Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications
Abstract
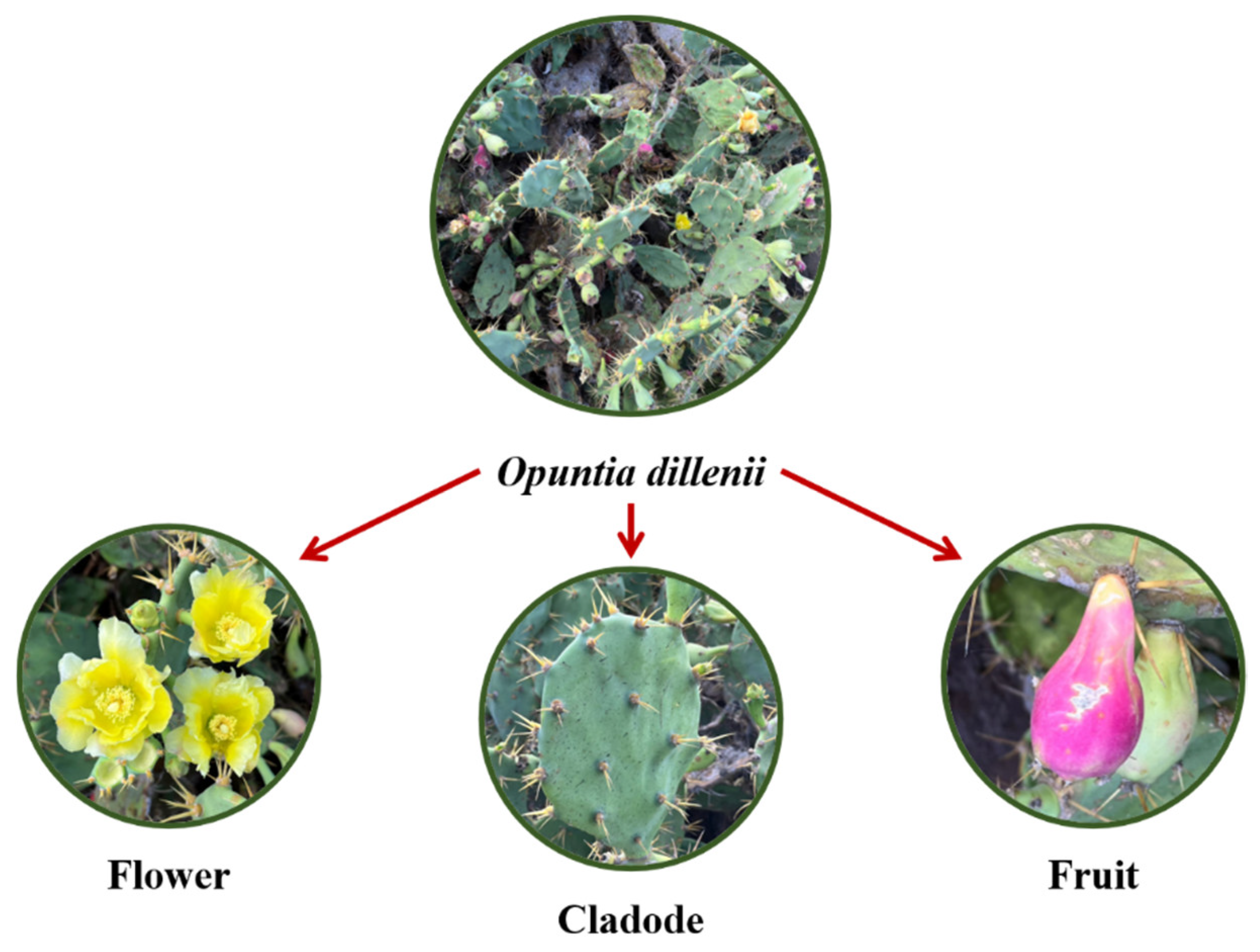
1. Introduction
2. Chemical Compounds of Opuntia dillenii
2.1. Fruits
2.2. Seeds
2.3. Cladodes
2.4. Flowers
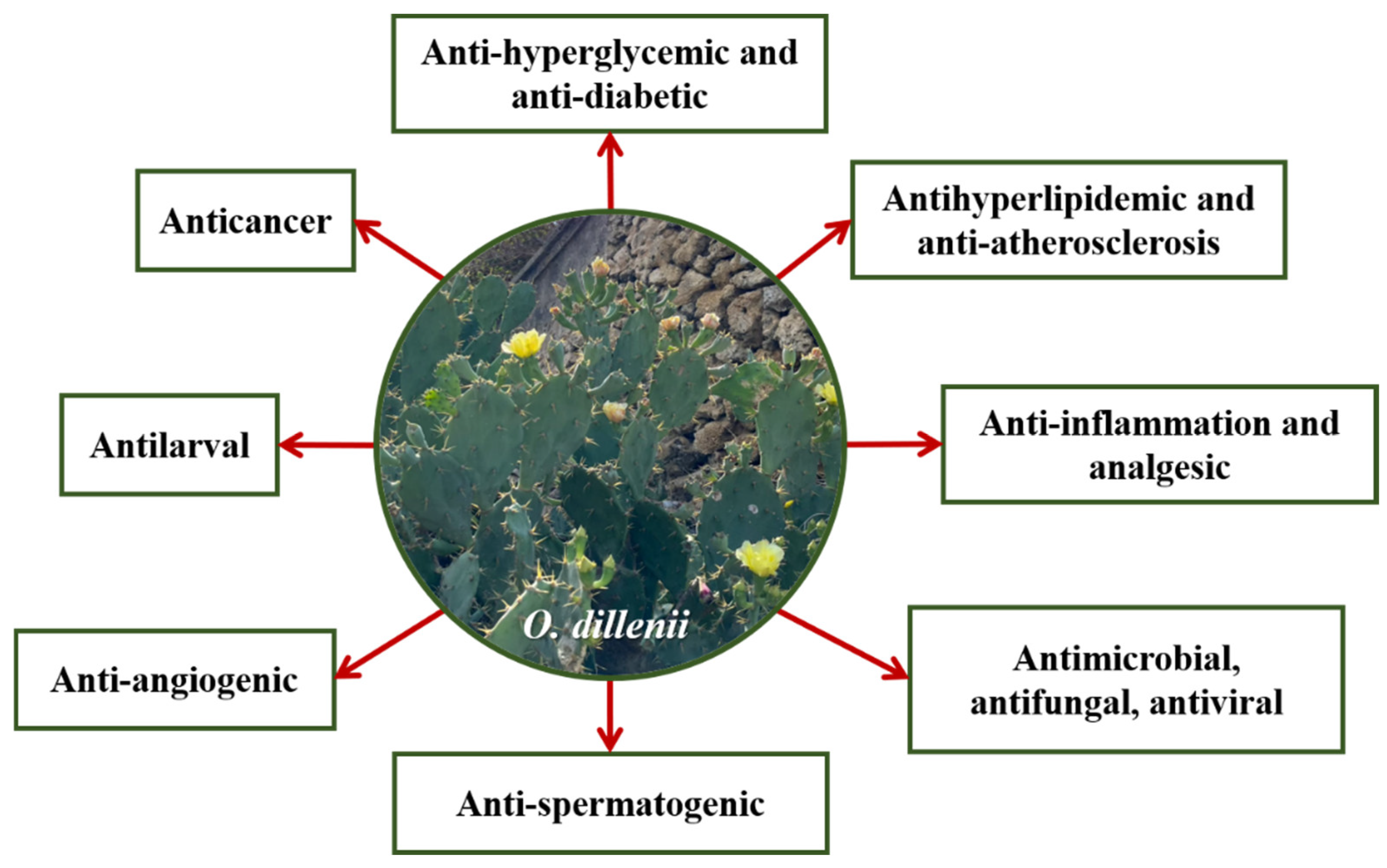
3. Biological Properties of Opuntia dillenii
3.1. Anti-Hyperglycemic and Anti-Diabetic Effects
3.2. Antihyperlipidemic and Anti-Atherosclerosis Effects
3.3. Anti-Inflammatory and Analgesic Effects
3.4. Antimicrobial, Antifungal, and Antiviral Activities
3.5. Anti-Spermatogenic Activity
3.6. Anticancer Activity
3.7. Antilarval Activity
3.8. Anti-Angiogenic Effects
3.9. Antioxidant Activity
4. Applications for Opuntia dillenii
4.1. Edible Coating
4.2. Food Products
4.3. Cosmetics
4.4. Gold and Silver Nanoparticles
4.5. Pharmaceutical Applications
4.6. Wastewater Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastola, T.; Poudel, M.; Lamichhane, S.; Sharma, G.; Poudel, P. Opuntia dillenii (Ker Gawl.) Haw. In Himalayan Fruits and Berries; Elsevier: Amsterdam, The Netherlands, 2023; pp. 303–312. [Google Scholar]
- Kalegowda, P.; Haware, D.J.; Rajarathnam, S.; Shashirekha, M.N. Minerals of cactus (Opuntia dillenii): Cladode and fruit. Curr. Sci. 2015, 109, 2295–2298. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Q.; Zhou, X.; Zeng, F.; Lu, X. Extraction of Opuntia dillenii Haw. polysaccharides and their antioxidant activities. Molecules 2016, 21, 1612. [Google Scholar] [CrossRef] [PubMed]
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia dillenii: A forgotten plant with promising pharmacological properties. J. Pharmacopunct. 2019, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-S.; Cheng, Y.-T.; Chan, Y.-J.; Lu, W.-C.; Yang, K.-m.; Li, P.-H. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci. Rep. 2023, 13, 501. [Google Scholar] [CrossRef]
- Raj, V. Plant Opuntia dillenii: A review on its traditional uses, phytochemical and pharmacological properties. EC Pharm. Sci. 2015, 1, 29–43. [Google Scholar]
- Majeed, S.; Zafar, M.; Ahmad, M.; Zafar, S.; Ghufran, A.; Ayoub, M.; Sultana, S.; Yaseen, G.; Raza, J. Morpho-palynological and anatomical studies in desert cacti (Opuntia dillenii and Opuntia monacantha) using light and scanning electron microscopy. Microsc. Res. Tech. 2022, 85, 2801–2812. [Google Scholar] [CrossRef]
- Ahmed, M.; Tanbouly, N.E.; Islam, W.; Sleem, A.; Senousy, A.E. Antiinflammatory flavonoids from Opuntia dillenii (Ker-Gawl) Haw. flowers growing in Egypt. Phytother. Res. 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Kumar, A.S.; Ganesh, M.; Peng, M.M.; Jang, H.T. Phytochemical, antioxidant, antiviral and cytotoxic evaluation of Opuntia dillenii flowers. Bangladesh J. Pharmacol. 2014, 9, 351–355. [Google Scholar] [CrossRef]
- Perfumi, M.; Tacconi, R. Antihyperglycemic effect of fresh Opuntia dillenii fruit from Tenerife (Canary Islands). Int. J. Pharmacogn. 1996, 34, 41–47. [Google Scholar] [CrossRef]
