The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review
Abstract
1. Introduction
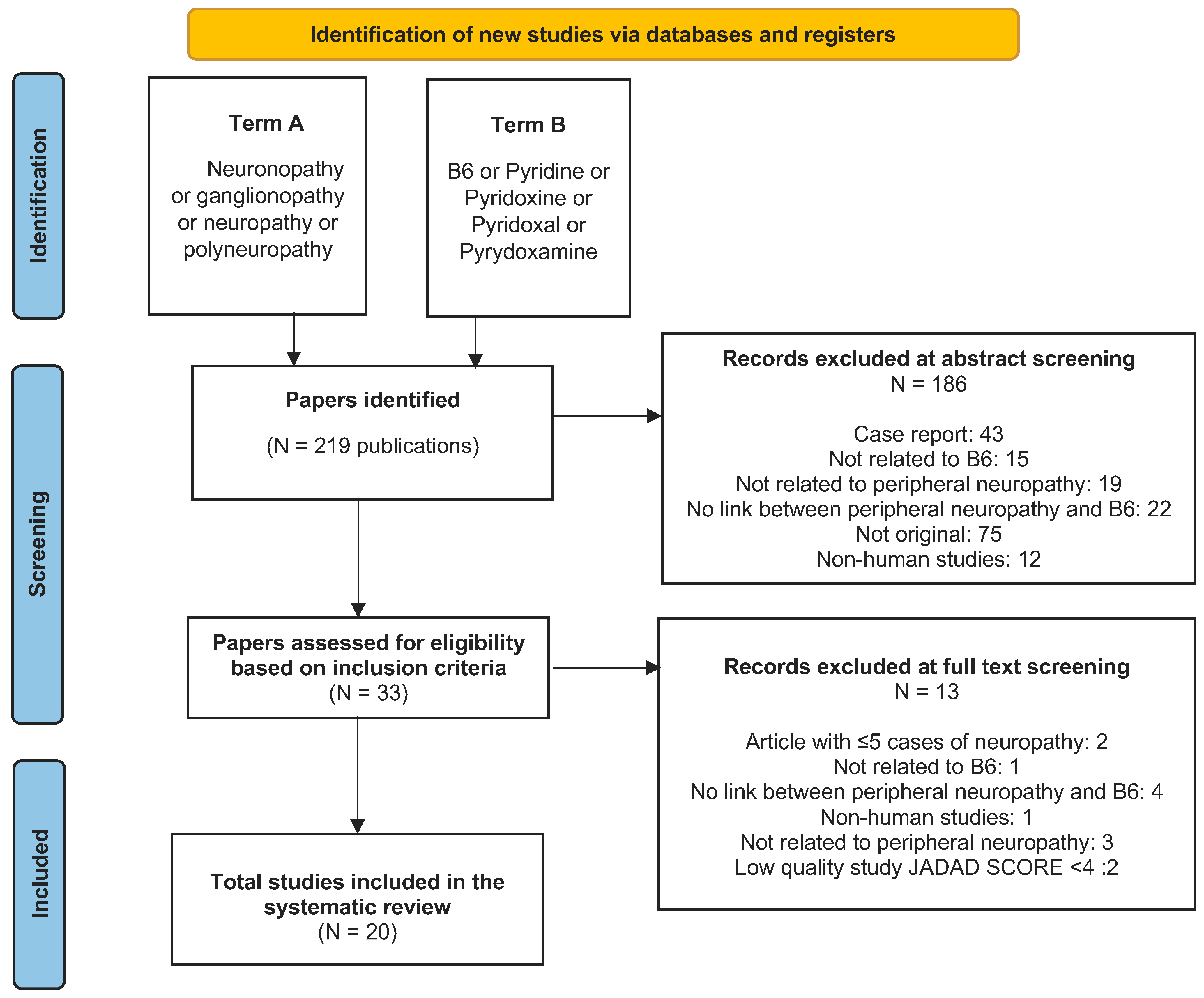
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
- The study was conducted using human subjects;
- The article was written in the English language;
- The study discussed possible links between B6 and PN.
