The Association of Metabolomic Profiles of a Healthy Lifestyle with Heart Failure Risk in a Prospective Study
Abstract
1. Introduction
2. Materials and Methods
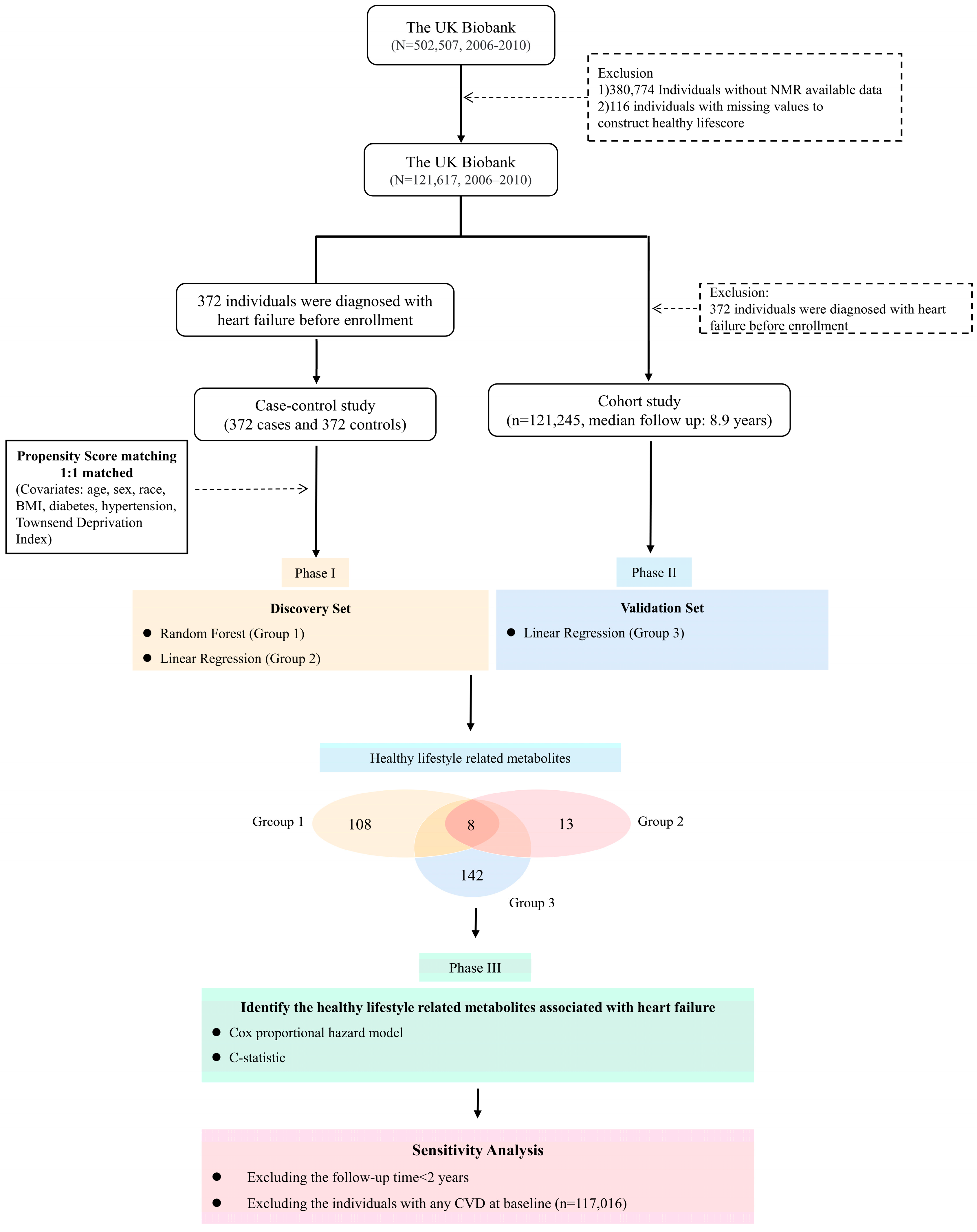
2.1. Study Design and Population
2.2. Healthy Lifestyle Score (HLS)
2.3. Metabolomics Profiling
2.4. Outcomes
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Identification of Healthy Lifestyle-Related Metabolites
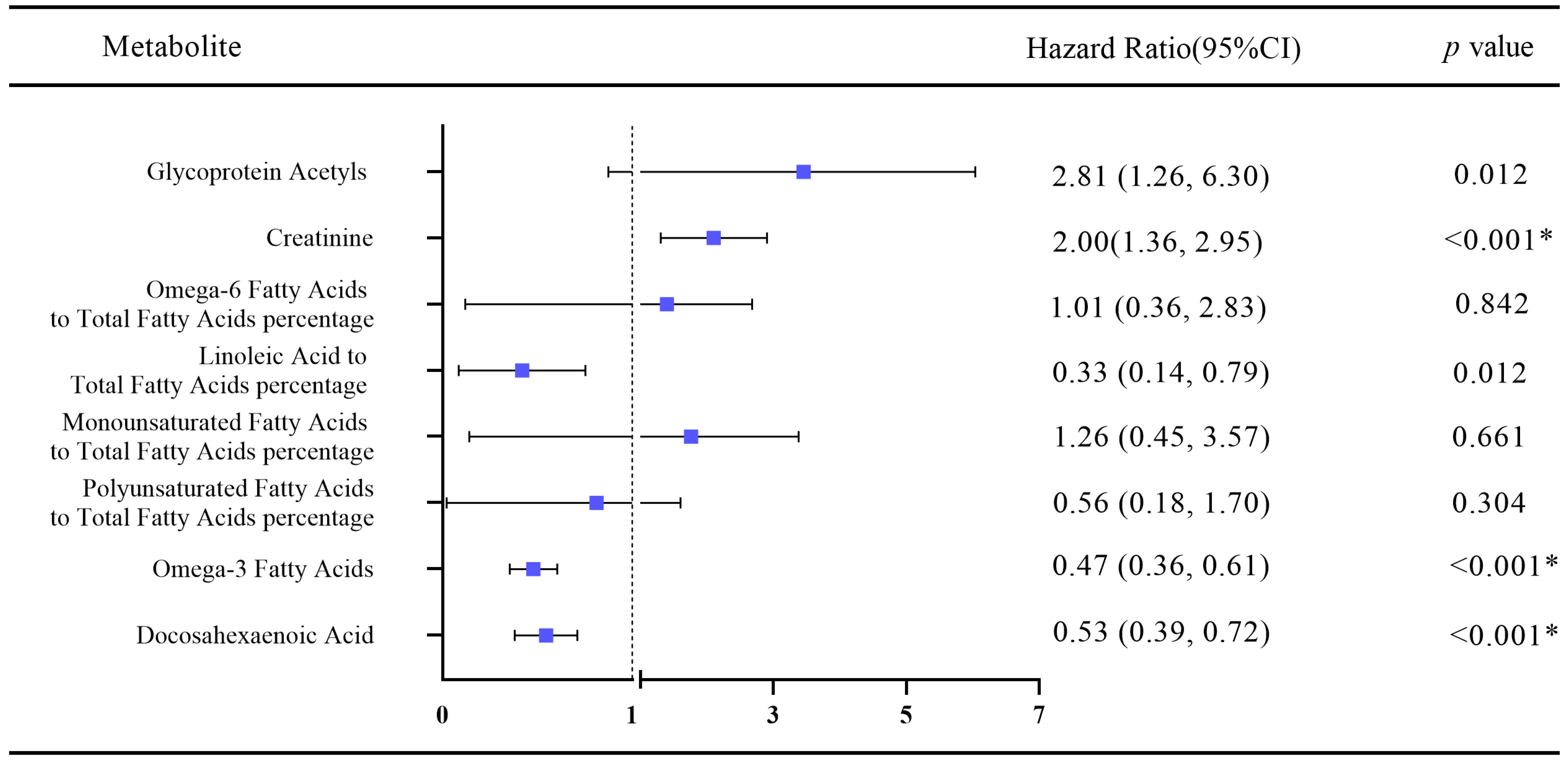
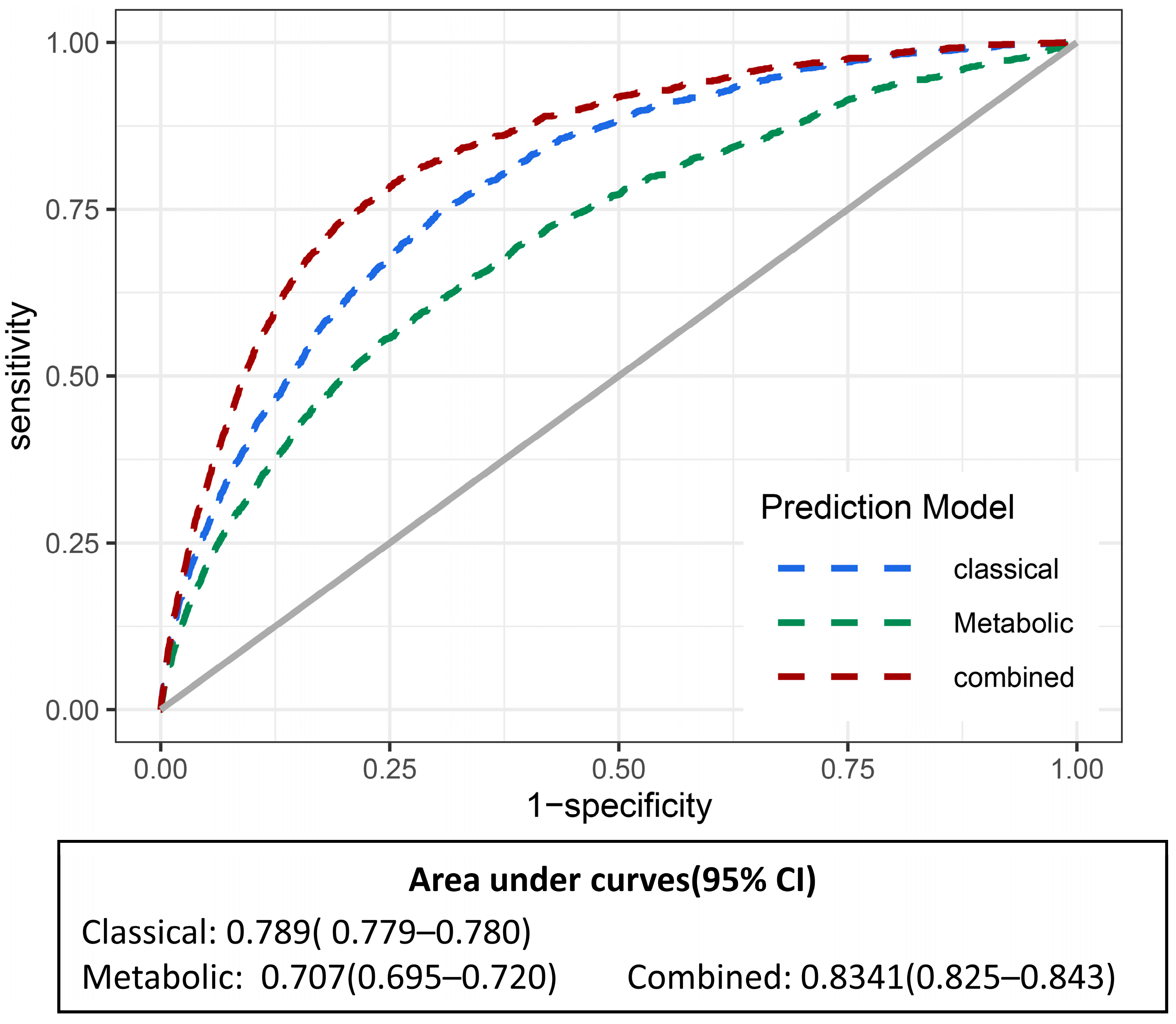
3.3. Association of Healthy Lifestyle-Related Metabolites with Heart Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Levy, D.; Kenchaiah, S.; Larson, M.G.; Benjamin, E.J.; Kupka, M.J.; Ho, K.K.; Murabito, J.M.; Vasan, R.S. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002, 347, 1397–1402. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Wang, Y.; Tuomilehto, J.; Jousilahti, P.; Antikainen, R.; Mähönen, M.; Katzmarzyk, P.T.; Hu, G. Lifestyle factors in relation to heart failure among Finnish men and women. Circ. Heart Fail. 2011, 4, 607–612. [Google Scholar] [CrossRef]
