Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children
Abstract
1. Introduction
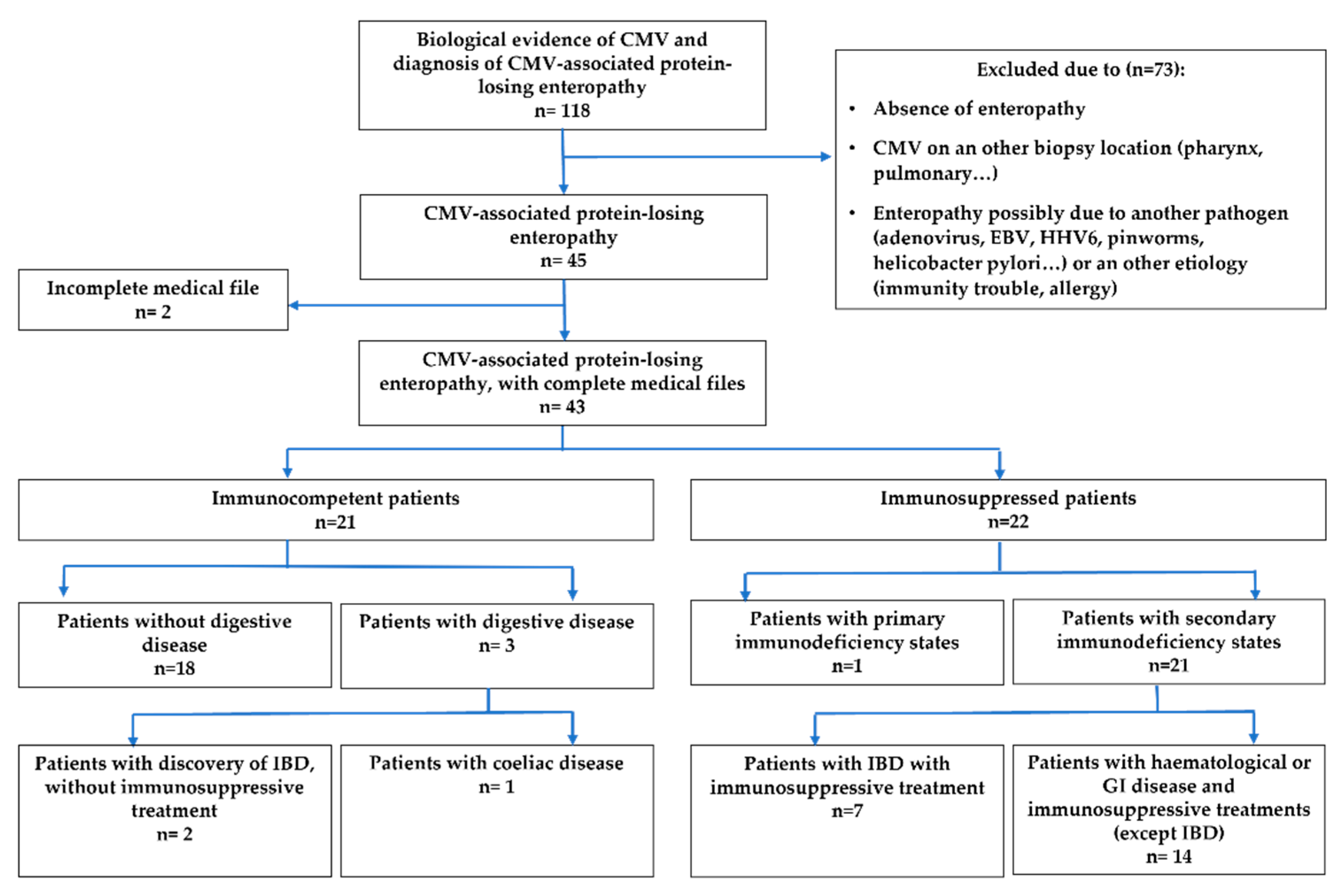
2. Materials and Methods
- Immunocompetent patients: patients who did not have an underlying disease or treatment which results in immunodeficiency.