- Loro, J.; Del Rio, I.; Perez-Santana, L. Preliminary studies of analgesic and anti-inflammatory properties of Opuntia dillenii aqueous extract. J. Ethnopharmacol. 1999, 67, 213–218. [Google Scholar] [CrossRef]
- Siddiqui, F.; Naqvi, S.; Abidi, L.; Faizi, S.; Avesi, L.; Mirza, T.; Farooq, A.D. Opuntia dillenii cladode: Opuntiol and opuntioside attenuated cytokines and eicosanoids mediated inflammation. J. Ethnopharmacol. 2016, 182, 221–234. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, M.; Azmat, A.; Ahmad, S.I.; Faizi, Z.; Abidi, L.; Faizi, S. Hypotensive activity, toxicology and histopathology of opuntioside-I and methanolic extract of Opuntia dillenii. Biol. Pharm. Bull. 2005, 28, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lan, Q.; Huang, Z.; Ouyang, L.; Zeng, F. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Bouhrim, M.; Daoudi, N.E.; Ouassou, H.; Benoutman, A.; Loukili, E.H.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Aziz, M.; Bnouham, M. Phenolic content and antioxidant, antihyperlipidemic, and antidiabetogenic effects of Opuntia dillenii seed oil. Sci. World J. 2020, 2020, 5717052. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.D.; Rodríguez, E.R.; Romero, C.D. Chemical characterization of Opuntia dillenii and Opuntia ficus indica fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Gómez-López, I.; Mendiola, J.A.; Portillo, M.P.; Cano, M.P. Pressurized green liquid extraction of betalains and phenolic compounds from Opuntia stricta var. Dillenii whole fruit: Process optimization and biological activities of green extracts. Innov. Food Sci. Emerg. Technol. 2022, 80, 103066. [Google Scholar] [CrossRef]
- Lataiefa, S.B.; Zourguia, M.-N.; Affia, W.; Daoudb, A.; Gharsallahb, N.; Zourgui, L. Phytochemical Composition and Evaluation of Antioxidant and Antimicrobial Activities of Tunisian Opuntia dillenii Peel Fruits. Eur. J. Pharm. Med. Res. 2020, 7, 149–160. [Google Scholar]
- Ghazi, Z.; Ramdani, M.; Tahri, M.; Rmili, R.; Elmsellem, H.; El Mahi, B.; Fauconnier, M.-L. Chemical composition and antioxidant activity of seeds oils and fruit juice of Opuntia ficus indica and Opuntia dillenii from Morocco. J. Mater. 2015, 6, 2338–2345. [Google Scholar]
- Chinedu, N.U.; Benjamin, A.; Peter, A. Chemical composition and physicochemical analysis of matured stems of Opuntia dillenii grown in Nigeria. Food Sci. Technol. 2017, 5, 106–112. [Google Scholar] [CrossRef]