- Agha, G.; Loucks, E.B.; Tinker, L.F.; Waring, M.E.; Michaud, D.S.; Foraker, R.E.; Li, W.; Martin, L.W.; Greenland, P.; Manson, J.E.; et al. Healthy lifestyle and decreasing risk of heart failure in women: The Women’s Health Initiative observational study. J. Am. Coll. Cardiol. 2014, 64, 1777–1785. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Kalantarian, S.; Imamura, F.; Lemaitre, R.; Siscovick, D.S.; Psaty, B.M.; Mozaffarian, D. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC Heart Fail. 2015, 3, 520–528. [Google Scholar] [CrossRef]
- Larsson, S.C.; Tektonidis, T.G.; Gigante, B.; Åkesson, A.; Wolk, A. Healthy Lifestyle and Risk of Heart Failure: Results from 2 Prospective Cohort Studies. Circ. Heart Fail. 2016, 9, e002855. [Google Scholar] [CrossRef]
- Ikegami, R.; Shimizu, I.; Yoshida, Y.; Minamino, T. Metabolomic Analysis in Heart Failure. Circ. J. 2017, 82, 10–16. [Google Scholar] [CrossRef]
- Clarke, C.J.; Haselden, J.N. Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol Pathol. 2008, 36, 140–147. [Google Scholar] [CrossRef]
- Goodacre, R. Metabolic profiling: Pathways in discovery. Drug Discov. Today 2004, 9, 260–261. [Google Scholar] [CrossRef]
- Aguilar, M.A.; McGuigan, J.; Hall, M.A. Semi-automated NMR Pipeline for Environmental Exposures: New Insights on the Metabolomics of Smokers versus Non-smokers. Pac. Symp. Biocomput. 2021, 26, 316–327. [Google Scholar]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef]
- Hang, D.; Zeleznik, O.A.; He, X.; Guasch-Ferre, M.; Jiang, X. Metabolomic Signatures of Long-term Coffee Consumption and Risk of Type 2 Diabetes in Women. Diabetes Care 2020, 43, 2588–2596. [Google Scholar] [CrossRef]
- Beuchel, C.; Dittrich, J.; Pott, J. Whole Blood Metabolite Profiles Reflect Changes in Energy Metabolism in Heart Failure. Metabolites 2022, 12, 216. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, T.; Huang, Y.; Zhao, X.; Ding, Y.; Zhu, M.; Ji, M. Genetic Risk for Overall Cancer and the Benefit of Adherence to a Healthy Lifestyle. Cancer Res. 2021, 81, 4618–4627. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Chen, C.; Pan, X.F.; Guo, J.; Li, Y.; Franco, O.H.; Liu, G.; Pan, A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ 2021, 373, n604. [Google Scholar] [CrossRef]
- Said, M.A.; Verweij, N.; van der Harst, P. Associations of Combined Genetic and Lifestyle Risks With Incident Cardiovascular Disease and Diabetes in the UK Biobank Study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ahola-Olli, A.V.; Mustelin, L.; Kalimeri, M.; Kettunen, J.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Havulinna, A.S.; Lehtimäki, T.; Kähönen, M.; et al. Circulating metabolites and the risk of type 2 diabetes: A prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 2019, 62, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Nightingale Health Metabolic Biomarkers: Phase 1 Release. Available online: https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/nmrm_companion_doc.pdf (accessed on 11 March 2022).