- Immunosuppressed patients divided in 2 sub-groups:
- Patients who have primary immunodeficiency diseases;
- Patients who have a secondary immunodeficiency state due to either haematologic disease, GI condition or immunosuppressive therapies in the context of organ or bone marrow transplant or inflammatory bowel disease (IBD).
- Normal;
- Non-specific aspect: signs of inflammation such as oedema of the chorion, ulceration, polynuclear infiltration, mononuclear cells infiltration, lymphoid follicles and villous atrophy;
- Evocative aspect of CMV: glandular abnormalities such as apoptosis, necrosis or crypt abscess or nuclear dystrophy;
- Typical feature of CMV: inclusions on histology with positive immunohistochemistry for CMV (antibodies against CMV).
- Benign course: a benign initial feature of the disease, with a good general condition, good hydration state and prescription of symptomatic treatment or a short hospitalisation without any treatment except hydration;
- Mild course: a severe initial clinical presentation (poor general condition, dehydration, need of blood transfusion) with subsequent good evolution and recovery;
- Severe course: an overall serious illness with the presence of one of the following criteria: hospitalisation in an intensive care unit, hypovolemic chock, parenteral nutrition or death.
Statistical Analyses
3. Results
3.1. Clinical and Biological Signs
3.2. CMV Identification
3.3. Medical Management and Clinical Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Endoscopic Results | Immunocompetent Patients | Immunosuppressed Patients | Overall Population | Confidence Interval | p |
|---|---|---|---|---|---|
| Esophagogastroduodenoscopy | |||||
| Macroscopic oesophagitis, n (%) | 3/18 (16.7) | 1/15 (6.7) | 4/33 (12.1) | [0.2; 157.2] | 0.61 |
| Macroscopic fundic gastritis, n (%) | 12/18 (66.7) | 3/15 (20) | 15/33 (45.5) | [1.3; 57.6] | 0.01 |
| Macroscopic antral gastritis, n (%) | 11/18 (61.1) | 6/15 (40) | 17/33 (51.5) | [0.5; 12] | 0.3 |
| Macroscopic duodenitis, n (%) | 1/18 (5.6) | 1/15 (6.7) | 2/33 (0.06) | [0.01; 69.2] | 1 |
| Macroscopic description of gastritis | |||||
| Hypertrophic folds, n (%) | 7/18 (38.9) | 0/15 (0) | 7/33 (21.2) | [1.5; +∞[ | 0.009 |
| Erythematosus, n (%) | 9/18 (50) | 6/15 (40) | 15/33 (45.5) | [0.3; 7.5] | 0.73 |
| Ulcers, n (%) | 4/18 (22.2) | 0/15 (0) | 4/33 (12.1) | [0.6; +∞[ | 0.11 |
| Coloscopy | |||||
| Rectocolitis, n (%) | 7/10 (70) | 13/20 (65) | 20/30 (66.7) | [0.2; 9.9] | 1 |
| Ulcers, n (%) | 5/10 (50) | 9/20 (45) | 14/30 (46.7) | [0.2; 7.3] | 1 |
| Histological examination | |||||
| Location of the lesions at histology | |||||
| Oesophagus, n (%) | 3/5 (60) | 0/1 (0) | 3/6 (50) | [0.03; +∞[ | 1 |
| Stomach, n (%) | 15/16 (93.8) | 9/14 (64.3) | 24/30 (80) | [0.7; 418.6] | 0.07 |
| Duodenum, n (%) | 10/16 (62.5) | 5/14 (35.7) | 15/30 (50) | [0.5; 17.3] | 0.27 |
| Colon/rectum, n (%) | 9/10 (90) | 18/20 (90) | 27/30 (90) | [0.05; 65.4] | 1 |
| Non-specific aspect | 19/19 (100) | 18/21 (85.7) | 37/40 (92.5) | [0.4; +∞] | 0.23 |
| Evocative aspect of CMV | 16/19 (84.2) | 15/21 (71.4) | 31/40 (77.5) | [0.4; 15.3] | 2.09 |
| Typical feature of CMV | 6/19 (31.6) | 6/22 (27.3) | 12/41 (29.3) | [0.3; 5.9] | 1 |
References
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Huynh, K.T.; van Zuylen, W.J.; Craig, M.E.; Rawlinson, W.D. Cytomegalovirus infection in day care centres: A systematic review and meta-analysis of prevalence of infection in children. Rev. Med. Virol. 2019, 29, e2011. [Google Scholar] [CrossRef] [PubMed]
- Frield, T.J. Epidemiology, Clinical Manifestations, and Treatment of Cytomegalovirus Infection in Immunocompetent Adults. UptoDate. 2019. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-treatment-of-cytomegalovirus-infection-in-immunocompetent-adults (accessed on 4 October 2019).
