Heavy Metal and Rice in Gluten-Free Diets: Are They a Risk?
Abstract
1. Introduction
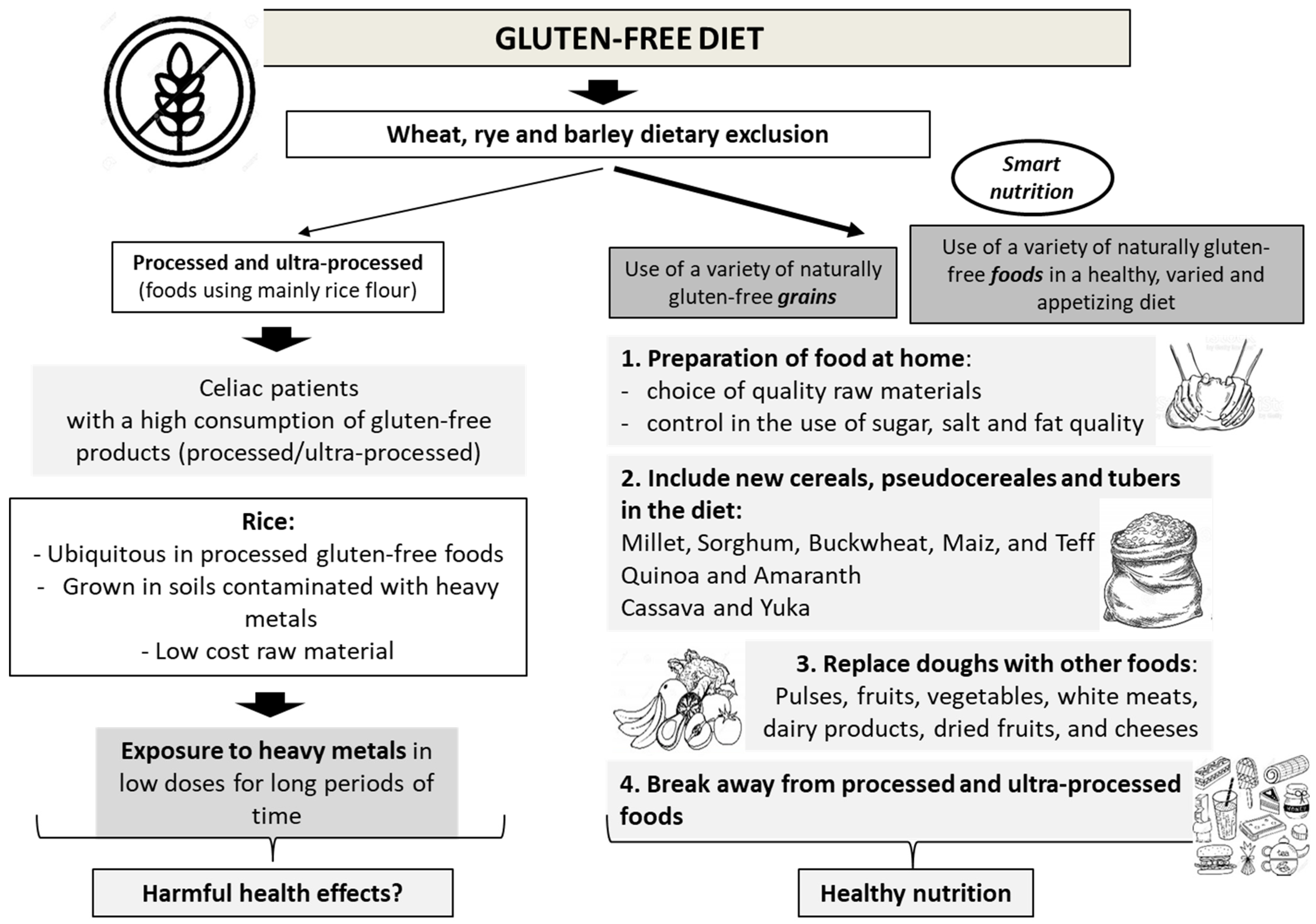
2. Gluten-Free Diet and Treatment of CD and GRDs
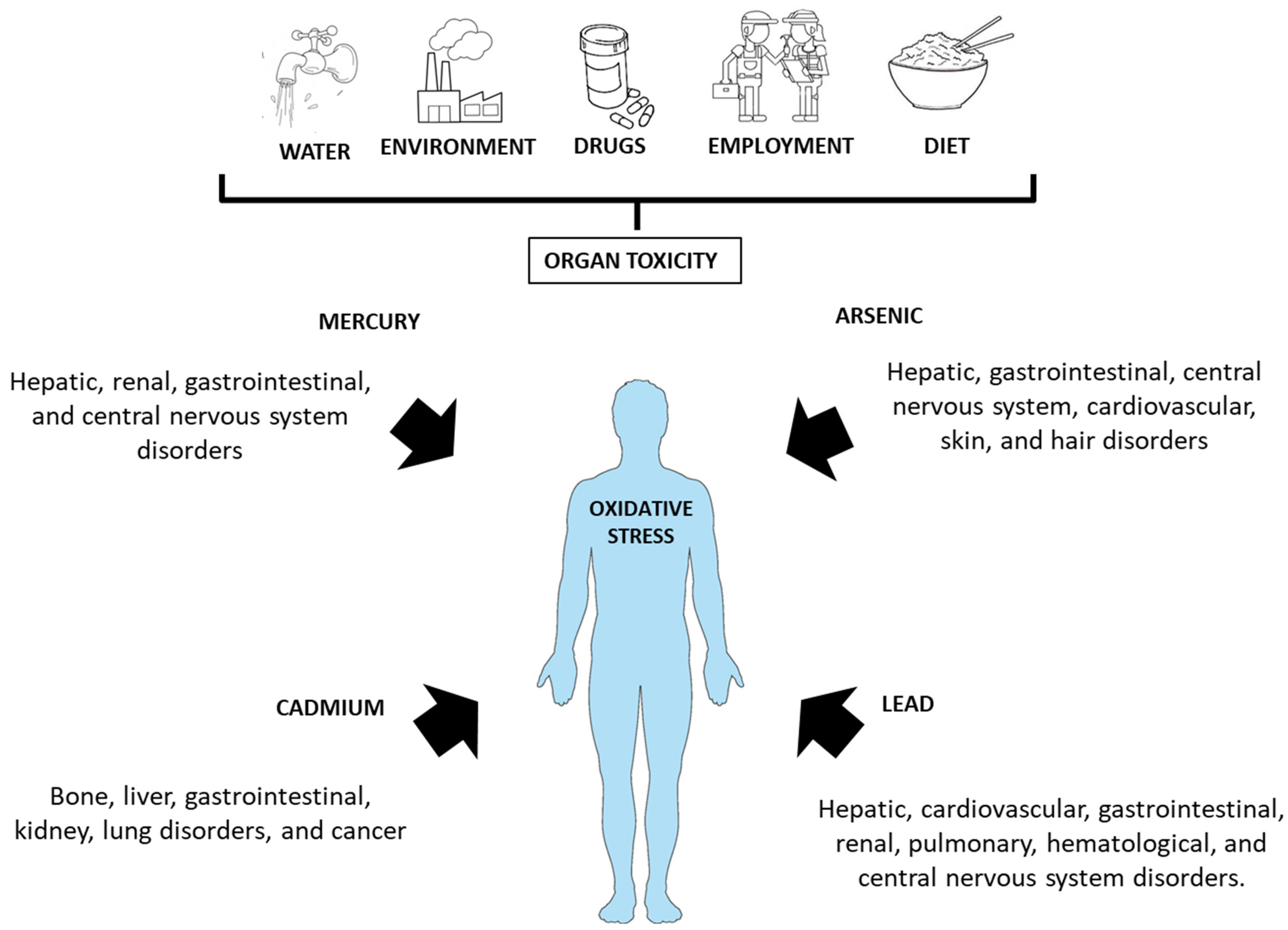
3. Bioaccumulation of Heavy Metal in Persons on a GFD
4. Metals in Gluten-Containing and Gluten-Free Foods
5. Rice Cultivars and As in Contaminated Soils
6. Future Perspectives
7. Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The Gluten-Free Diet: A Nutritional Risk Factor for Adolescents with Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Ballestero Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M. Celiac Disease and the Gluten-Free Diet: Consequences and Recommendations for Improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of High Sugar Intake, and Low Fibre and Mineral Intake, in the Gluten-Free Diet. Aliment Pharm. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Tomba, C.; Elli, L.; Sieri, S.; Grioni, S.; Bardella, M.T.; Agostoni, C.; Doneda, L.; et al. Evaluation of a Modified Italian European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire for Individuals with Celiac Disease. J. Acad. Nutr. Diet. 2016, 116, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Wünsche, J.; Lambert, C.; Gola, U.; Biesalski, H.K. Consumption of Gluten Free Products Increases Heavy Metal Intake. NFS J. 2018, 12, 11–15. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding Arsenic Dynamics in Agronomic Systems to Predict and Prevent Uptake by Crop Plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Proposes Limit for Inorganic Arsenic in Infant Rice Cereal; FDA: Silver Spring, MD, USA, 2016.
