Singh, S.; Sharma, N.; Aleya, L. Targeting NLRP3 inflammasome as a chief instigator of obesity, contributing to local adipose tissue inflammation and insulin resistance. Environ. Sci. Pollut. Res. 2021, 28, 43102–43113. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Hu, X.; Xu, Q.; Zhang, Y.; Liu, H.; Diao, Y.; Zhang, X.; Li, L.; Yu, J.; et al. Epigallocatechin-3-gallate prevents inflammation and diabetes-Induced glucose tolerance through inhibition of NLRP3 inflammasome activation. Int. Immunopharmacol. 2021, 93, 107412. [Google Scholar] [CrossRef]
- Lopez, E.O.; Ballard, B.D.; Jan, A. Cardiovascular Disease; StatPearls: Tampa, FL, USA, 2021. [Google Scholar]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef]
- Raheem, A.; Ahmed, S.; Kakar, A.W.; Majeed, H.; Tareen, I.; Tariq, K.; Rehman, Z.U.; Karim, M. Burden of Cardiovascular Diseases in South Asian Region from 1990 to 2019: Findings from the Global Burden of Disease Study. Pak. Heart J. 2022, 55, 15–21. [Google Scholar] [CrossRef]
- Ordunez, P.; Lombardi, C.; Picone, D.S.; Brady, T.M.; Campbell, N.R.C.; Moran, A.E.; Padwal, R.; Rosende, A.; Whelton, P.K.; Sharman, J.E. HEARTS in the Americas: A global example of using clinically validated automated blood pressure devices in cardiovascular disease prevention and management in primary health care settings. J. Hum. Hypertens. 2022, 37, 126–129. [Google Scholar] [CrossRef]
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S. Global progress in prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 7, S32. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Update on the Health Benefits of Green Tea. Beverages 2017, 3, 6. [Google Scholar] [CrossRef]
- Lange, K.W. Tea in cardiovascular health and disease: A critical appraisal of the evidence. Food Sci. Hum. Wellness 2022, 11, 445–454. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Barzegar, K. The Effect of Green Tea and Sour Tea on Blood Pressure of Patients with Type 2 Diabetes: A Randomized Clinical Trial. J. Diet. Suppl. 2013, 10, 105–115. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, R.; Wang, B.; Yu, X.; Yang, X.; Liu, K.; Mi, M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014, 4, srep06251. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Hamburg, N.M.; Anter, E.; Holbrook, M.; Kahn, D.F.; Elliott, J.G.; Keaney, J.F.; Vita, J.A. Acute EGCG Supplementation Reverses Endothelial Dysfunction in Patients with Coronary Artery Disease. J. Am. Coll. Nutr. 2013, 26, 95–102. [Google Scholar] [CrossRef]
- Szulińska, M.; Stępień, M.; Kręgielska-Narożna, M.; Suliburska, J.; Skrypnik, D.; Bąk-Sosnowska, M.; Kujawska-Łuczak, M.; Grzymisławska, M.; Bogdański, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61, 1295525. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, J.; Chen, X.; Zhu, X.; Liu, S.; Li, J.; Xu, X.; Ma, X.; Zhao, J.; Ji, X. (−)-Epigallocatechin-3-gallate (EGCG) attenuates salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Sci. Rep. 2020, 10, 4783. [Google Scholar] [CrossRef]
- Falsini, B.; Marangoni, D.; Salgarello, T.; Stifano, G.; Montrone, L.; Landro, S.; Guccione, L.; Balestrazzi, E.; Colotto, A. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: A short-term study by pattern electroretinogram. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, M.; Wang, X.; Wu, K.; Li, Y.; Li, L.; Yang, J.; Ruan, Y.; Bai, R.; Ma, C.; et al. Sex differences in the association between green tea consumption and hypertension in elderly Chinese adults. BMC Geriatr. 2021, 21, 486. [Google Scholar] [CrossRef]