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Overview of the diagnosis of cytomegalovirus infection. Infect. Disord. Drug Targets 2011, 11, 466–474. [Google Scholar] [CrossRef]
- Lopes, A.-A.; Champion, V.; Mitanchez, D. Nutrition of Preterm Infants and Raw Breast Milk-Acquired Cytomegalovirus Infection: French National Audit of Clinical Practices and Diagnostic Approach. Nutrients 2018, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Dioverti, M.V.; Razonable, R.R. Cytomegalovirus. Microbiol. Spectr. 2016, 4, 97–125. [Google Scholar] [CrossRef]
- Kotton, C.N. CMV: Prevention, Diagnosis and Therapy. Am. J. Transplant. 2013, 13, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddine, A.Y.; Frenette, C.T.; Konijeti, G.G. A Practical Review of Cytomegalovirus in Gastroenterology and Hepatology. Gastroenterol. Res. Pract. 2019, 2019, 6156581. [Google Scholar] [CrossRef]
- Urganci, N.; Gulec, S.; Kalyoncu, D.; Karaman, S. Evaluation of Paediatric Patients with Protein Losing Enteropathy: A Single Centre Experience. West Indian Med. J. 2013, 62, 186–189. [Google Scholar] [CrossRef]
- Liao, X.; Reed, S.L.; Lin, G.Y. Immunostaining Detection of Cytomegalovirus in Gastrointestinal Biopsies: Clinicopathological Correlation at a Large Academic Health System. Gastroenterol. Res. 2016, 9, 92–98. [Google Scholar] [CrossRef]
- Roussey-Kesler, G. [Syndrome Oedémateux]. Pas à Pas En Pédiatrie. 2017. Available online: http://pap-pediatrie.fr/cardiologie/syndrome-oedemateux (accessed on 4 October 2019).
- Hong, J.; Lee, S.; Shon, Y. Ménétrier’s Disease as a Gastrointestinal Manifestation of Active Cytomegalovirus Infection in a 22-Month-Old Boy: A Case Report with a Review of the Literature of Korean Pediatric Cases. Clin. Endosc. 2018, 51, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tfifha, M.; Ajmi, H.; Mabrouk, S.; Chemli, J.; Zouari, N.; Harbi, A. [Gastroentéropathie à CMV Chez l’enfant: À Propos de 4 cas]; Société Nationale Française de Ggastro-entérologie (SNFGE). Available online: http://www.snfge.org/content/gastroenteropathie-cmv-chez-lenfant-prop (accessed on 4 October 2019).
- Megged, O.; Schlesinger, Y. Cytomegalovirus-associated protein-losing gastropathy in childhood. Eur. J. Pediat. 2008, 167, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Cytomegalovirus (CMV) Colitis. PathologyOutlinesCom. 2019. Available online: http://www.pathologyoutlines.com/topic/colonCMV.html (accessed on 4 October 2019).
