The Mutual Interplay between Bone, Glucose and Lipid Metabolism: The Role of Vitamin D and PTH
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The interplay between bone and glucose metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J. Serum vitamin D status and metabolic syndrome: A systematic review and dose-response meta-analysis. Nutr. Res. Pract. 2021, 15, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Bahadorpour, S.; Hajhashemy, Z.; Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: A systematic review and dose-response meta-analysis of epidemiologic studies. Nutr. Rev. 2022, 81, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Cipriani, C.; Sonato, C.; Raimo, O.; Biamonte, F.; Minisola, S. Cardiovascular manifestations of primary hyperparathyroidism: A narrative review. Eur. J. Endocrinol. 2017, 177, R297–R308. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Liccardi, A.; Minotta, R.; Benevento, E.; Cannavale, G.; Colao, A. Parathyroid diseases and metabolic syndrome. J. Endocrinol. Investig. 2023, 46, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.J.; Chen, L.; Pan, Y.L.; Lu, Y.; Sun, L.H.; Zhao, H.Y.; Wang, W.Q.; Tao, B.; Liu, J.M. An inverted U-shaped relationship between parathyroid hormone and body weight, body mass index, body fat. Endocrine 2021, 72, 844–851. [Google Scholar] [CrossRef]
- Hagström, E.; Lundgren, E.; Lithell, H.; Berglund, L.; Ljunghall, S.; Hellman, P.; Rastad, J. Normalized dyslipidaemia after parathyroidectomy in mild primary hyperparathyroidism: Population-based study over five years. Clin. Endocrinol. 2022, 56, 253–260. [Google Scholar] [CrossRef]
- Ahlstrom, Y.; Hagström, E.; Larsson, A.; Rudberg, C.; Lind, L.; Hellman, P. Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin. Endocrinol. 2009, 71, 673–678. [Google Scholar] [CrossRef]
- Ferrone, F.; Pepe, J.; Danese, V.C.; Fassino, V.; Cecchetti, V.; De Lucia, F.; Biamonte, F.; Colangelo, L.; Ferrazza, G.; Panzini, E.; et al. The relative influence of serum ionized calcium and 25-hydroxyvitamin D in regulating PTH secretion in healthy subjects. Bone 2019, 125, 200–206. [Google Scholar] [CrossRef]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, assessment, and management of vitamin D inadequacy: Suggestions, recommendations, and warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef]