- European Commission. Commission Regulation (EU) 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs (Text with EEA Relevance); Publications Office of the European Union: Luxembourg, 2015; Volume 161, p. 16.
- Eur.Lex.Europa.Eu. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1006&from=EN (accessed on 20 March 2023).
- Davis, M.A.; Signes-Pastor, A.J.; Argos, M.; Slaughter, F.; Pendergrast, C.; Punshon, T.; Gossai, A.; Ahsan, H.; Karagas, M.R. Assessment of Human Dietary Exposure to Arsenic through Rice. Sci. Total Environ. 2017, 586, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Punshon, T.; Jackson, B.P. Essential Micronutrient and Toxic Trace Element Concentrations in Gluten Containing and Gluten-Free Foods. Food Chem. 2018, 252, 258–264. [Google Scholar] [CrossRef]
- Williams, P.N.; Price, A.H.; Raab, A.; Hossain, S.A.; Feldmann, J.; Meharg, A.A. Variation in Arsenic Speciation and Concentration in Paddy Rice Related to Dietary Exposure. Environ. Sci. Technol. 2005, 39, 5531–5540. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H.; Wang, H. Assessment of Heavy Metal (HM) Contamination in Agricultural Soil Lands in Northern Telangana, India: An Approach of Spatial Distribution and Multivariate Statistical Analysis. Environ. Monit. Assess. 2019, 191, 246. [Google Scholar] [CrossRef]
- Shifaw, E. Review of Heavy Metals Pollution in China in Agricultural and Urban Soils. J. Health Pollut. 2018, 8, 180607. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, J.; Zhang, J.; Zhang, H.; Qiao, L.; Men, Y. Heavy Metals in Rice and Garden Vegetables and Their Potential Health Risks to Inhabitants in the Vicinity of an Industrial Zone in Jiangsu, China. J. Environ. Sci. 2010, 22, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White Rice Consumption and Risk of Type 2 Diabetes: Meta-Analysis and Systematic Review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Kato, M.; Inoue, M.; Tsugane, S. Rice Intake and Type 2 Diabetes in Japanese Men and Women: The Japan Public Health Center-Based Prospective Study. Am. J. Clin. Nutr. 2010, 92, 1468–1477. [Google Scholar] [CrossRef]
- Villegas, R.; Liu, S.; Gao, Y.-T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective Study of Dietary Carbohydrates, Glycemic Index, Glycemic Load, and Incidence of Type 2 Diabetes Mellitus in Middle-Aged Chinese Women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Punshon, T.; Davis, M.; Bulka, C.M.; Slaughter, F.; Karalis, D.; Argos, M.; Ahsan, H. Rice Intake and Emerging Concerns on Arsenic in Rice: A Review of the Human Evidence and Methodologic Challenges. Curr. Environ. Health Rep. 2019, 6, 361–372. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Galipeau, H.J.; McCarville, J.L.; Huebener, S.; Litwin, O.; Meisel, M.; Jabri, B.; Sanz, Y.; Murray, J.A.; Jordana, M.; Alaedini, A.; et al. Intestinal Microbiota Modulates Gluten-Induced Immunopathology in Humanized Mice. Am. J. Pathol. 2015, 185, 2969–2982. [Google Scholar] [CrossRef]
- Burló, F.; Ramírez-Gandolfo, A.; Signes-Pastor, A.J.; Haris, P.I.; Carbonell-Barrachina, A.A. Arsenic Contents in Spanish Infant Rice, Pureed Infant Foods, and Rice. J. Food Sci. 2012, 77, T15–T19. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat Amylase Trypsin Inhibitors Drive Intestinal Inflammation via Activation of Toll-like Receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity after Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Bascuñán, K.A.; Alarcón-Sajarópulos, D.; Cabrera-Chávez, F.; Oyarzún, A.; Fernández, A.; Ontiveros, N. Living with Gluten and Other Food Intolerances: Self-Reported Diagnoses and Management. Nutrients 2020, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Pourpak, Z.; Mesdaghi, M.; Mansouri, M.; Kazemnejad, A.; Toosi, S.B.; Farhoudi, A. Which Cereal Is a Suitable Substitute for Wheat in Children with Wheat Allergy? Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2005, 16, 262–266. [Google Scholar] [CrossRef]
