Abstract
Osteoporosis is a major public health concern in Saudi Arabia’s aging population. There is particularly limited information on how diet affects bone loss in this ethnic group. The purpose of this study was to examine the association between dietary calcium (Ca) intake and osteoporosis risk in Saudi adults. A total of 1950 patients (416 males and 1534 females) with known risk factors for osteoporosis participated in this cross-sectional study. A short questionnaire (CaQ) was used to assess dietary Ca intakes in patients attending tertiary hospitals in Riyadh City. The prevalence of osteoporosis was 21.3% and was more common in females (93.5%). Patients with osteoporosis were older (p < 0.001) and had lower BMI (p < 0.001). Results showed that the overall mean Ca intake was only 445.1 mg/day (recommended dietary intake of 1300 mg/day). Tea intake (OR = 0.8 95%CI: 0.7–1.0; p = 0.02) and consumption of fish and eggs (OR = 0.9 95%CI: 0.8–1.0; p = 0.01) were significantly associated with a lower risk of osteoporosis. Furthermore, consumption of biscuits, cake and bread slices were significantly associated with higher incidence of osteoporosis (OR = 1.3 95%CI: 1.0–1.5; p = 0.02). In conclusion, extremely low dietary Ca intake was observed among Saudi adults already at risk of osteoporosis. A balanced diet including high amount of Ca, vitamin D and omega-3 fatty acids accompanied by limiting consumption of foods high in saturated fats and glycemic index may be helpful in reducing osteoporosis risk in the Saudi adult population.
1. Introduction
Osteoporosis is a common disease in Saudi Arabia (SA), resulting from decreased bone mass, strength and microarchitecture, which ultimately elevates the risk of fractures [1]. The World Health Organization (WHO) defined osteoporosis as a decrease in bone mineral density (BMD), measured by dual-energy X-ray absorptiometry (DXA), to at least 2.5 standard deviations below the mean peak BMD of a young adult woman [2]. In SA, the most recent hospital-based study showed that the prevalence of osteoporosis was as high as 64% in men and 58% in post-menopausal women above 50 years old [3]. Factors contributing to the high prevalence of osteoporosis in SA include advancing age, history of previous fracture, family history of low bone mass, menopause and use of corticosteroids [4].
Individuals diagnosed with osteoporosis have a 40% risk of fractures, which can result in mobility impairment and significantly reduce quality of life [1]. Available evidence suggests that bone loss related to age and/or post-menopause is influenced by the level of peak bone mass attained during childhood and adolescence, as well as the rate at which bone is lost [5]. Genetic factors can also explain between 60% and 80% of the variations in peak bone mass, with the remainder modulated by external factors such as nutrition [5,6,7]. In fact, consuming sufficient dietary calcium (Ca) and protein are necessary to attain ideal peak bone mass and prevent bone loss in adults [8].
Diet plays a significant role in the prevention and management of osteoporosis [8]. Consuming a diet that is rich in Ca, vitamin D, vitamin K and magnesium, and a balanced intake of protein, fruits, milk, yogurt, cheese, dark leafy green vegetables, such as spinach and kale, fish such as sardines, eggs and fortified foods such as breakfast cereals can contribute to good bone health by promoting bone formation and reducing bone loss [9,10,11,12,13]. However, cakes, biscuits and other foods that are high in added sugars and refined grains do not provide the same benefits for bone health and may be associated with an increased risk of inflammation and bone loss over time [7,8,9].
Several studies have investigated the association of dietary patterns with osteoporosis and BMD in different ethnic groups and have reported mixed results [14,15,16]. In contrast, there are limited studies conducted in Arabian populations, particularly in SA, as the available literature has focused mainly on osteopenia in post-menopausal women, extra-skeletal effects of vitamin D and more recently, biochemical osteomalacia in Arab adolescents [17,18,19]. In order to fill this gap, this study examined whether dietary patterns, and Ca intake in particular, are associated with osteoporosis risk in Saudi Arabian adults.
2. Materials and Methods
This multi-center cross-sectional study was taken from the Osteoporosis Registry database of the Chair for Biomarkers of Chronic Diseases (CBCD) in King Saud University (KSU), SA, in collaboration with the Ministry of Health. The project received approval from the Ethics Committee at the College of Medicine, KSU, Riyadh, SA (number H-01-R-012). In brief, 2185 records of all consenting Saudi adults visiting the major tertiary hospitals in Riyadh (King Fahad Medical City (KFMC), King Khalid University Hospital (KKUH) and King Salman Hospital (KSH)) who were advised to have their BMD assessed from 2013–2016 were included [20].