- Ben Lataief, S.; Zourgui, M.-N.; Rahmani, R.; Najjaa, H.; Gharsallah, N.; Zourgui, L. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from Opuntia dillenii cladodes. J. Food Meas. Charact. 2021, 15, 782–794. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Farooq, A.D.; Kabir, N.; Fatima, N.; Abidi, L.; Faizi, S. Toxicological assessment of Opuntia dillenii (Ker Gawl.) Haw. cladode methanol extract, fractions and its alpha pyrones: Opuntiol and opuntioside. J. Ethnopharmacol. 2021, 280, 114409. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hurtado, N.; Heredia, F.J. Potential use of new Colombian sources of betalains. Colorimetric study of red prickly pear (Opuntia dillenii) extracts under different technological conditions. Food Res. Int. 2015, 71, 91–99. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, stability, and bioaccessibility of Betalain and phenolic compounds from Opuntia stricta var. Dillenii fruits and products of their industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. 2022, 1–30. [Google Scholar] [CrossRef]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–117. [Google Scholar]
- Al-Naqeb, G.; Cafarella, C.; Aprea, E.; Ferrentino, G.; Gasparini, A.; Buzzanca, C.; Micalizzi, G.; Dugo, P.; Mondello, L.; Rigano, F. Supercritical Fluid Extraction of Oils from Cactus Opuntia ficus-indica L. and Opuntia dillenii Seeds. Foods 2023, 12, 618. [Google Scholar] [CrossRef]
- Rayan, A.M.; Morsy, N.E.; Youssef, K.M. Enrichment of rice-based extrudates with Cactus Opuntia dillenii seed powder: A novel source of fiber and antioxidants. J. Food Sci. Technol. 2018, 55, 523–531. [Google Scholar] [CrossRef]
- Alsaad, A.J.A.; Altemimi, A.B.; Aziz, S.N.; Lakhssassi, N. Extraction and identification of cactus Opuntia dillenii seed oil and its added value for human health benefits. Pharmacogn. J. 2019, 11, 579–587. [Google Scholar] [CrossRef]
- Atlas, D. International Diabetes Federation. In IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; Van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Han, Y.-L.; Jin, Z.-Y.; Xu, X.-M.; Zha, X.-Q.; Chen, H.-Q.; Yin, Y.-Y. Protective effect of polysaccharides from Opuntia dillenii Haw. fruits on streptozotocin-induced diabetic rats. Carbohydr. Polym. 2015, 124, 25–34. [Google Scholar] [CrossRef]
- Bouhrim, M.; Ouassou, H.; Loukili, E.H.; Ramdani, M.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Antidiabetic effect of Opuntia dillenii seed oil on streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2019, 9, 381. [Google Scholar]
- Bouhrim, M.; Ouassou, H.; Boutahiri, S.; Daoudi, N.E.; Mechchate, H.; Gressier, B.; Eto, B.; Imtara, H.; Alotaibi, A.A.; Al-Zharani, M. Opuntia dillenii (Ker Gawl.) Haw., seeds oil antidiabetic potential using in vivo, in vitro, in situ, and ex vivo approaches to reveal its underlying mechanism of action. Molecules 2021, 26, 1677. [Google Scholar] [CrossRef]
- Loukili, E.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Zidi, K.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia dillenii Fruits Collected from Morocco. J. Food Qual. 2022, 2022. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Huang, W.; Yuan, Q.-X.; Cheng, J.; Huang, Z.-C.; Ouyang, L.-J.; Zeng, F.-H. Hypolipidaemic effects and mechanisms of the main component of Opuntia dillenii Haw. polysaccharides in high-fat emulsion-induced hyperlipidaemic rats. Food Chem. 2012, 134, 964–971. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927. [Google Scholar] [PubMed]