- Lin, W.-R.; Su, M.-Y.; Hsu, C.-M.; Ho, Y.-P.; Ngan, K.-W.; Chiu, C.-T.; Chen, P.-C. Clinical and endoscopic features for alimentary tract cytomegalovirus disease: Report of 20 cases with gastrointestinal cytomegalovirus disease. Change Gung Med. J. 2005, 28, 476–484. [Google Scholar]
- Richard, P.; Le Tourneau, A.; Diebold, J.; Audouin, J.; Molina, T. Diagnostic histopathologiquedes infections virales à cytomégalovirus chez l’adulte. Rev. Francoph. Lab. 2007, 2007, 55–60. [Google Scholar] [CrossRef]
- Challier, P.; Huguet, P. P146—Œdèmes périphériques révélateurs d’une APLV: Deux observations. Arch. Pédiatrie 2010, 17, 86. [Google Scholar] [CrossRef]
- Jauhola, O.; Ronkainen, J.; Koskimies, O.; Ala-Houhala, M.; Arikoski, P.; Holtta, T.; Jahnukainen, T.; Rajantie, J.; Ormala, T.; Nuutinen, M. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: A 6-month prospective study. Arch. Dis. Child. 2010, 95, 871–876. [Google Scholar] [CrossRef]
- Higuchi, R.; Booka, M.; Suzuki, H.; Tsuno, H. Protein-losing enteropathy and erythema caused by egg allergy in a breast-fed infant. Pediatr. Int. 2016, 58, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- Vignes, S.; Bellanger, J. Primary intestinal lymphangiectasia (Waldmann’s disease). Orphanet J. Rare Dis. 2008, 3, 5. [Google Scholar] [CrossRef]
- Marks, M.; Lanza, M.; Kahlstrom, E.; Mikity, V.; Marks, S.; Kvalstad, R.; Marks, M.L.M.; Bair, J.; Russ, P.; Pretorius, D.; et al. Pediatric hypertrophic gastropathy. Am. J. Roentgenol. 1986, 147, 1031–1034. [Google Scholar] [CrossRef]
- Tard, C.; Madhi, F.; Verlhac, S.; Hagège, H.; Epaud, R.; Jung, C. Protein-losing gastropathy associated with cytomegalovirus in two sisters—Case reports and review of the literature. Arch. Pédiatrie 2019, 26, 232–235. [Google Scholar] [CrossRef]
- Eisenstat, D.D.; Griffiths, A.M.; Cutz, E.; Petric, M.; Drumm, B. Acute cytomegalovirus infection in a child with Ménétrier’s disease. Gastroenterology 1995, 109, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Kirsaclioglu, C.T.; Hizal, G.; Karakus, E.; Sayli, T.R. An Unusual Presentation of Cytomegalovirus Infection: Generalized Edema. Med. Princ. Pract. 2019, 29, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Kamath, B.; Chung, C.; Punnett, A. Menetrier’s disease (protein-losing gastropathy) in a child with acute lymphoblastic leukemia. Int. J. Pediatr. Adolesc. Med. 2019, 6, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Sezgin, E.; An, P.; Winkler, C.A. Host Genetics of Cytomegalovirus Pathogenesis. Front. Genet. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Diamond, D.J.; Ma, Y.; Wang, N.; Li, M.; Gao, S.; Wang, L.; Zheng, B.; Qi, Y.; Ruan, Q.; et al. The immune response to human CMV. Future Virol. 2012, 7, 279–293. [Google Scholar] [CrossRef]
- Mazeron, M.-C.; Ducancelle, A.; Alain, S. Diagnostic de l’infection à CMV chez les patients immunodéprimés. Rev. Française Lab. 2002, 2002, 29–34. [Google Scholar] [CrossRef]
- Sue, P.K.; Salazar-Austin, N.; McDonald, O.G.; Rishi, A.; Cornish, T.; Arav-Boger, R. Cytomegalovirus Enterocolitis in Immunocompetent Young Children: A Report of Two Cases and Review of the Literature. Pediatr. Infect. Dis. J. 2016, 35, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Oderda, G.; Cinti, S.; Cangiotti, A.M.; Forni, M.; Ansaldi, N. Increased Tight Junction Width in Two Children with Ménétrierʼs Disease. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.J.; Coffey, R.J.; Washington, M.K. Ménétrier’s Disease: Its Mimickers and Pathogenesis. J. Pathol. Transl. Med. 2016, 50, 10–16. [Google Scholar] [CrossRef]
- Azer, S.A.; Limaiem, F. Cytomegalovirus Colitis; StatPearls Publishing: StatPearls, FL, USA, 2020. [Google Scholar]
- Oh, S.J.; Lee, C.K.; Kim, Y.-W.; Jeong, S.J.; Park, Y.M.; Oh, C.H.; Kim, J.-W.; Kim, H.J. True cytomegalovirus colitis is a poor prognostic indicator in patients with ulcerative colitis flares: The 10-year experience of an academic referral inflammatory bowel disease center. Scand. J. Gastroenterol. 2019, 54, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Buck, Q.; Cho, S.; Walsh, S.M.; Schady, D.; Kellermayer, R. Routine Histology-Based Diagnosis of CMV Colitis Was Rare in Pediatric Patients. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 462–465. [Google Scholar] [CrossRef]
- Kalkan, I.H.; Dağli, U. What is the most accurate method for the diagnosis of cytomegalovirus (CMV) enteritis or colitis? Turk. J. Gastroenterol. 2010, 21, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Haute Aurorité de Santé (HAS). Diagnostic par Sérologie et/ou par Recherche du Génome Viral de L’infection Congénitale à Cytomégalovirus. 2015. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2015-11/argumentaire_cmv_me_vd.pdf (accessed on 22 September 2019).