2.1. Study Design and Participants
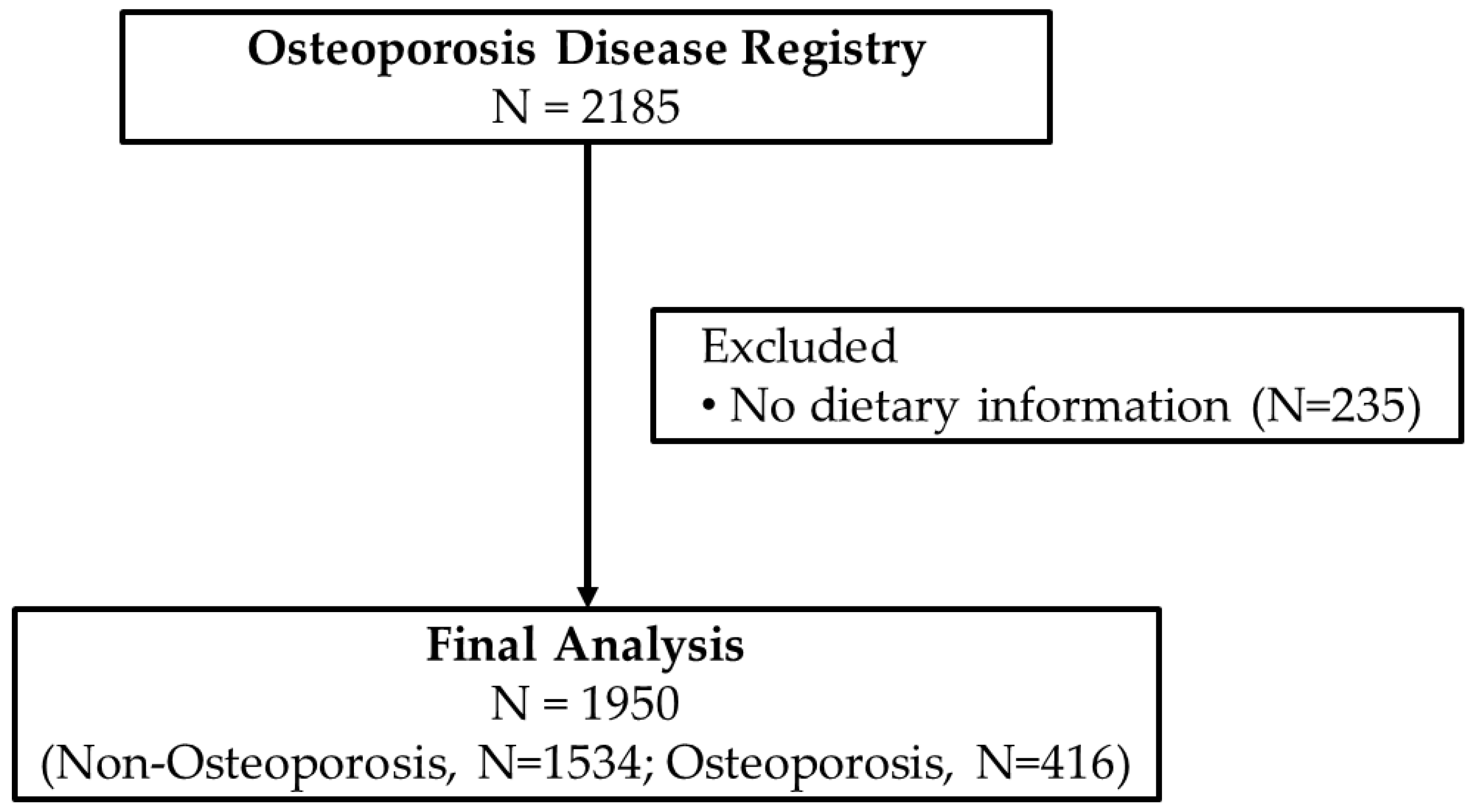
Data collection for the Osteoporosis Registry started in 2013 and ended in August 2016. Screening of participants for inclusion in the registry were overseen by trained physicians in their respective radiology departments in participating hospitals where a BMD (DXA) scan is available. The inclusion criteria were those preferably, but not limited to, post-menopausal women, those with history of fracture in the last 5 years, diminished height in the last 2 years, men and women with history of malabsorption and nutritional deficiencies (vitamin D and Ca), who had endocrine disorders affecting skeletal mass (e.g., hyperparathyroidism), were at risk for bone density loss (family history of osteoporosis) and had an available BMD scan. All participants were asked for their consent before inclusion in the registry. Participants were excluded if they were <35 years of age or if they have any malignancy, cardiac disorders, lung disease or any disease that required immediate medical attention. For this particular study, participants were excluded if there were no dietary data available. Figure 1 shows the flowchart of participants.
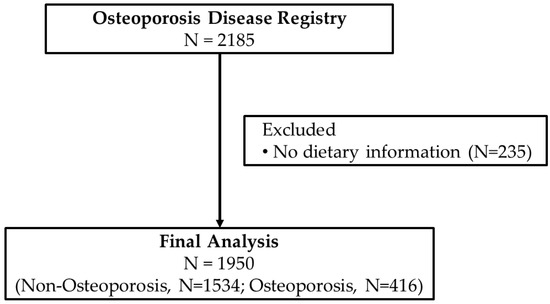
Figure 1.