- Wang, Y.; Qi, Z.; Liu, Z.; Li, T.; Cui, H.; Wang, B.; Chi, N. Therapeutic effects and mechanisms of Opuntia dillenii Haw on atherosclerosis of rats. Acta Pharm. Sin 2015, 50, 453–458. [Google Scholar]
- Babitha, S.; Bindu, K.; Nageena, T.; Veerapur, V. Fresh fruit juice of Opuntia dillenii Haw. attenuates acetic acid–induced ulcerative colitis in rats. J. Diet. Suppl. 2019, 16, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Shirazinia, R.; Golabchifar, A.A.; Rahimi, V.B.; Jamshidian, A.; Samzadeh-Kermani, A.; Hasanein, P.; Hajinezhad, M.; Askari, V.R. Protective effect of Opuntia dillenii haw fruit against lead acetate-induced hepatotoxicity: In vitro and in vivo studies. Evid.-Based Complement. Altern. Med. 2021, 2021, 6698345. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Zhou, X.; Chen, H. A Study on the Time–Effect and Dose–Effect Relationships of Polysaccharide from Opuntia dillenii against Cadmium-Induced Liver Injury in Mice. Foods 2022, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Abidi, L.; Poh, C.F.; Naqvi, S.; Faizi, S.; Dar Farooq, A. Analgesic Potential of Opuntia dillenii and its Compounds Opuntiol and Opuntioside Against Pain Models in Mice. Rec. Nat. Prod. 2016, 10, 721–734. [Google Scholar]
- Kumaar, A.S.; Vanitha, J.; Venkateshwaran, K.; Reddy, K.S.; Karthikeyan, D. Antibacterial and antifungal activity of Opuntia dillenii (Cactaceae) fruit extract. J. Environ. Nanotechnol. 2013, 2, 16–19. [Google Scholar]
- Katanić, J.; Yousfi, F.; Caruso, M.C.; Matić, S.; Monti, D.M.; Loukili, E.H.; Boroja, T.; Mihailović, V.; Galgano, F.; Imbimbo, P. Characterization of bioactivity and phytochemical composition with toxicity studies of different Opuntia dillenii extracts from Morocco. Food Biosci. 2019, 30, 100410. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, R.; Sharma, A.; Chaudhudery, R.; Bhatnager, A.; Dobhal, M.; Joshi, Y.; Sharma, M. Antispermatogenic effect and chemical investigation of Opuntia dillenii. Pharm. Biol. 2002, 40, 411–415. [Google Scholar] [CrossRef]
- Niyaz, A.M.; Ravikumar, S. Antilarval and in vitro anticancer efficacy of Cladode extracts of Opuntia dillenii (Ker Gawl.) Haw., Cereus pterogonus Lem. and Acanthocereus tetragonus (L.) Hummelinck. Res. J. Pharm. Technol. 2022, 15, 2877–2882. [Google Scholar] [CrossRef]
- Ejaz, S.; Anwar, K.; Taj, R.; Ashraf, M. A novel link between angiogenesis and natural products: Anti-angiogenic effects of Opuntia dillenii. Open Life Sci. 2014, 9, 298–308. [Google Scholar] [CrossRef]