- Non original studies (i.e., reviews/letters to the editor/opinion papers);
- Case reports or case series with fewer than five participants with B6-related PN;
- Papers not related to PN;
- Papers not related to B6;
- Full texts not in English;
- Low-quality trials measured as by Jadad score, where applicable.
2.3. Data Collection Process
2.4. Risk of Bias in Individual Studies
2.5. Synthesis of Results
2.6. Compliance with Ethical Guidelines
2.7. Study Registration
3. Results
3.1. Nature of Included Studies
3.2. Neuropathy due to B6 Deficiency
3.3. Neuropathy due to B6 Toxicity
3.4. B6 Used for the Treatment of Neuropathy
3.5. Genetic Predisposition
4. Conclusions
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrell, K.; Smith, A.G. Peripheral Neuropathy. Med. Clin. N. Am. 2019, 103, 383–397. [Google Scholar] [CrossRef]
- Hanewinckel, R.; Drenthen, J.; van Oijen, M.; Hofman, A.; van Doorn, P.A.; Ikram, M.A. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 2016, 87, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.M.; Staff, N.P.; Robb, J.M.; St Sauver, J.L.; Dyck, P.J.; Klein, C.J. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology 2015, 84, 1644–1651. [Google Scholar] [CrossRef]
- Zis, P.; Sarrigiannis, P.G.; Rao, D.G.; Hewamadduma, C.; Hadjivassiliou, M. Chronic idiopathic axonal polyneuropathy: A systematic review. J. Neurol. 2016, 263, 1903–1910. [Google Scholar] [CrossRef]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Hageman, G.J.; van de Koppel, S.; van Hunsel, F.; Bast, A. Inter-individual differences in pharmacokinetics of vitamin B6: A possible explanation of different sensitivity to its neuropathic effects. PharmaNutrition 2020, 12, 100188. [Google Scholar] [CrossRef]
- National Research Council Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
- Pietrzik, K.; Bronstrup, A. Vitamins B12, B6 and folate as determinants of homocysteine concentration in the healthy population. Eur. J. Pediatr. 1998, 157 (Suppl. 2), S135–S138. [Google Scholar] [CrossRef] [PubMed]
- Hammond, N.; Wang, Y.; Dimachkie, M.M.; Barohn, R.J. Nutritional neuropathies. Neurol. Clin. 2013, 31, 477–489. [Google Scholar] [CrossRef]
- Ghavanini, A.A.; Kimpinski, K. Revisiting the evidence for neuropathy caused by pyridoxine deficiency and excess. J. Clin. Neuromuscul. Dis. 2014, 16, 25–31. [Google Scholar] [CrossRef]
- Van Hunsel, F.; van de Koppel, S.; van Puijenbroek, E.; Kant, A. Vitamin B6 in Health Supplements and Neuropathy: Case Series Assessment of Spontaneously Reported Cases. Drug. Saf. 2018, 41, 859–869. [Google Scholar] [CrossRef]
- Moher, D.; Jones, A.; Cook, D.J.; Jadad, A.R.; Moher, M.; Tugwell, P.; Klassen, T.P. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998, 352, 609–613. [Google Scholar] [CrossRef]
- Stewart, S.L.; Thomas, S.; Höke, E.; Simpson, D.; Singleton, J.R.; Höke, A. Vitamin B6 levels do not correlate with severity of neuropathy in chronic idiopathic axonal polyneuropathy. J. Peripher. Nerv. Syst. 2022, 27, 31–37. [Google Scholar] [CrossRef]
- Court, R.; Centner, C.M.; Chirehwa, M.; Wiesner, L.; Denti, P.; de Vries, N.; Harding, J.; Gumbo, T.; Maartens, G.; McIlleron, H. Neuropsychiatric toxicity and cycloserine concentrations during treatment for multidrug-resistant tuberculosis. Int. J. Infect. Dis. 2021, 105, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Köker, S.A.; Gözmen, S.; Demirağ, B.; Ünalp, A.; Karapinar, T.H.; Oymak, Y.; Gürbüz, G.; Öner, E.I.; Vergin, R.C. Effect of pyridoxine plus pyridostigmine treatment on vincristine-induced peripheral neuropathy in pediatric patients with acute lymphoblastic leukemia: A single-center experience. Neurol. Sci. 2021, 42, 3681–3686. [Google Scholar] [CrossRef]