Flowchart of participants.
2.2. Diagnosis of Osteoporosis
Diagnosis of osteoporosis was undertaken by measuring BMD through a DXA scan of the femoral neck or spine and calculating the T-score. A patient with a T-score of ≤−2.5 (standard deviations, SD, from the mean value of young normal Caucasian women) is classified as having osteoporosis [21,22]. DXA scans were overseen by a qualified technician certified in densitometry, while the machine itself was calibrated using a standard phantom provided by the manufacturer. Upon completion of the test, the BMD findings and corresponding T-scores were forwarded to the Chair for Biomarkers in Chronic Diseases (CBCD) at King Saud University, SA, for classification according to osteoporosis status and data entry.
2.3. Anthropometry, Blood Collection and Sample Analysis
Clinical data were obtained by trained nurses using standard techniques to measure anthropometrics which included height (cm), waist/hip measurements (cm) and weight (kg). Body mass index (BMI) was computed as weight (in kilograms) divided by height (in squared meters). Blood pressure (mmHg) was recorded twice, with a 10-min interval between readings, using a mercurial sphygmomanometer, and the average reading was noted. Fasting blood samples were extracted and subsequently sent to the CBCD laboratory for biochemical analysis using an automated biochemical analyzer (Konelab 20, Thermo-Fischer Scientific, Espoo, Finland). This analysis included serum lipid profiling as well as quantification of fasting glucose, Ca and albumin levels.
2.4. Dietary Intake
Participants were invited for the administration of a short questionnaire (CaQ) adopted from Macdonald et al. [23]. The CaQ is a validated instrument to assess daily dietary Ca intakes [23]. The information collected was on weekly consumption of food and beverages that are common sources of Ca in SA which included milk, tea with milk, coffee with milk, bread, biscuits, cakes, eggs, fish, cheese, yogurt, green vegetables, porridge, pancakes and lasagna. The participants were asked to provide weekly standard servings of the mentioned foods and beverages. Then, weekly Ca intake was calculated by multiplying the amount of Ca present in the standard serving of the food/beverage.
2.5. Data Analysis
Data were analyzed using SPSS, version 21.0. The continuous variables were expressed as the mean with the standard deviation or as the median with the quartile range. The categorical variables were presented as frequencies and percentages. To determine significance, the independent sample t-test and Mann–Whitney U test were used for normal and non-normal variables, respectively. Age and BMI were adjusted for using univariate analysis of variance. Prior to conducting parametric testing, non-normal variables were log-transformed. Multivariate logistic regression analysis was used to obtain both unadjusted and adjusted odds ratios (OR). A correlation matrix was performed among different sources of dietary Ca prior to univariate modelling to confirm absence of multicollinearity. Significance was set at p < 0.05.
3. Results
A total of 1950 patients (N = 1534 (389 males and 1145 females) without osteoporosis and N = 416 (27 males and 389 females with osteoporosis)) were included in this observational study. The prevalence of osteoporosis was 21.3%, of which 93.5% were females. Patients with osteoporosis were significantly older (p < 0.001) and had lower BMI (p < 0.001). Table 1 shows the anthropometrics, clinical characteristics and biochemical parameters according to osteoporosis status.

Table 1.
Anthropometrics and biochemical parameters according to osteoporosis status.
After adjusting for age and BMI, the osteoporosis group appeared to have significantly lower systolic blood pressure (p = 0.05), diastolic blood pressure (p = 0.005), glucose level (p = 0.01) and triglycerides (p = 0.001) than the non-osteoporosis group. However, the osteoporosis group have significantly higher 25(OH)D concentrations than the non-osteoporosis group (p < 0.001).