- Tringali, A.; Balassone, V.; De Angelis, P.; Landi, R. Complications in pediatric endoscopy. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.E.; Narkewicz, M.R. Adverse Events Following Gastrointestinal Endoscopy in Children: Classifications, Characterizations, and Implications. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Najafi, N.; Veyckemans, F.; Vanhonacker, D.; Legrand, C.; Van de Velde, A.; Vandenplas, Y.; Poelaert, J. Incidence and risk factors for adverse events during monitored anaesthesia care for gastrointestinal endoscopy in children: A prospective observational study. Eur. J. Anaesthesiol. 2019, 36, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, E.; Farooq, U.; Yanes, B.; Magalhaes-Silverman, M. Repeat Endoscopy Affects Patient Management in Gastrointestinal Graft-Versus-Host Disease. Clin. Hematol. Int. 2020, 2, 69. [Google Scholar] [CrossRef]
- Blanco-Velasco, G.; Palos-Cuellar, R.; Domínguez-García, M.; Solórzano-Pineda, O.; Zamarripa-Mottú, R.; Martínez-Camacho, C.; González-Bautista, M.; Contreras-Serratos, M.; Murcio-Pérez, E.; Blancas-Valencia, J.; et al. Utility of capsule endoscopy in the diagnosis of gastrointestinal graft-versus-host disease. Rev. Gastroenterol. Mex. (Engl. Ed.) 2021, 86, 215–219. [Google Scholar] [CrossRef]
- Crowell, K.R.; Patel, R.A.; Fluchel, M.; Lowichik, A.; Bryson, S.; Pohl, J.F. Endoscopy in the diagnosis of intestinal graft-versus-host disease: Is lower endoscopy with biopsy as effective in diagnosis as upper endoscopy combined with lower endoscopy?: Endoscopy and Graft-Versus-Disease. Pediatr. Blood Cancer 2013, 60, 1798–1800. [Google Scholar] [CrossRef]
| Clinicopathological Features | Immunocompetent Patients (n = 21) | Immunosuppressed Patients (n = 22) | Overall Population (n = 43) | Confidence Interval | p |
|---|---|---|---|---|---|
| Age in months, median [min-max] | 29.7 [1–133.7] | 105.3 [3.4–179.4] | 45.9 [1–179.4] | p < 0.001 | |
| Age < 24 months, n (%) | 9 (42.9) | 2 (9) | 11 (25.6) | [1.2; 78.8] | 0.02 |
| Gender, n (%) | |||||
| Male | 13 (61.9) | 14 (63.6) | 27 (62.8) | [0.2; 3.8] | 1 |
| Female | 8 (38) | 8 (36.3) | 16 (37.2) | [0.3; 4.4] | |
| Chronic digestive disease, n (%) | |||||
| Coeliac disease | 1 (4.7) | 0 (0) | 1 (2.3) | [0.03; +∞[ | 0.5 |
| Inflammatory bowel disease | 2 (9.5) | 7 (31.8) | 9 (20.9) | [0.02; 1.5] | 0.13 |
| Immune deficiency, n (%) | |||||
| Primary immunodeficiency | / | 1 (4.5) | 1 (2.3) | 1 | |
| Secondary immunodeficiency | / | 21 (95.4) | 21 (48.8) | p < 0.001 |
| Clinical Symptoms | Immunocompetent Patients (n = 21) | Immunosuppressed Patients (n = 22) | Overall Population (n = 43) | Confidence Interval | p |