- Loens, S.; Chorbadzhieva, E.; Kleimann, A.; Dressler, D.; Schrader, C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav. 2017, 7, e00698. [Google Scholar] [CrossRef] [PubMed]
- Alsabah, A.; Al Sabah, S.; Al-Sabah, S.; Al-Serri, A.; Al Haddad, E.; Renno, W.M. Investigating Factors Involved in Post Laparoscopic Sleeve Gastrectomy (LSG) Neuropathy. Obes. Surg. 2016, 27, 1271–1276. [Google Scholar] [CrossRef]
- Latov, N.; Vo, M.L.; Chin, R.L.; Carey, B.T.; Langsdorf, J.A.; Feuer, N.T. Abnormal Nutritional Factors in Patients Evaluated at a Neuropathy Center. J. Clin. Neuromuscul. Dis. 2016, 17, 212–214. [Google Scholar] [CrossRef]
- Van der Watt, J.J.; Benatar, M.G.; Harrison, T.B.; Carrara, H.; Heckmann, J.M. Isoniazid exposure and pyridoxine levels in human immunodeficiency virus associated distal sensory neuropathy. Int. J. Tuberc. Lung Dis. 2015, 19, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.M.; Carrara, H.; Harrison, T.B.; Benatar, M.; Heckmann, J. Sensory polyneuropathy in human immunodeficiency virus-infected patients receiving tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2014, 18, 27–33. [Google Scholar] [CrossRef]
- Trippe, B.S.; Barrentine, L.W.; Curole, M.V.; Tipa, E. Nutritional management of patients with diabetic peripheral neuropathy with L-methylfolate-methylcobalamin-pyridoxal-5-phosphate: Results of a real-world patient experience trial. Curr. Med. Res. Opin. 2016, 32, 219–227. [Google Scholar] [CrossRef]
- Visser, N.A.; Notermans, N.C.; Degen, L.A.R.; de Kruijk, J.R.; Berg, L.H.V.D.; Vrancken, A.F.J.E. Chronic idiopathic axonal polyneuropathy and vitamin B6: A controlled population-based study. J. Peripher. Nerv. Syst. 2014, 19, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A.M.D.; Lavery, L.A.; Thethi, T.K.; Daoud, Y.; DeSouza, C.; Ovalle, F.; Denham, D.S.; Bottiglieri, T.; Sheehan, P.; Rosenstock, J. Metanx in Type 2 Diabetes with Peripheral Neuropathy: A Randomized Trial. Am. J. Med. 2013, 126, 141–149. [Google Scholar] [CrossRef]
- Scott, K.; Zeris, S.; Kothari, M.J. Elevated B6 levels and peripheral neuropathies. Electromyogr. Clin. Neurophysiol. 2008, 48, 219–223. [Google Scholar] [PubMed]
- Peters, T.J.; Kotowicz, J.; Nyka, W.; Kozubski, W.; Kuznetsov, V.; Vanderbist, F.; DE Niet, S.; Marcereuil, D.; Coffiner, M. Treatment of alcoholic polyneuropathy with vitamin b complex: A randomised controlled trial. Alcohol Alcohol. 2006, 41, 636–642. [Google Scholar] [CrossRef]
- Moriwaki, K.; Kanno, Y.; Nakamoto, H.; Okada, H.; Suzuki, H. Vitamin B6 deficiency in elderly patients on chronic peritoneal dialysis. Adv. Perit. Dial. 2000, 16, 308–312. [Google Scholar] [PubMed]
- Okada, H.; Moriwaki, K.; Kanno, Y.; Sugahara, S.; Nakamoto, H.; Yoshizawa, M.; Suzuki, H. Vitamin B6 supplementation can improve peripheral polyneuropathy in patients with chronic renal failure on high-flux haemodialysis and human recombinant erythropoietin. Nephrol. Dial. Transplant. 2000, 15, 1410–1413. [Google Scholar] [CrossRef]
- Parry, G.J.; Bredesen, D.E. Sensory neuropathy with low-dose pyridoxine. Neurology 1985, 35, 1466–1468. [Google Scholar] [CrossRef]
- McCann, V.J.; Davis, R.E. Serum pyridoxal concentrations in patients with diabetic neuropathy. Aust. N. Z. J. Med. 1978, 8, 259–261. [Google Scholar] [CrossRef]