- Cleeman, J.I.; Grundy, S.M.; Becker, D.; Clark, L. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L.; Olarte, O.R. Calcifediol (25OH Vitamin D3) deficiency: A risk factor from early to old age. Nutrients 2022, 14, 1168. [Google Scholar] [CrossRef]
- Scragg, R.; Sluyter, J. Is there proof of extraskeletal benefits from vitamin D supplementation fom recent mega tials of vitamin D? JBMR Plus 2021, 5, e10459. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Rosen, C. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol. 2023, 11, 362–374. [Google Scholar] [CrossRef]
- Ghozali, N.M.; Giribabu, N.; Salleh, N. Mechanisms linking vitamin D deficiency to impaired metabolism: An overview. Int. J. Endocrinol. 2022, 2022, 6453882. [Google Scholar] [CrossRef]
- Kauser, H.; Palakeel, J.; Ali, M.; Chaduvula, P.; Chhabra, S.; Lamichhane, S.L.; Ramesh, V.; Opara, C.O.; Khan, F.Y.; Kabiraj, G.; et al. Factors showing the gowing relation between vitamin D, metabolic syndrome, and obesity in the adult population: A systematic review. Cureus 2022, 14, e27335. [Google Scholar] [CrossRef]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Sadat Mirenayat, F.; Saneei, P. Serum vitamin D levels in relation to metabolic syndrome: A systematic review and dose–response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13223. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P. Is hypovitaminosis D related to incidence of type 2 diabetes and high fasting glucose level in healthy subjects: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P. Insulin resistance is inversely associated with the status of vitamin D in both diabetic and non-diabetic populations. Nutrients 2021, 13, 1742. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease rsk factors: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The action of vitamin D in adipose tissue: Is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, l.S.; Ranjbar, G.; Norouzy, A.; Arabi, S.M. Vitamin D supplementation effects on the clinical outcomes of patients with coronary artery disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12923. [Google