|---|---|---|---|---|---|
| Median time of onset of digestive symptoms before the first consultation, in days [interquartile range] | 3 [1–6] | 1 [0–4.8] | 1 [0–5] | 0.33 | |
| Oedema, n (%) | 13 (61.9) | 1 (4.5) | 14 (32.6) | [3.6; 1502.4] | p < 0.001 |
| Fever, low-grade fever, n (%) | 10 (47.6) | 12 (54.5) | 22 (51.2) | [0.2; 2.9] | 0.76 |
| Vomiting, n (%) | 18 (85.7) | 11 (50) | 29 (67.4) | [1.2; 39.2] | 0.02 |
| Abdominal pain, n (%) | 11 (52.4) | 11 (50) | 22 (51.2) | [0.3; 4.3] | 1 |
| Acute diarrhoea, n (%) | 14 (66.7) | 19 (86.4) | 33 (76.7) | [0.05; 1.7] | 0.16 |
| Dehydration, n (%) | 9 (42.8) | 6 (27.3) | 15 (34.9) | [0.5; 8.8] | 0.35 |
| Gastrointestinal bleeding, n (%) | 11 (52.4) | 11 (50) | 22 (51.2) | [0.3; 4.3] | 1 |
| Anorexia, n (%) | 14 (66.7) | 15 (68.2) | 29 (67.4) | [0.2; 4] | 1 |
| Asthenia, n (%) | 11 (52.4) | 11 (50) | 22 (51.2) | [0.3; 4.3] | 1 |
| Signs of organ failure, n (%) | Immunocompetent patients (n = 21) | Immunosuppressed patients (n = 22) | Overall population (n = 43) | p | |
| Cardiac failure signs | 0 (0) | 0 (0) | 0 (0) | / | 1 |
| Renal failure signs | 0 (0) | 0 (0) | 0 (0) | / | 1 |
| Liver failure signs | 0 (0) | 1 (4.5) | 1 (2.3) | / | 1 |
| Biological signs | Immunocompetent patients (n = 21) | Immunosuppressed patients (n = 22) | Overall population (n = 43) | p | |
| Development of hypoalbuminemia at any time, n (%) | 18 (85.7) | 21 (95.5) | 39 (90.7) | [0.005; 4] | 0.34 |
| Median duration of symptoms at the time of hypoalbuminemia, in days [interquartile range] | 9.5 [6.3–18] | 14 [2–18] | 12 [5.5–18] | 0.88 | |
| Median serum albumin, g/L [interquartile range] | 21.2 [17.6–25.7] | 29.6 [24.9–33.9] | 25.4 [19.2–31.8] | 0.01 | |
| Natremia ≤ 135 mmol/L, n (%) | 12 (57.1) | 5 (22.7) | 17 (39.5) | [1; 21.4] | 0.03 |
| CRP > 10 mg/L, n (%) | 7 (33.3) | 15 (68.2) | 22 (51.2) | [0.05; 1] | 0.03 |
| CMV Identification | Immunocompetent Patients (n = 21) | Immunosuppressed Patients (n = 22) | Overall Population (n = 43) | Confidence Interval | p |
|---|---|---|---|---|---|
| CMV PCR positive on bodily fluids, n (%) | 13/16 (81.2) | 21/22 (95.5) | 34/38 (89.5) | [0.004; 3] | 0.30 |
| CMV PCR positive on digestive biopsy, n (%) | 15/20 (75) | 22/22 (100) | 37/42 (88.1) | / | 0.02 |
| CMV serology | |||||
| Detection of CMV IgM antibodies, n (%) | 9/10 (90) | 2/5 (40) | 11/15 (73.3) | [0.6; 778.9] | 0.08 |
| Detection of CMV IgG antibodies, n (%) | 9/10 (90) | 5/5 (100) | 14/15 (93.3) | / | 1 |
| pp65 antigenemia, n (%) | 1/1 (100) | 3/4 (75) | 4/5 (80) | [0.006; +∞[ | 1 |
| Biological evidence of CMV infection | |||||