- Cabanillas, B. Gluten-Related Disorders: Celiac Disease, Wheat Allergy, and Nonceliac Gluten Sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Muhammad, H.; Reeves, S.; Ishaq, S.; Mayberry, J.; Jeanes, Y.M. Adherence to a Gluten Free Diet Is Associated with Receiving Gluten Free Foods on Prescription and Understanding Food Labelling. Nutrients 2017, 9, 705. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Kamycheva, E.; Goto, T.; Camargo, C.A.J. Blood Levels of Lead and Mercury and Celiac Disease Seropositivity: The US National Health and Nutrition Examination Survey. Environ. Sci. Pollut. Res. Int. 2017, 24, 8385–8391. [Google Scholar] [CrossRef] [PubMed]
- Batres-Marquez, S.P.; Jensen, H.H.; Upton, J. Rice Consumption in the United States: Recent Evidence from Food Consumption Surveys. J. Am. Diet. Assoc. 2009, 109, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Rossi, V.; Conte, D.; Ronchi, A.; Tomba, C.; Passoni, M.; Bardella, M.T.; Roncoroni, L.; Guzzi, G. Increased Mercury Levels in Patients with Celiac Disease Following a Gluten-Free Regimen. Gastroenterol. Res. Pract. 2015, 2015, 953042. [Google Scholar] [CrossRef]
- Raehsler, S.L.; Choung, R.S.; Marietta, E.V.; Murray, J.A. Accumulation of Heavy Metals in People on a Gluten-Free Diet. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple Metal Exposures and Metabolic Syndrome: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Park, S.K. Associations between Rice Consumption, Arsenic Metabolism, and Insulin Resistance in Adults without Diabetes. Int. J. Hyg. Environ. Health 2021, 237, 113834. [Google Scholar] [CrossRef]
- Orzechowska-Wylęgała, B.; Obuchowicz, A.; Malara, P.; Fischer, A.; Kalita, B. Cadmium and Lead Accumulate in the Deciduous Teeth of Children with Celiac Disease or Food Allergies. Int. J. Stomatol. Occlusion Med. 2011, 4, 28–31. [Google Scholar] [CrossRef]
- Mawia, A.M.; Hui, S.; Zhou, L.; Li, H.; Tabassum, J.; Lai, C.; Wang, J.; Shao, G.; Wei, X.; Tang, S.; et al. Inorganic Arsenic Toxicity and Alleviation Strategies in Rice. J. Hazard. Mater. 2021, 408, 124751. [Google Scholar] [CrossRef]
- Heinrich-Salmeron, A.; Cordi, A.; Brochier-Armanet, C.; Halter, D.; Pagnout, C.; Abbaszadeh-fard, E.; Montaut, D.; Seby, F.; Bertin, P.N.; Bauda, P.; et al. Unsuspected Diversity of Arsenite-Oxidizing Bacteria as Revealed by Widespread Distribution of the AoxB Gene in Prokaryotes. Appl. Environ. Microbiol. 2011, 77, 4685–4692. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Santini, J.M.; vanden Hoven, R.N. Molybdenum-Containing Arsenite Oxidase of the Chemolithoautotrophic Arsenite Oxidizer NT-26. J. Bacteriol. 2004, 186, 1614–1619. [Google Scholar] [CrossRef]
- Šlejkovec, Z.; Gorše, L.; Grobler, A.; Jagodic, M.; Falnoga, I. Arsenic Speciation and Elemental Composition of Rice Samples from the Slovenian Market. Food Chem. 2021, 342, 128348. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluation of Certain Food Additive and Contaminants; WHO Technical Report Series; WHO: Geneva, Switzerland, 2011; pp. 1–226.
- World Health Organization. Arsenic: Fact Sheet; World Health Organization: Geneva, Switzerland, 2022.
- European Food Safety Authority. Panel on Contaminants in the Food Chain (CONTAM): Scientific Opinion on Arsenic in Food; European Food Safety Authority: Parma, Italy, 2009.