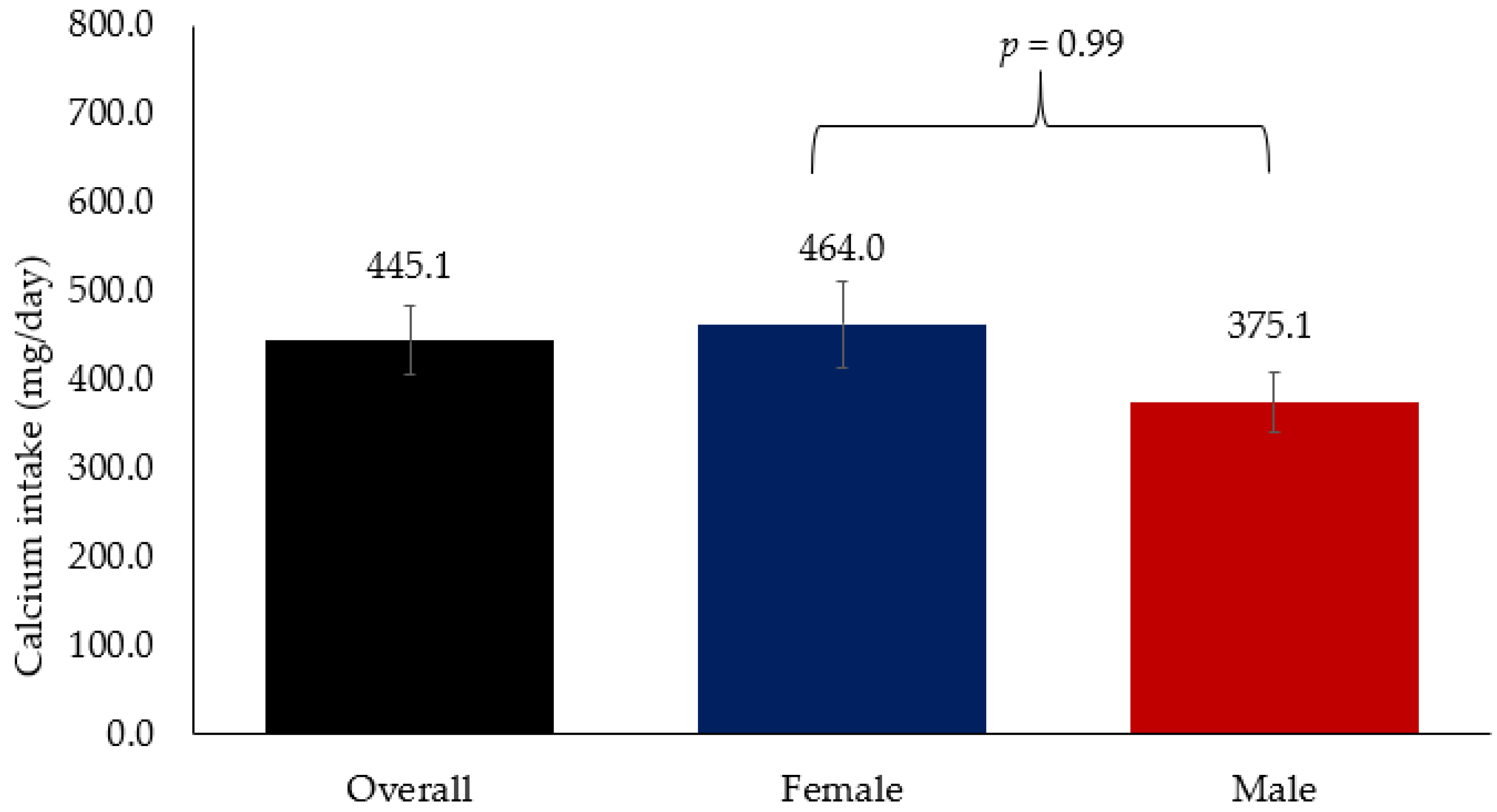
Figure 2 shows the overall mean Ca intake which was 445.1 mg/day, which is far below the recommended dietary intake of 1300 mg/day. Mean Ca intake among women was 464.0 mg/day while in men it was 375.1 mg/day. No significant difference in Ca intake was observed between men and women.
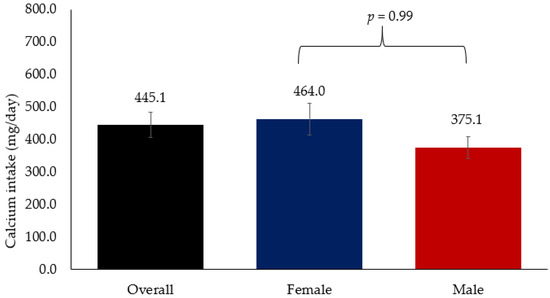
Figure 2.
Mean Ca intake among Arab adults.
Table 2 shows the Ca intake from food groups according to osteoporosis status. The Ca intake from tea with milk was significantly higher in the non-osteoporosis group than the osteoporosis groups with odds ratio (OR) of 0.7 (0.6–0.8) (p < 0.001). Similarly, the Ca intake of coffee with milk was significantly higher in the non-osteoporosis group than the osteoporosis group with an OR 0.6 (0.5–0.8) (p < 0.001). Furthermore, Ca intake from fish and eggs was also significantly higher in the non-osteoporosis group than the osteoporosis group with an OR 0.8 (0.7–0.9) (p < 0.001). Ca intake from porridge and oats was also significantly higher in the non-osteoporosis group than osteoporosis group with OR 0.8 (0.8–0.9) (p = 0.005). On the other hand, Ca intake from biscuits, cake and bread slices were significantly higher in the osteoporosis group than the non-osteoporosis group (p < 0.001). After adjusting for age and BMI, only consumption of tea with milk, biscuits, cake and bread slices and fish and eggs remained significantly different between groups, while consumption of coffee with milk showed borderline significance (p = 0.05).

Table 2.
Ca intake from different food groups according to Osteoporosis status.