- Si, J.; Li, J.; Yu, C.; Guo, Y.; Bian, Z.; Millwood, I.; Yang, L.; Walters, R.; Chen, Y.; Du, H.; et al. Improved lipidomic profile mediates the effects of adherence to healthy lifestyles on coronary heart disease. Elife 2021, 10, e60999. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Velandia, M.; Gonzalez-Marrachelli, V.; Domingo-Relloso, A.; Galvez-Fernandez, M.; Grau-Perez, M.; Olmedo, P.; Galan, I.; Rodriguez-Artalejo, F.; Amigo, N.; Briongos-Figuero, L.; et al. Healthy lifestyle, metabolomics and incident type 2 diabetes in a population-based cohort from Spain. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 8. [Google Scholar] [CrossRef]
- Assi, N.; Gunter, M.J.; Thomas, D.C.; Leitzmann, M.; Stepien, M.; Chajès, V.; Philip, T.; Vineis, P.; Bamia, C.; Boutron-Ruault, M.C.; et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am. J. Clin. Nutr. 2018, 108, 117–126. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Posma, J.M.; Gibson, R.; Chambers, E.S.; Hansen, T.H.; Vestergaard, H.; Hansen, T.; Beckmann, M.; Pedersen, O.; Elliott, P.; et al. Objective assessment of dietary patterns by use of metabolic phenotyping: A randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017, 5, 184–195. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, J.; Djukovic, D.; Daniel-MacDougall, C.; Gu, H.; Wu, X.; Chow, W.H. Metabolomics-identified metabolites associated with body mass index and prospective weight gain among Mexican American women. Obes. Sci. Pract. 2016, 2, 309–317. [Google Scholar] [CrossRef]
- Würtz, P.; Cook, S.; Wang, Q.; Tiainen, M.; Tynkkynen, T.; Kangas, A.J.; Soininen, P.; Laitinen, J.; Viikari, J.; Kähönen, M.; et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int. J. Epidemiol. 2016, 45, 1493–1506. [Google Scholar] [CrossRef]
- Kujala, U.M.; Mäkinen, V.P.; Heinonen, I.; Soininen, P.; Kangas, A.J.; Leskinen, T.H.; Rahkila, P.; Würtz, P.; Kovanen, V.; Cheng, S.; et al. Long-term leisure-time physical activity and serum metabolome. Circulation 2013, 127, 340–348. [Google Scholar] [CrossRef]
- Li, Y.; Gray, A.; Xue, L.; Farb, M.G.; Ayalon, N.; Andersson, C.; Ko, D.; Benjamin, E.J.; Levy, D.; Vasan, R.S.; et al. Metabolomic Profiles, Ideal Cardiovascular Health, and Risk of Heart Failure and Atrial Fibrillation: Insights From the Framingham Heart Study. J. Am. Heart Assoc. 2023, 12, e028022. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Wilk, J.B.; Tsai, M.Y.; Hanson, N.Q.; Gaziano, J.M.; Djoussé, L. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am. J. Clin. Nutr. 2012, 96, 882–888. [Google Scholar] [CrossRef]
- Berliner, D.; Mattern, S.; Wellige, M.; Malsch, C.; Güder, G.; Brenner, S.; Morbach, C.; Deubner, N.; Breunig, M.; Kiefl, R.; et al. The omega-3 index in patients with heart failure: A prospective cohort study. Prostaglandins Leukot. Essent. Fat. Acids. 2019, 140, 34–41. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- Barbarawi, M.; Lakshman, H.; Barbarawi, O.; Alabdouh, A.; Al Kasasbeh, M.; Djousse, L.; Manson, J.E. Omega-3 supplementation and heart failure: A meta-analysis of 12 trials including 81,364 participants. Contemp. Clin. Trials. 2021, 107, 106458. [Google Scholar] [CrossRef]