- Wu, S. Extending shelf-life of fresh-cut potato with cactus Opuntia dillenii polysaccharide-based edible coatings. Int. J. Biol. Macromol. 2019, 130, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Li, S.; Li, C. Effects of cactus Opuntia dillenii polysaccharide-based coatings loaded with glutathione on the preservation of freshly cut Chinese water chestnut. Food Chem. 2023, 401, 134187. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Jäger, H.; Knorr, D.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Impact of pulsed electric fields, high hydrostatic pressure, and thermal pasteurization on selected characteristics of Opuntia dillenii cactus juice. LWT 2017, 79, 534–542. [Google Scholar] [CrossRef]
- El-Samahy, S.; Gaballah, A.; Embaby, H.; Hamed, Y.; Khalil, R. A novel low fat ice cream based on the use of preparation of cactus pear (Opuntia dillenii) pulp. Egypt. J. Dairy Sci. 2015, 43, 91–104. [Google Scholar]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Ahmed, A.; Rauf, A.; Hemeg, H.A.; Qureshi, M.N.; Sharma, R.; Aljohani, A.S.; Alhumaydhi, F.A.; Khan, I.; Alam, A.; Rahman, M.M. Green synthesis of gold and silver nanoparticles using Opuntia dillenii aqueous extracts: Characterization and their antimicrobial assessment. J. Nanomater. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- De Paz, P.L.P.; Medina, I.M. Catalogo de las Plantas Medicinales de la Flora Canaria: Applicaciones Populares; Instituto de Estudios Canarios: La Laguna, Spain, 1988. [Google Scholar]
- Khan, M.P.Z.; Ahmad, M.; Zafar, M.; Sultana, S.; Ali, M.I.; Sun, H. Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pakistan. J. Ethnopharmacol. 2015, 173, 191–203. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Azam, M.N.K.; Rahman, M.M.; Seraj, S.; Mahal, M.J.; Mou, S.M.; Nasrin, D.; Khatun, Z.; Islam, F.; Chowdhury, M.H. A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am.-Eurasian J. Sustain. Agric. AEJSA 2011, 5, 350–357. [Google Scholar]
- Pooja, S.; Vidyasagar, G. Phytochemical screening for secondary metabolites of Opuntia dillenii Haw. J. Med. Plants Stud. 2016, 4, 39–43. [Google Scholar]
- Kanwal, F.; Rehman, R.; Mushtaq, M.W.; Batool, A.; Naseem, S. Use of Opuntia dillenii seeds for sorptive removal of acidic textile dyes from water in benign way. Asian J. Chem. 2013, 25, 7710. [Google Scholar] [CrossRef]
- Nougbodé, Y.A.E.I.; Agbangnan, C.P.; Koudoro, A.Y.; Dèdjiho, C.A.; Aïna, M.P.; Mama, D.; Sohounhloué, D.C.K. Evaluation of the Opuntia dillenii as natural coagulant in water clarification: Case of treatment of highly turbid surface water. J. Water Resour. Prot. 2013, 5, 1242. [Google Scholar] [CrossRef]

| Part | Chemical Composition | Value | Ref. |
|---|---|---|---|
| Fruit | Fiber (%) | 9.49 ± 1.51 | [16] |
| Protein (%) | 0.52 ± 0.12 | ||
| Fat (%) | 0.71 ± 0.19 | ||
| Ash (%) | 0.437 ± 0.062 | ||
| Acidity (g/100 g) | 1.23 ± 0.272 | ||
| Ascorbic acid (mg/100 g) | 29.7 ± 2.95 | ||
| Phenolics (mg/100 g) | 117 ± 10 | ||
| Na (mg/kg) | 153 ± 162 | ||
| K (mg/kg) | 908 ± 251 | ||