Table 3 shows the odds of osteoporosis according to Ca intake from different food groups obtained from multivariate logistic regression analysis. All these variables were log transformed and added simultaneously to the logistic model adjusting for one another. Results showed that consuming tea (p = 0.005), coffee with milk (p = 0.007) and fish and eggs (p = 0.002) were significantly associated with lower risk of osteoporosis. After adjustment with age and BMI, consumption of biscuits, cake and bread slices remained significantly associated with higher risk of osteoporosis (p = 0.02).

Table 3.
Odds of Osteoporosis according to Ca intake from food groups.
4. Discussion
The present study aimed to determine the association between dietary Ca intake and osteoporosis risk in Saudi adults with known risk factors for osteoporosis. Results showed that the overall mean Ca intake, which was 445.1 mg/day, was substantially below the recommended dietary intake of 1300 mg/day. Mean Ca intake of women was 464.0 mg/day, while in men it was 375.1 mg/day. Dietary Ca intake from tea with milk, fish and eggs were significantly associated with lower risk of osteoporosis while biscuits, cake and bread slices showed a significant association with higher risk of osteoporosis independent of age and BMI.
Ca is an essential divalent cation that is mainly present outside cells and plays a major role in a multitude of vital functions such as muscle contraction, neuronal excitability, neurotransmitter release and blood coagulation. Additionally, the Ca reservoir significantly contributes to the mineral matrix of bone, an indication that the reservoir can influence bone strength [24] and serve as a buffer when required [25]. It is well-established that consuming enough Ca during the period of growth is the most significant nutritional factor for attaining optimal peak bone mass [25,26,27]. A 2017 systematic review found that the average Ca intake in national diets ranged from 175 to 1233 mg/day, as reported in 78 studies from 74 countries [28]. Similar to the present study, the average dietary Ca intake in many Asian countries is less than 500 mg/day, while countries in Africa and South America generally ranged from 400 to 700 mg/day [28].
Additionally, a more recent systematic review was conducted on the diets of individuals aged 25 and older in 195 countries, which used national or sub-national representative nutrition surveys and multiple dietary data sources from the Global Health Data Exchange [29]. According to this review, the global average daily Ca intake is approximately 400 mg/day, with lower intakes observed in Sub-Saharan African countries and Southeast Asia (around 200 mg/day) as compared to high-income countries (around 600–800 mg/day) [29]. In this study, the average Ca intake in adult males was 375.1 mg/d, while for females it was 464.0 mg/d. Unfortunately, inadequate Ca intake and suboptimal levels of vitamin D are prevalent among the elderly population of SA, with the latter improving over time across all demographic groups due to successful public health campaigns [30]. It is expected that this improvement in vitamin D levels may also bring a general improvement in Ca intake through continuous public health campaigns.
This study suggests that Ca intake from tea with milk is significantly associated with decreased risk of osteoporosis. Some studies reported that frequent tea and coffee intake had little influence on BMD and were not associated with fracture risk among postmenopausal women with osteoporosis [31,32]. In a meta-analysis which included 17 studies [14], the odds of osteoporosis for the highest versus the lowest categories of tea consumption was 0.62 (95%CI, 0.46–0.83). Furthermore, the risk-lowering effect of tea consumption on osteoporosis was consistent in males and females, different study periods, study designs and multiple geographic locations [14]. This beneficial effect of tea consumption with milk may be due to the presence of polyphenols that are responsible for improving BMD by reducing oxidative stress and inflammation [33]. Additionally, polyphenols are known to be sequestered into physiological bone, which may help protect it from degradation [14,33]. While more research is needed to understand the exact mechanisms by which polyphenols may help protect bone health, it is suggested that consuming polyphenol-rich foods, such as tea, may help protect against bone loss.
The present study also found the protective effects of fish and eggs consumption on osteoporosis risk. Both fish and eggs are known to be great sources of protein which help build and maintain strong bones and muscles. Pujia et al. [17] also reported a positive association between whole egg consumption and bone health. Furthermore, numerous studies have shown that fish consumption is linked to decreased osteoporosis risk, increased BMD and decreased fracture risk for both men and women—more so in Asian populations [34,35,36,37] than Caucasians, as observed in the Cardiovascular Health Study [38].
In the present study, Ca intake from cake, biscuits and bread contributed to increased osteoporosis risk. These results are consistent with other observations which found that high glycemic index (GI) foods had negative consequences for bone health, including decreased osteoblast activity, increased bone resorption, oxidative stress and inflammation, as well as the induction of acidosis [39,40]. In contrast, complex carbohydrates such as those found in vegetables, legumes and whole grains can increase Ca absorption and neutralize metabolic acid loads in the body [9]. Inulin-type fructans are a form of non-digestible carbohydrate found in various vegetables and fruits, which cannot be broken down by mammalian enzymes [9]. Inulin-type fructans alter intestinal Ca absorption, enhance Ca solubility in the lumen and amplify bioavailability, eventually leading to improved passive transport of into the body [9,41]. While the present study did not observe an association between vegetable and fruit consumption on osteoporosis risk, it should not supersede previous findings on the beneficial effects of vegetables and fruits consumption on bone health [41,42,43].