- Liu, J.; Ayada, I.; Zhang, X.; Wang, L.; Li, Y.; Wen, T.; Ma, Z.; Bruno, M.J.; de Knegt, R.J.; Cao, W.; et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin. Gastroenterol. Hepatol. 2022, 20, e573–e582. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.F.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin gallate protects mice against methionine-choline-deficient-diet-induced nonalcoholic steatohepatitis by improving gut microbiota to attenuate hepatic injury and regulate metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Yang, S.; Kwak, S.; Lee, J.H.; Kang, S.; Lee, S.P. Nonalcoholic fatty liver disease is an early predictor of metabolic diseases in a metabolically healthy population. PLoS ONE 2019, 14, e0224626. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Review article: Shared disease mechanisms between non-alcoholic fatty liver disease and metabolic syndrome—Translating knowledge from systems biology to the bedside. Aliment. Pharmacol. Ther. 2019, 49, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, J.; Kang, X.; Xu, C.; Sun, C.; Tao, L.; Zheng, D.; Han, Y.; Li, Q.; Guo, X.; et al. Hyperuricemia precedes non-alcoholic fatty liver disease with abdominal obesity moderating this unidirectional relationship: Three longitudinal analyses. Atherosclerosis 2020, 311, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green Tea and Epigallocatechin Gallate (EGCG) for the Management of Nonalcoholic Fatty Liver Diseases (NAFLD): Insights into the Role of Oxidative Stress and Antioxidant Mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Ushiroda, C.; Mizushima, K.; Inoue, R.; Yasukawa, Z.; Abe, A.; Takagi, T. Epigallocatechin-3-gallate (EGCG) attenuates non-alcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J. Clin. Biochem. Nutr. 2020, 67, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sapper, T.N.; Mah, E.; Rudraiah, S.; Schill, K.E.; Chitchumroonchokchai, C.; Moller, M.V.; Mcdonald, J.D.; Rohrer, P.R.; Manautou, J.E.; et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 858–870. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Chen, C.H.; Hong, T.; Yang, J.R.; Huang, X.J.; Geng, F.; Hu, J.L.; Nie, S.P. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chem. 2023, 404, 134591. [Google Scholar] [CrossRef]
- Fallah, S.; Musa-Veloso, K.; Cao, J.; Venditti, C.; Lee, H.Y.; Hamamji, S.; Hu, J.; Appelhans, K.; Frankos, V. Liver biomarkers in adults: Evaluation of associations with reported green tea consumption and use of green tea supplements in U.S. NHANES. Regul. Toxicol. Pharmacol. 2022, 129, 105087. [Google Scholar] [CrossRef]
- Yates, A.A.; Erdman, J.W.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Siblini, H.; Al-Hendy, A.; Segars, J.; González, F.; Taylor, H.S.; Singh, B.; Flaminia, A.; Flores, V.A.; Christman, G.M.; Huang, H.; et al. Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women. Nutr. 2023, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 3: Teratogenicity and reproductive toxicity studies in rats. Food Chem. Toxicol. 2006, 44, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, N.O.A.; Ahmed, L.A.; Abdallah, D.M.; El-Sayeh, B.M. Nephro-toxic effects of intraperitoneally injected EGCG in diabetic mice: Involvement of oxidative stress, inflammation and apoptosis. Sci. Rep. 2017, 7, 40617. [Google Scholar] [CrossRef]