| Ca (mg/kg) | 535 ± 187 | ||
| Mg (mg/kg) | 454 ± 102 | ||
| Fe (mg/kg) | 1.53 ± 0.31 | ||
| Cu (mg/kg) | 0.334 ± 0.054 | ||
| Zn (mg/kg) | 1.29 ± 0.49 | ||
| Mn (mg/kg) | 5.09 ± 3.80 | ||
| Ni (mg/kg) | 0.204 ± 0.082 | ||
| Cr (mg/kg) | 0.144 ± 0.036 | ||
| Whole fruit | Major betalains | [17] | |
| Betanin (mg/g) | 2.75 ± 0.00 | ||
| Isobetanin (mg/g) | 1.60 ± 0.08 | ||
| 2′-O-apiosyl-4-O-phyllocactin (mg/g) | 1.13 ± 0.08 | ||
| 5″-O-E-sinapoyl-2′-apyosil-phyllocactin (mg/g) | 2.82 ± 0.02 | ||
| Neobetanin (mg/g) | 1.67 ± 0.00 | ||
| Neobetanin isomer III v | 0.23 ± 0.01 | ||
| Total betalains (mg/g) | 10.19 ± 0.13 | ||
| Phenolic acid | |||
| Protocatechuic acid derivative (mg/g) | 3.26 ± 0.18 | ||
| Piscidic acid (mg/g) | 0.93 ± 0.00 | ||
| Total phenolic acids (mg/g) | 4.19 ± 0.18 | ||
| Major flavanoids | |||
| Quercetin-3-O-rhamnosyl-rutinoside (QG3) (mg/g) | 0.03 ± 0.00 | ||
| Quercetin hexose pentoside (QG2) (mg/g) | 0.02 ± 0.00 | ||
| Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) (mg/g) | 0.28 ± 0.00 | ||
| Fruit peel (Aqueous extract) | Phenolic acids | [18] | |
| Quinic acid (μg/g) | 118.95 | ||
| Caffeic acid (μg/g) | 0.041 | ||
| P-coumaric acid (μg/g) | 0.046 | ||
| Trans ferulic acid (μg/g) | 0.042 | ||
| Flavonoids | |||
| Rutin (μg/g) | 0.046 | ||
| Naringin (μg/g) | 0.007 | ||
| Luteolin (μg/g) | 0.014 | ||
| Fruit peel (Ethanolic extract) | Phenolic acids | [18] | |
| Quinic acid (μg/g) | 1437.03 | ||
| Protocatechuic acid (μg/g) | 1 | ||
| Caffeic acid (μg/g) | 0.5 | ||
| P-coumaric acid (μg/g) | 1.27 | ||
| Trans ferulic acid (μg/g) | 2.4 | ||
| Cinnamic acid (μg/g) | 40.19 | ||
| Flavonoids | |||
| Catechin (μg/g) | 0.36 | ||
| Hyperoside (μg/g) | 0.92 | ||
| Naringin (μg/g) | 0.34 | ||
| Quercetrin (μg/g) | 0.36 | ||
| Quercetin (μg/g) | 9.27 | ||
| Naringenin (μg/g) | 0.29 | ||
| Apeginin (μg/g) | 0.095 | ||
| Luteolin (μg/g) | 6.16 | ||
| Cirsiliol (μg/g) | 7.18 | ||
| Acacetin (μg/g) | 0.19 | ||
| Fruit peel | Compounds | [18] | |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (area %) | 2.21 | ||
| 2-Furancarboxaldehyde-5-(hydroxymethyl)- (area %) | 32.91 | ||
| Phenol, 2-methyl-5-(1-methylethyl)- (area %) | 1.36 | ||
| α-D-Glucopyranoside, α-D-glucopyranosyl (area %) | 2.36 | ||
| Phen-1,4-diol, 2,3-dimethyl-5-trifluoromethyl- (area %) | 0.20 | ||
| n-Hexadecanoic acid (area %) | 3.52 | ||
| Ethyl iso-allocholate (area %) | 1.13 | ||
| Vitamin E (area %) | 1.11 | ||
| β-Sitosterol (area %) | 9.57 | ||
| Dry seeds | Macroelements | [19] | |
| Ca (mg/100 g) | 408.28 | ||
| Mg (mg/100 g) | 240.30 | ||
| Na (mg/100 g) | 18.18 | ||
| K (mg/100 g) | 201.96 | ||
| P (mg/100 g) | 970.15 | ||
| Trace elements | |||
| Fe (mg/100 g) | 1.98 | ||
| Cu (mg/100 g) | 1.18 | ||
| Zn (mg/100 g) | 78.26 | ||
| Mn (mg/100 g) | 4.35 | ||
| Cr (mg/100 g) | 1.58 | ||
| Ni (mg/100 g) | 2.76 | ||