Scholar] [CrossRef]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The impacts of vitamin D supplementation in adults with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of vitamin D supplementation on glycemic control in prediabetes: A meta-analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Y.; Shen, S. Association between vitamin D and prediabetes—A PRISMA-compliant meta-analysis. Medicine 2020, 99, 8. [Google Scholar] [CrossRef]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and risk for type 2 diabetes in people with prediabetes: A systematic review and meta-analysis of individual participant data from 3 randomized clinical trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Beysel, S.; Caliskan, M.; Kizilgul, M.; Apaydin, M.; Kan, S.; Ozbek, M.; Cakal, E. Parathyroidectomy improves cardiovascular risk factors in normocalcemic and hypercalcemic primary hyperparathyroidism. BMC Cardiov Dis. 2019, 19, 106. [Google Scholar] [CrossRef]
- Ejlsmark-Svensson, H.; Rolighed, L.; Rejnmark, L. Effect of parathyroidectomy on cardiovascular risk factors in primary hyperparathyroidism: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2019, 104, 3223–3232. [Google Scholar] [CrossRef]
- Procopio, M.; Barale, M.; Bertaina, S.; Sigrist, S.; Mazzetti, R.; Loiacono, M.; Mengozzi, G.; Ghigo, E.; Maccario, M. Cardiovascular risk and metabolic syndrome in primary hyperparathyroidism and their correlation to different clinical forms. Endocrine 2014, 47, 581–589. [Google Scholar] [CrossRef]
- He, Y.; Liu, R.; Zhu, M.; Shen, W.; Xie, J.; Zhang, Z.; Chen, N.; Shan, C.; Guo, X.; Lu, Y.; et al. The browning of white adipose tissue and body weight loss in primary hyperparathyroidism. EBioMedicine 2019, 40, 56–66. [Google Scholar] [CrossRef]
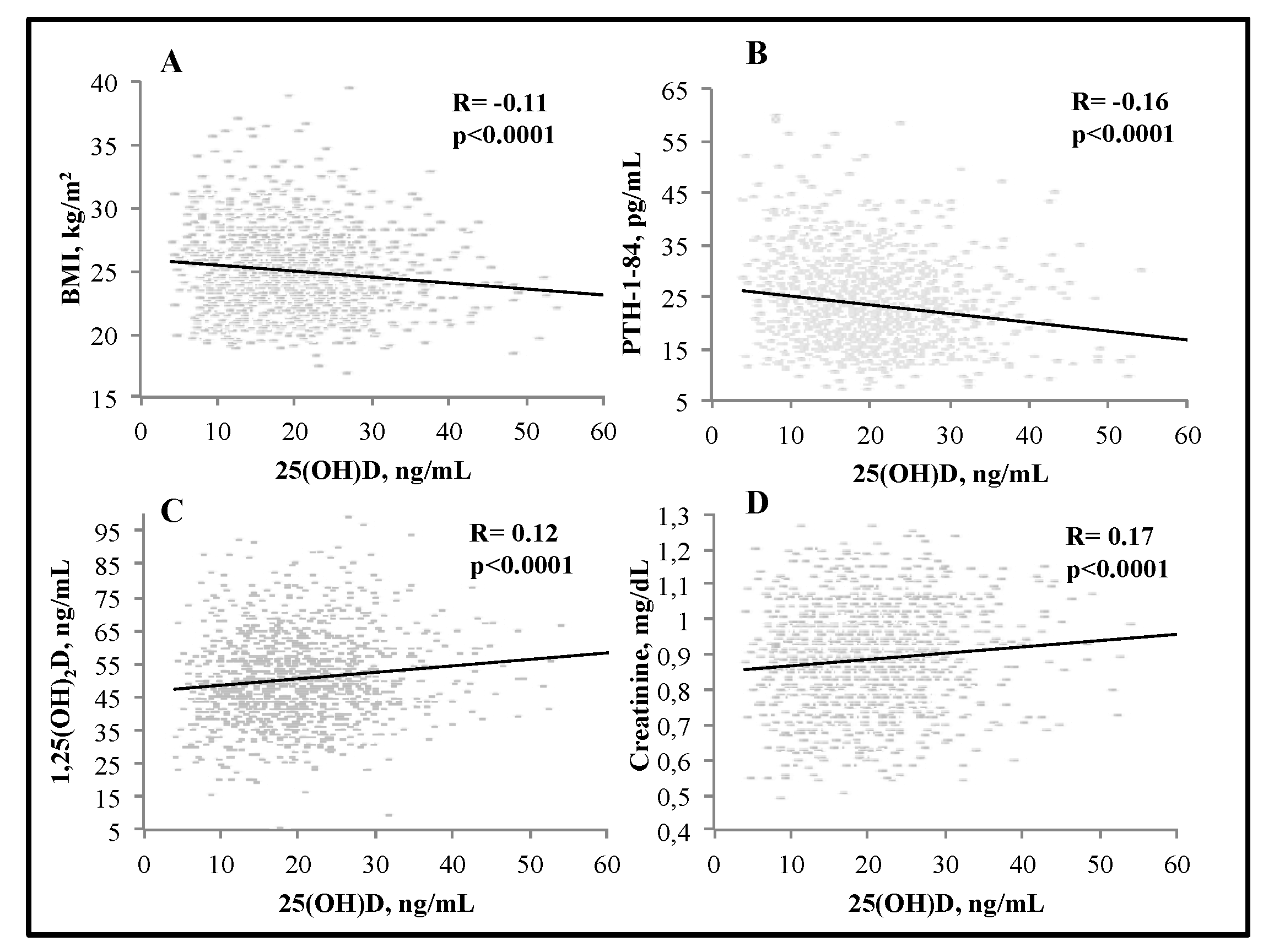
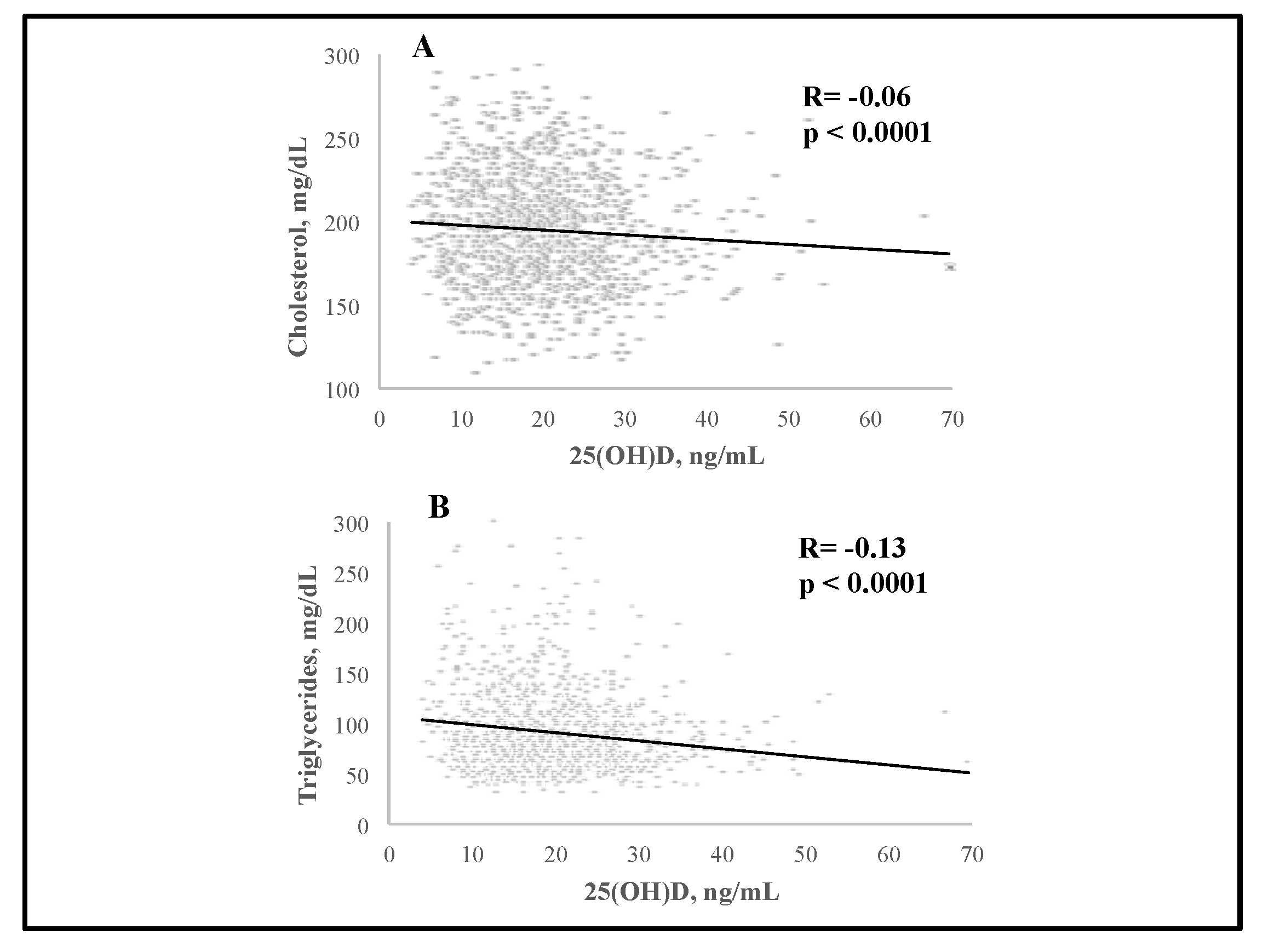



| Parameter | All Subjects (n = 1240) | Normal Range |
|---|---|---|
| Age, years | 41.9 ± 11.7 | - |
| Female/male ratio | 1/3.2 | - |
| Men, n | 949 | |
| Pre-menopausal women, n | 228 | |
| Post-menopausal women, n | 63 | |
| Weight, kg | 76.7 ± 13 | - |
| Height, cm | 175 ± 8 | - |
| BMI, kg/m2 | 24.9 ± 3.3 | 18.5–25 |
| 19.9 ± 8.4 | >20 | |
| PTH, pg/mL | 23.5 ± 8.3 | 6.5–36.6 |