- Matsuo, N.; Miyoshi, T. High Plasma Docosahexaenoic Acid Associated to Better Prognoses of Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction. Nutrients 2021, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Cook, N.R.; Pester, J.; Moorthy, M.V.; Ridge, C.; Danik, J.S.; Gencer, B.; Siddiqi, H.K.; Ng, C.; Gibson, H.; et al. Effect of Marine Omega-3 Fatty Acid and Vitamin D Supplementation on Incident Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2021, 325, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, L.; Okoye, G.C. Therapeutic effect of continuous exercise training program on serum creatinine concentration in men with hypertension: A randomized controlled trial. Ghana Med. J. 2014, 48, 135–142. [Google Scholar] [CrossRef]
- Metra, M.; Cotter, G.; Senger, S.; Edwards, C.; Cleland, J.G.; Ponikowski, P.; Cursack, G.C.; Milo, O.; Teerlink, J.R.; Givertz, M.M.; et al. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circ. Heart Fail. 2018, 11, e004644. [Google Scholar] [CrossRef]
- Swolinsky, J.S.; Nerger, N.P.; Leistner, D.M.; Edelmann, F.; Knebel, F.; Tuvshinbat, E.; Lemke, C.; Roehle, R.; Haase, M.; Costanzo, M.R.; et al. Serum creatinine and cystatin C-based estimates of glomerular filtration rate are misleading in acute heart failure. ESC Heart Fail. 2021, 8, 3070–3081. [Google Scholar] [CrossRef]
- Roh, J.D.; Houstis, N.; Yu, A.; Chang, B.; Yeri, A.; Li, H. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell 2020, 19, e13159. [Google Scholar] [CrossRef]
- Grassi, B.; Majerczak, J.; Bardi, E.; Buso, A.; Comelli, M.; Chlopicki, S.; Guzik, M.; Mavelli, I.; Nieckarz, Z.; Salvadego, D.; et al. Exercise training in Tgα(q)*44 mice during the progression of chronic heart failure: Cardiac vs. peripheral (soleus muscle) impairments to oxidative metabolism. J. Appl. Physiol. 2017, 123, 326–336. [Google Scholar] [CrossRef]
- Wang, B.; Xu, M.; Li, W.; Li, X.; Zheng, Q.; Niu, X. Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation. PLoS ONE. 2017, 12, e0179648. [Google Scholar] [CrossRef]
- Qin, R.; Murakoshi, N.; Xu, D.; Tajiri, K.; Feng, D.; Stujanna, E.N.; Yonebayashi, S.; Nakagawa, Y.; Shimano, H.; Nogami, A.; et al. Exercise training reduces ventricular arrhythmias through restoring calcium handling and sympathetic tone in myocardial infarction mice. Physiol. Rep. 2019, 7, e13972. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Kang, G.J.; Kim, E.J.; Lee, C.H. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants 2020, 9, 1259. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef]
- McCommis, K.S.; Kovacs, A.; Weinheimer, C.J.; Shew, T.M.; Koves, T.R.; Ilkayeva, O.R. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat. Metab. 2020, 2, 1232–1247. [Google Scholar] [CrossRef]
- Lavi, S.; Prasad, A.; Yang, E.H.; Mathew, V.; Simari, R.D.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation 2007, 115, 2621–2627. [Google Scholar] [CrossRef]
- Kangavari, S.; Matetzky, S.; Shah, P.K.; Yano, J.; Chyu, K.Y.; Fishbein, M.C.; Cercek, B. Smoking increases inflammation and metalloproteinase expression in human carotid atherosclerotic plaques. J. Cardiovasc. Pharmacol. Ther. 2004, 9, 291–298. [Google Scholar] [CrossRef]