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Investigating the Roles of Ascorbate-Glutathione Cycle and Thiol Metabolism in Arsenate Tolerance in Ridged Luffa Seedlings. Protoplasma 2015, 252, 1217–1229. [Google Scholar] [CrossRef]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and Characterization of Maize and Barley Lsi2-like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, S.; Kontogianni, M.D. The Economic Burden of Gluten-Free Products and Gluten-Free Diet: A Cost Estimation Analysis in Greece. J. Hum. Nutr. Diet. 2017, 30, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Perinelli, D.R.; Camilletti, D.; Carotenuto, F.; Belli, L.; Polzonetti, V. Nutritional Properties of Rice Varieties Commonly Consumed in Italy and Applicability in Gluten Free Diet. Foods 2021, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Zhang, Q.-C.; Yan, C.-A.; Tang, G.-Y.; Zhang, M.-Y.; Ma, L.Q.; Gu, R.-H.; Xiang, P. Heavy Metal(Loid)s in Agriculture Soils, Rice, and Wheat across China: Status Assessment and Spatiotemporal Analysis. Sci. Total Environ. 2023, 882, 163361. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Elli, L.; Vecchi, M.; Scricciolo, A.; Mascaretti, F.; Parisi, M.; Doneda, L.; Lombardo, V.; Araya, M.; Roncoroni, L. Mediterranean Gluten-Free Diet: Is It a Fair Bet for the Treatment of Gluten-Related Disorders? Front. Nutr. 2020, 7, 583981. [Google Scholar] [CrossRef]
- Drosdak, A.; Satyavada, S.; Ismail, M.; Shah, R.; Cooper, G. Obesity Prevalence in Celiac Disease in the United States from 2014 to 2018. Int. J. Obes. 2022, 46, 441–443. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Tang, Z.; Sun, S.; Ma, J.F.; Huang, X.-Y.; Zhao, F.-J. OASTL-A1 Functions as a Cytosolic Cysteine Synthase and Affects Arsenic Tolerance in Rice. J. Exp. Bot. 2020, 71, 3678–3689. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of Zinc Oxide Nanoparticles on Arsenic Stress in Rice (Oryza Sativa L.): Germination, Early Growth, and Arsenic Uptake. Environ. Sci. Pollut. Res. Int. 2020, 27, 26974–26981. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO(2) Nanoparticles to Reduce Bioaccumulation of Arsenic in Rice Seedlings (Oryza Sativa L.): A Mechanistic Study. J. Hazard Mater. 2021, 405, 124047. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Mazumdar, M.; Bellinger, D.C.; Gregas, M.; Abanilla, K.; Bacic, J.; Needleman, H.L. Low-Level Environmental Lead Exposure in Childhood and Adult Intellectual Function: A Follow-up Study. Environ. Health A Glob. Access Sci. Source 2011, 10, 24. [Google Scholar] [CrossRef]
- Davis, M.A.; Gilbert-Diamond, D.; Karagas, M.R.; Li, Z.; Moore, J.H.; Williams, S.M.; Frost, H.R. A Dietary-Wide Association Study (DWAS) of Environmental Metal Exposure in US Children and Adults. PLoS ONE 2014, 9, e104768. [Google Scholar] [CrossRef]
- Munera-Picazo, S.; Ramírez-Gandolfo, A.; Burló, F.; Carbonell-Barrachina, A.A. Inorganic and Total Arsenic Contents in Rice-Based Foods for Children with Celiac Disease. J. Food Sci. 2014, 79, T122–T128. [Google Scholar] [CrossRef]
- Limmer, M.A.; Seyfferth, A.L. Altering the Localization and Toxicity of Arsenic in Rice Grain. Sci. Rep. 2022, 12, 5210. [Google Scholar] [CrossRef]
- Hasanato, R.M.; Almomen, A.M. Unusual Presentation of Arsenic Poisoning in a Case of Celiac Disease. Ann. Saudi Med. 2015, 35, 165–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bascuñán, K.A.; Orosteguí, C.; Rodríguez, J.M.; Roncoroni, L.; Doneda, L.; Elli, L.; Araya, M. Heavy Metal and Rice in Gluten-Free Diets: Are They a Risk? Nutrients 2023, 15, 2975. https://doi.org/10.3390/nu15132975
Bascuñán KA, Orosteguí C, Rodríguez JM, Roncoroni L, Doneda L, Elli L, Araya M. Heavy Metal and Rice in Gluten-Free Diets: Are They a Risk? Nutrients. 2023; 15(13):2975. https://doi.org/10.3390/nu15132975
Chicago/Turabian StyleBascuñán, Karla A., Claudia Orosteguí, Juan Manuel Rodríguez, Leda Roncoroni, Luisa Doneda, Luca Elli, and Magdalena Araya. 2023. "Heavy Metal and Rice in Gluten-Free Diets: Are They a Risk?" Nutrients 15, no. 13: 2975. https://doi.org/10.3390/nu15132975
APA StyleBascuñán, K. A., Orosteguí, C., Rodríguez, J. M., Roncoroni, L., Doneda, L., Elli, L., & Araya, M. (2023). Heavy Metal and Rice in Gluten-Free Diets: Are They a Risk? Nutrients, 15(13), 2975. https://doi.org/10.3390/nu15132975