| Matured stems | Proximate composition | [20] | |
| Ash content (%) | 3.33 ± 0.18 | ||
| Moisture content (%) | 11.20 ± 0.13 | ||
| Crude protein (%) | 11.60 ± 0.21 | ||
| Fat (%) | 4.42 ± 0.19 | ||
| Crude fiber (%) | 4.40 ± 0.06 | ||
| Carbohydrate (%) | 64 ± 0.14 | ||
| Phytochemical analysis | |||
| Anthocyanin (μg/mL) | 0.04 ± 0.02 | ||
| Oxalate (μg/mL) | 1.07 ± 0.01 | ||
| Tanin (μg/mL) | 13.62 ± 0.05 | ||
| Rutin (μg/mL) | 12.41 ± 0.26 | ||
| Phenol (μg/mL) | 4.66 ± 0.08 | ||
| Lunamarine (μg/mL) | 34.43 ± 0.35 | ||
| Saponin (μg/mL) | 118.08 ± 0.57 | ||
| Sapogenin (μg/mL) | 11.88 ± 0.09 | ||
| Ribalinidine (μg/mL) | 3.75 ± 0.09 | ||
| Phytate (μg/mL) | 0.18 ± 0.04 | ||
| Kaempferol (μg/mL) | 7.90 ± 0.06 | ||
| Catechin (μg/mL) | 44.90 ± 0.38 | ||
| Fatty acid composition | |||
| Lauric acid (%) | 7.78 ± 0.06 | ||
| Myristic acid (%) | 41.24 ± 0.55 | ||
| Palmitic acid (%) | 5.48 ± 0.0 | ||
| Heptadecanoic acid (%) | 11.32 ± 0.06 | ||
| Stearic acid (%) | 9.25 ± 0.03 | ||
| Arachidic acid (%) | 1.21 ± 0.06 | ||
| Linoleic acid (%) | 13.95 ± 0.02 | ||
| Cladode (Aqueous extract) | Phenolic acid and flavonoid | [21] | |
| Quinic acid (μg/g dw) | 18.40 | ||
| Protocatechuic acid (μg/g dw) | 0.44 | ||
| Caffeic acid (μg/g dw) | 0.025 | ||
| Syringic acid (μg/g dw) | 0.003 | ||
| P-coumaric acid (μg/g dw) | 0.106 | ||
| Naringin (μg/g dw) | 0.01 | ||
| Trans ferulic acid (μg/g dw) | 0.16 | ||
| Cinnamic acid (μg/g dw) | 0.023 | ||
| Compounds | |||
| Aromadendrene | Not mentioned | ||
| 9,9-Dimethoxybicyclo [3.3.1] nona-2,4-dion | Not mentioned | ||
| 6,7-Dimethylthieno [2,3-b] quinolin-3-ylamine | Not mentioned | ||
| 2-Hydroxypentadecyl propanoate | Not mentioned | ||
| 2-Hexyl-1-octanol | Not mentioned | ||
| 1-Eicosene | Not mentioned | ||
| Cladode (Ethanolic extract) | Phenolic acid and flavonoid | [21] | |
| Quinic acid (μg/g dw) | 58.78 | ||
| Gallic acid (μg/g dw) | 0.72 | ||
| Protocatechuic acid (μg/g dw) | 0.38 | ||
| Rutin (μg/g dw) | 7.02 | ||
| Hyperoside (μg/g dw) | 0.24 | ||
| P-coumaric acid (μg/g dw) | 1.49 | ||
| Naringin (μg/g dw) | 0.15 | ||
| Quercetrin (μg/g dw) | 0.50 | ||
| 1,3-di-O-cafeoylquinic acid (μg/g dw) | 0.77 | ||
| Apegenin-7-O-glucoside (μg/g dw) | 0.09 | ||
| Trans ferulic acid (μg/g dw) | 1.56 | ||
| Salviolonic acid (μg/g dw) | 0.28 | ||
| Quercetin (μg/g dw) | 11.5 | ||
| Kampherol (μg/g dw) | 0.21 | ||
| Naringenin (μg/g dw) | 0.16 | ||
| Apeginin (μg/g dw) | 0.10 | ||
| Luteolin (μg/g dw) | 4.93 | ||
| Cirsiliol (μg/g dw) | 3.22 | ||
| Cirsilineol (μg/g dw) | 0.15 | ||
| Acacetin (μg/g dw) | 0.29 | ||
| Compounds | |||
| n-Hexadecanoic acid | Not mentioned | ||
| Oleic acid | Not mentioned | ||
| Octadecanoic acid | Not mentioned | ||
| Vitamin A | Not mentioned | ||
| 4,6-Cholestadien-3β-ol | Not mentioned | ||
| Stigmastan-3,5-diene | Not mentioned | ||
| Retinol, acetate | Not mentioned | ||