- Nakamura, K.; Barzi, F.; Huxley, R.; Lam, T.H.; Suh, I.; Woo, J.; Kim, H.C.; Feigin, V.L.; Gu, D.; Woodward, M. Does cigarette smoking exacerbate the effect of total cholesterol and high-density lipoprotein cholesterol on the risk of cardiovascular diseases? Heart 2009, 95, 909–916. [Google Scholar] [CrossRef]
- Bristow, M.R. beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000, 101, 558–569. [Google Scholar] [CrossRef]
- Adams, M.A.; Hirst, M. Metoprolol suppresses the development of ethanol-induced cardiac hypertrophy in the rat. Can. J. Physiol. Pharmacol. 1990, 68, 562–567. [Google Scholar] [CrossRef]
- Piano, M.R. Alcohol and heart failure. J. Card. Fail. 2002, 8, 239–246. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Incident Heart Failure | p Value | ||
|---|---|---|---|---|
| Total Subjects | Yes | No | ||
| No. of participants | 121,245 | 1718 | 119,527 | |
| Age (yrs), mean (SD) | 56.5 (8.1) | 62.1 (6.1) | 56.4 (8.1) | <0.001 |
| Sex, % | <0.001 | |||
| Male | 45.8 | 66.3 | 45.6 | |
| Female | 54.2 | 33.7 | 54.4 | |
| Race | 0.007 | |||
| (White, %) | 94.3 | 94.1 | 94.3 | |
| Smoking Status, % | <0.001 | |||
| Never | 54.5 | 36.7 | 54.8 | |
| Past | 34.6 | 45.3 | 34.4 | |
| Current | 10.4 | 17.3 | 10.3 | |
| Missing | 0.5 | 0.7 | 0.5 | |
| Alcohol drinking, % | <0.001 | |||
| Never | 4.3 | 6.2 | 4.3 | |
| Past | 3.6 | 8.2 | 3.5 | |
| Current | 91.8 | 85.2 | 92.0 | |
| Missing | 0.3 | 0.4 | 0.2 | |
| Physical activity | <0.001 | |||
| (MET min/week), mean (SD) | 2647.4 (2437.6) | 2349.7 (2239.8) | 2651.7 (2440.1) | |
| BMI, % | <0.001 | |||
| Normal (<25 kg/m2) | 33.1 | 18.0 | 33.3 | |
| Overweight (25 to 29.9 kg/m2) | 42.4 | 36.9 | 42.5 | |
| Obesity (≥30 kg/m2) | 24.2 | 43.8 | 23.9 | |
| Missing | 0.3 | 1.3 | 0.3 | |
| Townsend deprivation index | −1.3 | −0.5 | −1.3 | <0.001 |
| Diabetes, % | <0.001 | |||
| Yes | 5.2 | 19.9 | 5.0 | |
| No | 94.4 | 79.5 | 94.6 | |
| Missing | 0.4 | 0.6 | 0.4 | |
| Hypertension, % | <0.001 | |||
| Yes | 22.2 | 73.9 | 21.5 | |
| No | 77.8 | 26.1 | 78.5 | |
| Family history of CVD, % | <0.001 | |||
| Yes | 56.5 | 65.1 | 56.3 | |
| No | 36.8 | 25.6 | 37.0 | |
| Missing | 6.7 | 9.3 | 6.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Chu, M.; Fu, Z.; Liu, Q.; Liang, J.; Xu, J.; Weng, Z.; Chen, X.; Xu, C.; Gu, A. The Association of Metabolomic Profiles of a Healthy Lifestyle with Heart Failure Risk in a Prospective Study. Nutrients 2023, 15, 2934. https://doi.org/10.3390/nu15132934
Ma Y, Chu M, Fu Z, Liu Q, Liang J, Xu J, Weng Z, Chen X, Xu C, Gu A. The Association of Metabolomic Profiles of a Healthy Lifestyle with Heart Failure Risk in a Prospective Study. Nutrients. 2023; 15(13):2934. https://doi.org/10.3390/nu15132934
Chicago/Turabian StyleMa, Yuanyuan, Maomao Chu, Zuqiang Fu, Qian Liu, Jingjia Liang, Jin Xu, Zhenkun Weng, Xiu Chen, Cheng Xu, and Aihua Gu. 2023. "The Association of Metabolomic Profiles of a Healthy Lifestyle with Heart Failure Risk in a Prospective Study" Nutrients 15, no. 13: 2934. https://doi.org/10.3390/nu15132934
APA StyleMa, Y., Chu, M., Fu, Z., Liu, Q., Liang, J., Xu, J., Weng, Z., Chen, X., Xu, C., & Gu, A. (2023). The Association of Metabolomic Profiles of a Healthy Lifestyle with Heart Failure Risk in a Prospective Study. Nutrients, 15(13), 2934. https://doi.org/10.3390/nu15132934