| Positive viral test including CMV PCR on digestive tissue, n (%) | 21 (100) | 22 (100) | 43 (100) | / | 1 |
| Performed viral test excluding digestive CMV PCR, n (%) | 18 (85.7) | 22 (100) | 40 (93) | / | 0.1 |
| Positive viral test excluding digestive CMV PCR, n (%) | 16/18 (88.9) | 21/22 (95.5) | 37/40 (92.5) | [0.006; 8.1] | 0.58 |
| Treatments | Immunocompetent Patients (n = 21) | Immunosuppressed Patients (n = 22) | Overall Population (n = 43) | Confidence Interval | p |
|---|---|---|---|---|---|
| In depth-treatment, n (%) | |||||
| Preventive antiviral drug | 0 (0) | 2 (9) | 2 (4.7) | / | 0.50 |
| Corticosteroids | 0 (0) | 16 (72.3) | 16 (37.2) | / | p < 0.001 |
| Emergency treatment (<24 h), n (%) | |||||
| Fast vascular filing | 4 (19) | 3 (13.6) | 7 (16.3) | [0.2; 11.6] | 0.70 |
| Intravenous or nasogastric rehydration | 11 (52.4) | 12 (54.5) | 23 (53.5) | [0.2; 3.6] | 1 |
| Treatment during hospitalisation, n (%) | |||||
| Albumin infusion | 8 (38) | 7 (31.8) | 15 (34.9) | [0.3; 5.6] | 0.75 |
| Curative antiviral drug | 5 (23.8) | 22 (100) | 27 (62.8) | / | p < 0.001 |
| Parenteral nutrition | 5 (23.8) | 10 (45.5) | 15 (34.9) | [0.08; 1.6] | 0.2 |
| Blood transfusion | 6 (28.6) | 7 (31.8) | 13 (30.2) | [0.2; 3.8] | 1 |
| Immunoglobulin infusion | 2 (9.5) | 7 (31.8) | 9 (20.9) | [0.02; 1.5] | 0.13 |
| Intravenous corticosteroids | 2 (9·5) | 9 (40.9) | 11 (25.6) | [0.01; 0.9] | 0.03 |
| Clinical course, n (%) | Immunocompetent patients (n = 21) | Immunosuppressed patients (n = 22) | Overall population (n = 43) | p | |
| Global clinical course | / | 0.02 | |||
| Benign course | 10 (47.6) | 4 (18.2) | 14 (32.6) | [0.9; 21.7] | 0.05 |
| Mild course | 6 (28.6) | 6 (27.3) | 12 (27.9) | [0.2; 5] | 1 |
| Severe course | 5 (23.8) | 12 (54.5) | 17 (39.5) | [0.06; 1.1] | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrua, C.; Lemoine, A.; Mosca, A.; Lopes, A.-A. Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children. Nutrients 2023, 15, 2844. https://doi.org/10.3390/nu15132844
Ferrua C, Lemoine A, Mosca A, Lopes A-A. Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children. Nutrients. 2023; 15(13):2844. https://doi.org/10.3390/nu15132844
Chicago/Turabian StyleFerrua, Claire, Anais Lemoine, Alexis Mosca, and Anne-Aurélie Lopes. 2023. "Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children" Nutrients 15, no. 13: 2844. https://doi.org/10.3390/nu15132844
APA StyleFerrua, C., Lemoine, A., Mosca, A., & Lopes, A.-A. (2023). Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children. Nutrients, 15(13), 2844. https://doi.org/10.3390/nu15132844