| 1,25(OH)2D, pg/mL | 50.5 ± 13.2 | 19.9–79.3 |
| Ca++, mmol/L | 1.29 ± 0.03 | 1.17–1.33 |
| Mg++, mmol/L | 0.53 ± 0.05 | 0.45–0.6 |
| Creatinine, mg/dL | 0.89 ± 0.15 | 0.7–1.2 |
| Total cholesterol, mg/dL | 195 ± 34.2 | 80–232 |
| LDL cholesterol, mg/dL | 116.4 ± 32 | 69–118 |
| HDL cholesterol, mg/dL | 60 ± 15.2 | 44–64 |
| Triglycerides, mg/dL | 91 ± 46.5 | 45–163 |
| Glucose, mg/dL | 86.2 ± 9.5 | 68–99 |
| Parameter | 25(OH)D | PTH |
|---|---|---|
| Total cholesterol | OR 0.99 (95% CI 0.97–1) p = 0.06 | OR 1 (95% CI 0.99–1.03) p = 0.06 |
| LDL cholesterol | OR 0.99 (95% CI 0.98–1.01) p = NS | OR 1 (95% CI 1.01–1.04) p < 0.01 |
| HDL cholesterol | OR 0.97 (95% CI 0.94–0.99) p < 0.03 | OR 1 (95% CI 0.99–1.04) p = 0.05 |
| Triglycerides | OR 0.94 (95% CI 0.91–0.96) p < 0.0001 | OR 1.02 (95% CI 1–1.05) p < 0.03 |
| Glucose | OR 1 (95% CI 0.97–1.02) p = NS | OR 1 (95% CI 0.99–1.05) p = 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danese, V.C.; Pepe, J.; Ferrone, F.; Colangelo, L.; De Martino, V.; Nieddu, L.; Ferrazza, G.; Panzini, E.; Pascone, R.; Blocki, F.; et al. The Mutual Interplay between Bone, Glucose and Lipid Metabolism: The Role of Vitamin D and PTH. Nutrients 2023, 15, 2998. https://doi.org/10.3390/nu15132998
Danese VC, Pepe J, Ferrone F, Colangelo L, De Martino V, Nieddu L, Ferrazza G, Panzini E, Pascone R, Blocki F, et al. The Mutual Interplay between Bone, Glucose and Lipid Metabolism: The Role of Vitamin D and PTH. Nutrients. 2023; 15(13):2998. https://doi.org/10.3390/nu15132998
Chicago/Turabian StyleDanese, Vittoria Carmela, Jessica Pepe, Federica Ferrone, Luciano Colangelo, Viviana De Martino, Luciano Nieddu, Giancarlo Ferrazza, Enrico Panzini, Roberto Pascone, Frank Blocki, and et al. 2023. "The Mutual Interplay between Bone, Glucose and Lipid Metabolism: The Role of Vitamin D and PTH" Nutrients 15, no. 13: 2998. https://doi.org/10.3390/nu15132998
APA StyleDanese, V. C., Pepe, J., Ferrone, F., Colangelo, L., De Martino, V., Nieddu, L., Ferrazza, G., Panzini, E., Pascone, R., Blocki, F., Minisola, S., & Cipriani, C. (2023). The Mutual Interplay between Bone, Glucose and Lipid Metabolism: The Role of Vitamin D and PTH. Nutrients, 15(13), 2998. https://doi.org/10.3390/nu15132998