| Vitamin E | Not mentioned | ||
| β-Sitosterol | Not mentioned | ||
| Cladode | Neutral sugars | [22] | |
| Rhamnose (%) | 15.70 | ||
| Arabinose (%) | 38.80 | ||
| Xylose (%) | 5.10 | ||
| Galactose (%) | 33.00 | ||
| Glucose (%) | 5.10 | ||
| Uronic acid (%) | 2.50 | ||
| Cladode (Methanolic extract) | Pure α-pyrone compounds | [23] | |
| Opuntiol (%) | 1.04 | ||
| Opuntioside (%) | 5.34 | ||
| Flowers | Phytochemicals | [8] | |
| Flavonoids | Not mentioned | ||
| Tannins | Not mentioned | ||
| Alkaloids | Not mentioned | ||
| Anthraquinone glycosides | Not mentioned | ||
| Steroids | Not mentioned | ||
| Sample | Method | Concentration | Inhibition Ratio | Ascorbic Acid | Ref. |
|---|---|---|---|---|---|
| Seed oil | DPPH | 5 μL/mL | 21.04 ± 0.071 | 21.02 ± 0.066 | [19] |
| 10 μL/mL | 26.19 ± 0.076 | 29.96 ± 0.091 | |||
| 15 μL/mL | 29.81 ± 0.066 | 40.15 ± 0.060 | |||
| 20 μL/mL | 42.60 ± 0.061 | 63.85 ± 0.064 | |||
| IC50 | 27.21 ± 0.075 | 16.56 ± 0.019 | |||
| Whole fruit | ORAC | μmol Trolox eq./g dry weight | 151.81 ± 1.86 | - | [17] |
| Fruit juice | DPPH | 5 μL/mL | 39.15 ± 0.095 | 63.85 ± 0.064 | [19] |
| 10 μL/mL | 56.11 ± 0.080 | 63.85 ± 0.064 | |||
| 15 μL/mL | 73.54 ± 0.164 | 63.85 ± 0.064 | |||
| 20 μL/mL | 91.94 ± 0.031 | 63.85 ± 0.064 | |||
| IC50 | 8.18 ± 0.010 | 16.56 ± 0.019 | |||
| Aqueous cladode extract | DPPH | IC50 mg/mL | 0.54 ± 0.001 | 0.015 ± 0.00 | [21] |
| NO | IC50 mg/mL | 0.15 ± 0.005 | 0.04 ± 0.001 | ||
| FRAP | 700 nm | 1.39 ± 0.00 | 2.41 ± 0.00 | ||
| TEAC | mM Trolox/g | 0.46 ± 0.15 | 0.85 ± 0.02 | ||
| TAC | mg AAE/g | 60.44 ± 3.65 | 81.24 ± 0.14 | ||
| Ethanolic cladode extract | DPPH | IC50 mg/mL | 0.60 ± 0.005 | 0.015 ± 0.00 | [21] |
| NO | IC50 mg/mL | 0.06 ± 0.003 | 0.04 ± 0.001 | ||
| FRAP | 700 nm | 1.97 ± 0.00 | 2.41 ± 0.00 | ||
| TEAC | mM Trolox/g | 0.59 ± 0.75 | 0.85 ± 0.02 | ||
| TAC | mg AAE/g | 62.99 ± 1.18 | 81.24 ± 0.14 | ||
| Seed oil | DPPH | IC50 mg/mL | 0.38 ± 0.08 | 0.23 ± 0.01 μg/mL | [15] |
| Flower | DPPH | IC50 μg/mL | 58.7 ± 0.00 | 1.2 ± 0.00 | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-C.; Chiu, C.-S.; Chan, Y.-J.; Mulio, A.T.; Li, P.-H. Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications. Nutrients 2023, 15, 2962. https://doi.org/10.3390/nu15132962
Lu W-C, Chiu C-S, Chan Y-J, Mulio AT, Li P-H. Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications. Nutrients. 2023; 15(13):2962. https://doi.org/10.3390/nu15132962
Chicago/Turabian StyleLu, Wen-Chien, Chien-Shan Chiu, Yung-Jia Chan, Amanda Tresiliana Mulio, and Po-Hsien Li. 2023. "Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications" Nutrients 15, no. 13: 2962. https://doi.org/10.3390/nu15132962
APA StyleLu, W.-C., Chiu, C.-S., Chan, Y.-J., Mulio, A. T., & Li, P.-H. (2023). Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications. Nutrients, 15(13), 2962. https://doi.org/10.3390/nu15132962