Large populations across the globe consider milk and dairy products, including cheese, butter, cream and yogurt, to be major sources of Ca, which can increase BMD and potentially reduce the risk of fractures, particularly in postmenopausal women [15,40,44]. A recent 2021 systematic review of 8 case-control studies, 10 cross-sectional studies, 17 prospective cohort studies and 14 randomized clinical trials (RCT) reported that dairy consumption had a moderate effect on bone health in older adults aged 50 years or more [44]. All case-control studies showed reduced hip fracture risk with the exception of one study; the included cross-sectional studies showed favorable impact of milk intake on BMD; and RCTs showed a beneficial impact of fortified dairy products [44]. In the same review, however, there is ambiguity as to the life period in which milk intake is most advantageous [44].
It is important to note that the relationship between dairy intake and bone health is also influenced by other factors such as exercise, genetics and overall nutrition [45,46]. For instance, hip fracture risk in the elderly was found to be higher in those who self-reported consuming milk and dairy products, specifically at the age of 20 years, suggesting a potential negative impact of high dairy intake [46]. In Feskanich et al.’s investigation, Ca supplements may help prevent osteoporotic fractures in certain bone areas, but regularly consuming high amounts of Ca-rich foods during middle age is unlikely to confer any protective benefits against hip or forearm fractures, at least for white middle-aged women in the US [45]. The mentioned study was followed-up after 6 years and involved analysis of 72,337 postmenopausal women where a total of 603 hip fractures occurred due to low or moderate trauma during the period from 1980 to 1998 [47]. The risk of hip fracture was not linked to either milk intake or total Ca consumed, and instead there was a lower risk of osteoporotic hip fractures in postmenopausal women with sufficient intake of vitamin D [47]. The results of our study also showed no association between Ca intake from dairy products and risk of osteoporosis. These inconsistencies in the research regarding a potential link between milk consumption and bone health can be attributed, in part, to the biases and inaccuracies inherent in dietary data collection [48].
The present study has certain limitations which should be taken into consideration when interpreting findings. First, the results are based on observational study; thus, causality cannot be inferred. Second, Ca intake from supplements and medications in general were not recorded and this could have explained why the osteoporosis group appeared to have a better metabolic profile (e.g., lower BP and glucose, higher 25(OH)D) than the non-osteoporosis group. Finally, the findings of the present study are most relevant to Arab adults already at risk of osteoporosis. Similar studies using other populations (e.g., pregnant, pre-menopausal) in the same ethnic group may be able to provide additional information on the over-all dietary deficiencies present in the modern-day Saudi diet.
5. Conclusions
In conclusion, extremely low dietary Ca intake was observed among Saudi adults at risk of osteoporosis. Ca intake from tea with milk, fish and eggs decrease, while Ca intake from cakes, biscuits and bread increase, the risk of osteoporosis. These findings suggest an urgent need to promote Ca supplementation in this high-risk population since diet alone is clearly not enough to meet daily Ca needs. A balanced diet that meets the recommended intake of Ca, vitamin D and omega-3 fatty acids, as well as limiting foods high in saturated fats and/or glycemic index, may be effective in reducing osteoporosis risk in the Saudi adult population.
Author Contributions
Conceptualization S.S.; methodology, S.S., S.D.H., A.M.A. and N.A.; formal analysis, S.D.H. writing—original draft preparation, S.D.H.; writing—review and editing, S.S. and N.M.A.-D.; supervision, N.M.A.-D.; funding acquisition, N.M.A.-D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research (IFKSURC-1-1604).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (approval number H-01-R-012).