- Ramachandran, B.; Jayavelu, S.; Murhekar, K.; Rajkumar, T. Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicol. Rep. 2016, 3, 336–345. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Bausch, J.; Edwards, J.A.; Wolz, E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: Genotoxicity. Food Chem. Toxicol. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible controversy over dietary polyphenols: Benefits vs risks. Chem. Res. Toxicol. 2007, 20, 583–585. [Google Scholar] [CrossRef]
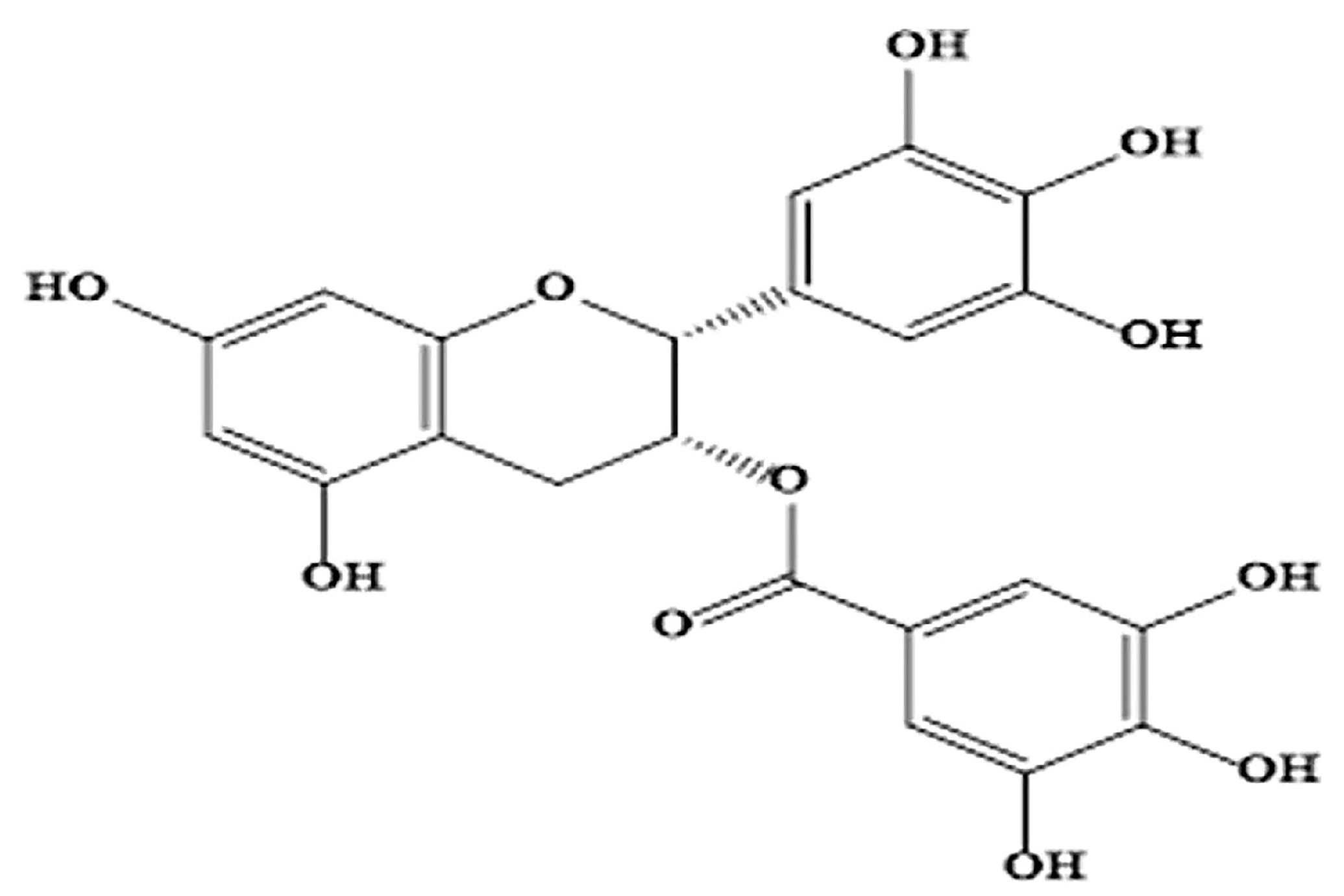

| Molecular Mechanism | Molecular Targets | References |
|---|---|---|
| Antioxidation | Protect against metal-induced oxidation by chelating metal ions Cell sulfur metabolism | [34] |
| ROS scavenging including superoxide anion and hydroxyl radicals | [35,36] | |
| Uric acid metabolism | Inhibitory activity on xanthine oxidase (XOD) and adenosine deaminase (ADA) enzymes | [14,37] |
| Upregulation of uric acid secretion transporters and downregulating uric acid reabsorption | [14,38] | |
| Glucose metabolism | Inhibition of α-glucosidase, an enzyme that hydrolyzes disaccharide and oligosaccharides Increase glucagon-like peptide-1 for lowering blood glucose and improving glucose homeostasis | [39,40] |
| Insulin sensitivity | Restores phosphorylation of protein kinase B (AKT)/glycogen synthetase kinase and insulin receptor substrate-1 (IRS-1) through AMPK-activated pathway Restore and decrease ROS-induced pathway Stimulate glycogen synthesis | [41,42] |
| Hepatic gluconeogenesis | Phosphorylation of insulin receptor substrate-1 (IRS-1) and suppresses hepatic gluconeogenesis | [43,44] |
| Lipid metabolism | Increase serum glucagon-like peptide-1 | [44] |
| Reduction of total cholesterol Increase the HDL levels Reduce LDL cell uptake and inhibit LDL cholesterol oxidation | [36,45] | |
| Free fatty acid oxidation | Activation of AMPK and upregulation of succinate dehydrogenase activity to maintain cell energy homeostasis | [43] |
| Anti-inflammatory | Interferes and suppresses activation of inflammatory nuclear factor kappa-B (NF-κB) and tumor necrosis factor-α (TNF-α) | [45,46] |
| Liver functioning | Lower expression of hepatic alanine aminotransaminase (ALT) and aspartate transaminase (AST), the hepatic enzymes indicative of liver injury | [46] |
| Gut microbiota | Targets gut microbiota by providing prebiotics function. Protects against the gut barrier Enhance the formation of beneficial short chain fatty acids (SCFAs) and amino acids | [47,48] |
| Bile acids metabolism | Targets bile acids metabolism, which mediates various metabolic pathways via G-protein-coupled receptor 1 and farnesoid X-activated receptor (FXR) | [49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. https://doi.org/10.3390/nu15133022
James A, Wang K, Wang Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients. 2023; 15(13):3022. https://doi.org/10.3390/nu15133022
Chicago/Turabian StyleJames, Armachius, Ke Wang, and Yousheng Wang. 2023. "Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates" Nutrients 15, no. 13: 3022. https://doi.org/10.3390/nu15133022
APA StyleJames, A., Wang, K., & Wang, Y. (2023). Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients, 15(13), 3022. https://doi.org/10.3390/nu15133022