Informed Consent Statement
Written Informed consent was obtained from each participant involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research (IFKSURC-1-1604).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Daghri, N.M.; Sabico, S.; Al-Saleh, Y.; Sulimani, R.; Aljohani, N.J.; Sheshah, E.; Alodhayani, A.; Harvey, N.C.; Liu, E.; Lorentzon, M.; et al. The application of FRAX in Saudi Arabia. Arch. Osteoporos. 2021, 16, 166. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., 3rd; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; AlZamami, J.F.; AlNaimi, S.N.; Al-Noaimi, D.A.; AlDakheel, D.A.; AlSayed, H.N.; Al-Turki, H.A.; AlOmran, A.S. Osteoporosis: Is the prevalence increasing in Saudi Arabia. Ann. Afr. Med. 2022, 21, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Al Hamam, N.M.; Al-Moaibed, G.F.; Alfayez, E.H.; Alfayez, E.H.; Al-Mubaddil, M.S.; Alramadhan, N.A. Prevalence and risk factors for osteoporotic fracture among adults with comorbidities in Al-Ahsaa, Saudi Arabia. J. Fam. Med. Prim. Care 2020, 9, 877–882. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bianchi, M.L.; Garabedian, M.; McKay, H.A.; Moreno, L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef]
- Ferrari, S.; Rizzoli, R.; Bonjour, J.P. Genetic aspects of osteoporosis. Curr. Opin. Rheumatol. 1999, 11, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutrition: Its role in bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef]
- Lorincz, C.; Manske, S.L.; Zernicke, R. Bone health: Part 1, nutrition. Sport. Health 2009, 1, 253–260. [Google Scholar] [CrossRef]
- Munoz-Garach, A.; Garcia-Fontana, B.; Munoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Malmir, H.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2018, 57, 2147–2160. [Google Scholar] [CrossRef]
- Matia-Martin, P.; Torrego-Ellacuria, M.; Larrad-Sainz, A.; Fernandez-Perez, C.; Cuesta-Triana, F.; Rubio-Herrera, M.A. Effects of Milk and Dairy Products on the Prevention of Osteoporosis and Osteoporotic Fractures in Europeans and Non-Hispanic Whites from North America: A Systematic Review and Updated Meta-Analysis. Adv. Nutr. 2019, 10, S120–S143. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Feskanich, D. Dietary patterns and risk of hip fractures in postmenopausal women and men over 50 years. Osteoporos. Int. 2015, 26, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between tea consumption and osteoporosis: A meta-analysis. Medicine 2017, 96, e9034. [Google Scholar] [CrossRef]
- Pujia, R.; Ferro, Y.; Maurotti, S.; Mare, R.; Arturi, F.; Montalcini, T.; Pujia, A.; Mazza, E. Relationship between Osteoporosis, Multiple Fractures, and Egg Intake in Healthy Elderly. J. Midlife Health 2021, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garcia, J.F.; Moran, J.M.; Roncero-Martin, R.; Rey-Sanchez, P.; Rodriguez-Velasco, F.J.; Pedrera-Zamorano, J.D. Dietary habits, nutrients and bone mass in Spanish premenopausal women: The contribution of fish to better bone health. Nutrients 2012, 5, 10–22. [Google Scholar] [CrossRef]
- Sabico, S.; Wani, K.; Grant, W.B.; Al-Daghri, N.M. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients 2023, 15, 551. [Google Scholar] [CrossRef]
- Alissa, E.M.; Qadi, S.G.; Alhujaili, N.A.; Alshehri, A.M.; Ferns, G.A. Effect of diet and lifestyle factors on bone health in postmenopausal women. J. Bone Miner. Metab. 2011, 29, 725–735. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Yakout, S.; Sabico, S.; Wani, K.; Hussain, S.D.; Aljohani, N.; Uday, S.; Hogler, W. Establishing the Prevalence of Osteomalacia in Arab Adolescents Using Biochemical Markers of Bone Health. Nutrients 2022, 14, 5354. [Google Scholar] [CrossRef]
- Wani, K.; Yakout, S.M.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alokail, M.S.; Sheshah, E.; Aljohani, N.J.; Al-Saleh, Y.; Reginster, J.Y.; et al. Metabolic Syndrome in Arab Adults with Low Bone Mineral Density. Nutrients 2019, 11, 1405. [Google Scholar] [CrossRef]
- Al-Saleh, Y.; Al-Daghri, N.M.; Sabico, S.; Alessa, T.; Al Emadi, S.; Alawadi, F.; Al Qasaabi, S.; Alfutaisi, A.; Al Izzi, M.; Mukhaimer, J.; et al. Diagnosis and management of osteoporosis in postmenopausal women in Gulf Cooperation Council (GCC) countries: Consensus statement of the GCC countries’ osteoporosis societies under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Arch. Osteoporos. 2020, 15, 109. [Google Scholar] [CrossRef]
- Al-Saleh, Y.; Sulimani, R.; Sabico, S.; Alshahrani, F.M.; Fouda, M.A.; Almohaya, M.; Alaidarous, S.B.; Alkhawashki, H.M.; Alshaker, M.; Alrayes, H.; et al. Diagnosis and management of osteoporosis in Saudi Arabia: 2023 key updates from the Saudi Osteoporosis Society. Arch. Osteoporos. 2023, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Garland, A.; Burr, J.; Strachan, A.; Wood, A.D.; Jamil, N.A.; McLernon, D.; Black, A.J. Validation of a short questionnaire for estimating dietary calcium intakes. Osteoporos. Int. 2014, 25, 1765–1773. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef]
- Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: Dairy intake and bone health: A viewpoint from the state of the art. J. Dairy Sci. 2011, 94, 5249–5262. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.; Tylavsky, F.A.; Halioua, L.; Metz, J.A. Determinants of peak bone mass in young adult women: A review. Osteoporos. Int. 1993, 3 (Suppl. S1), 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Galuska, D.A. Epidemiology of bone mass in premenopausal women. Epidemiol. Rev. 1993, 15, 374–398. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Hussain, S.D.; Ansari, M.G.A.; Khattak, M.N.K.; Aljohani, N.; Al-Saleh, Y.; Al-Harbi, M.Y.; Sabico, S.; Alokail, M.S. Decreasing prevalence of vitamin D deficiency in the central region of Saudi Arabia (2008–2017). J. Steroid Biochem. Mol. Biol. 2021, 212, 105920. [Google Scholar] [CrossRef]
- Chen, Z.; Pettinger, M.B.; Ritenbaugh, C.; LaCroix, A.Z.; Robbins, J.; Caan, B.J.; Barad, D.H.; Hakim, I.A. Habitual tea consumption and risk of osteoporosis: A prospective study in the women’s health initiative observational cohort. Am. J. Epidemiol. 2003, 158, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Chang, J.C.; Edelstein, S.L. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. JAMA 1994, 271, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Han, D.W.; Suh, H.; Ryu, G.H.; Hyon, S.H.; Cho, B.K.; Park, J.C. Protective effects of green tea polyphenol against reactive oxygen species-induced oxidative stress in cultured rat calvarial osteoblast. Cell Biol. Toxicol. 2003, 19, 325–337. [Google Scholar] [CrossRef]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef]
- Nawata, K.; Yamauchi, M.; Takaoka, S.; Yamaguchi, T.; Sugimoto, T. Association of n-3 polyunsaturated fatty acid intake with bone mineral density in postmenopausal women. Calcif. Tissue Int. 2013, 93, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Xue, W.Q.; Wu, B.H.; He, M.G.; Xie, H.L.; Ouyang, W.F.; Tu, S.L.; Chen, Y.M. Higher fish intake is associated with a lower risk of hip fractures in Chinese men and women: A matched case-control study. PLoS ONE 2013, 8, e56849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Ho, S.C.; Lam, S.S. Higher sea fish intake is associated with greater bone mass and lower osteoporosis risk in postmenopausal Chinese women. Osteoporos. Int. 2010, 21, 939–946. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mozaffarian, D.; Cauley, J.A.; Mukamal, K.J.; Robbins, J.; Siscovick, D.S. Fish consumption, bone mineral density, and risk of hip fracture among older adults: The cardiovascular health study. J. Bone Miner. Res. 2010, 25, 1972–1979. [Google Scholar] [CrossRef]
- Nouri, M.; Mahmoodi, M.; Shateri, Z.; Ghadiri, M.; Rajabzadeh-Dehkordi, M.; Vali, M.; Gargari, B.P. How do carbohydrate quality indices influence on bone mass density in postmenopausal women? A case-control study. BMC Womens Health 2023, 23, 42. [Google Scholar] [CrossRef]
- Garcia-Gavilan, J.F.; Bullo, M.; Camacho-Barcia, L.; Rosique-Esteban, N.; Hernandez-Alonso, P.; Basora, J.; Martinez-Gonzalez, M.A.; Estruch, R.; Fito, M.; Salas-Salvado, J. Higher dietary glycemic index and glycemic load values increase the risk of osteoporotic fracture in the PREvencion con DIeta MEDiterranea (PREDIMED)-Reus trial. Am. J. Clin. Nutr. 2018, 107, 1035–1042. [Google Scholar] [CrossRef]
- Coxam, V. Current data with inulin-type fructans and calcium, targeting bone health in adults. J. Nutr. 2007, 137, 2527S–2533S. [Google Scholar] [CrossRef] [PubMed]
- Raschka, L.; Daniel, H. Mechanisms underlying the effects of inulin-type fructans on calcium absorption in the large intestine of rats. Bone 2005, 37, 728–735. [Google Scholar] [CrossRef]
- Qiu, R.; Cao, W.T.; Tian, H.Y.; He, J.; Chen, G.D.; Chen, Y.M. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Bailey, R.L.; Lappe, J.; O’Brien, K.O.; Wang, D.D.; Sahni, S.; Weaver, C.M. Dairy intake and bone health across the lifespan: A systematic review and expert narrative. Crit. Rev. Food Sci. Nutr. 2021, 61, 3661–3707. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A. Milk, dietary calcium, and bone fractures in women: A 12-year prospective study. Am. J. Public Health 1997, 87, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G.; Klineberg, R.J. Case-control study of risk factors for hip fractures in the elderly. Am. J. Epidemiol. 1994, 139, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Willett, W.C.; Colditz, G.A. Calcium, vitamin D, milk consumption, and hip fractures: A prospective study among postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Nel, J.H.; Steyn, N.P.; Senekal, M. Illustration of the Importance of Adjustment for within- and between-Person Variability in Dietary Intake Surveys for Assessment of Population Risk of Micronutrient Deficiency/Excess Using an Example Data Set. Nutrients 2022, 14, 285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).