- Devadatta, S.; Gangadharam, P.R.J.; Andrews, R.H.; Fox, W.; Ramakrishnan, C.V.; Selkon, J.B.; Velu, S. Peripheral neuritis due to isoniazid. Bull. World Health Organ. 1960, 23, 587–598. [Google Scholar]
- Pauls, K.A.M.; Toppila, J.; Koivu, M.; Eerola-Rautio, J.; Udd, M.; Pekkonen, E. Polyneuropathy monitoring in Parkinson’s disease patients treated with levodopa/carbidopa intestinal gel. Brain Behav. 2021, 11, e2408. [Google Scholar] [CrossRef]
- Chelban, V.; Wilson, M.P.; Warman Chardon, J.; Vandrovcova, J.; Zanetti, M.N.; Zamba-Papanicolaou, E.; Efthymiou, S.; Pope, S.; Conte, M.R.; Care4Rare Canada Consortium and the SYNaPS Study Group; et al. PDXK mutations cause polyneuropathy responsive to pyridoxal 5′-phosphate supplementation. Ann. Neurol. 2019, 86, 225–240. [Google Scholar] [CrossRef] [PubMed]
| Study (Reference) | Study Type | Participants | Country | B6 Link | Type of Neuropathy | Assessment Tool for Neuropathy | Main Outcome |
|---|---|---|---|---|---|---|---|
| 1-(Stewart et al., 2022) [13] | Cross-sectional study | Patients with chronic idiopathic axonal polyneuropathy (CIAP), n = 261 (56% males) | USA | Management of PN | Sensory motor neuropathy | Total Neuropathy Score-reduced (TNSr) + Nerve Conduction Studies (NCS) | Mild to moderate elevation of B6 (100 to 200 μg/L range) was not related to worse PN symptoms in chronic idiopathic axonal polyneuropathy (CIAP) |
| 2-(Court et al., 2021) [14] | Prospective, observational study | Patients with tuberculosis treated with terizidone receiving prophylactic supplementation with B6 (150 or 200 mg daily), n = 144 (60% male) | South Africa | Excess B6 | Sensory neuropathy | The Brief PN Rating Screen (BPNS) | Patients prescribed higher B6 doses had 2.78 times the risk of PN compared to those on lower dose (p = 0.012) |
| 3-(Aydin Köker et al., 2021) [15] | Prospective, observational study | Pediatric patients with VIPN (vincristine-induced peripheral neuropathy) receiving prophylactic supplementation with pyridoxine 150 mg/m2 BID and pyridostigmine 3 mg/kg BID, n = 23 (44% male) | Turkey | Management of PN | Sensory motor neuropathy | World Health Organization (WHO) neurotoxicity score (sensory/motor) and the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) + NCS | Statistically significant improvement in PN symptoms with B6 supplementation |
| 4-(Loens et al., 2017) [16] | Retrospective, observational study | Patients with advanced idiopathic Parkinson’s disease (IPD) receiving oral levodopa therapy or levodopa/carbidopa intestinal gel (LCIG), n = 21 (52% male) | Germany | Low B6 | Axonal sensory or sensory motor neuropathy | NCS | Lower levels of B6 due to levodopa therapy were associated with presence of more severe PN |
| 5-(Alsabah et al., 2016) [17] | Retrospective, case-control study | Post-LSG (laparoscopic sleeve gastrectomy) patients receiving B6 supplementation 202 mg daily, n = 32 (16% male) | Kuwait | Excess B6 | Not specified | Assessment was mainly based on symptoms of PN | Higher levels of B6 were associated with presence of PN |
| 6-(Latov et al., 2016) [18] | Retrospective, observational study | Patients with recently diagnosed PN caused by pathological nutritional status, n = 23 | USA | Excess B6 | Sensory small fiber neuropathy (SFN) | Signs and symptoms of PN + NCS and biopsy | High pyridoxal phosphate (PYP) levels were identified in patients with neuropathy; 50% were diagnosed with SFN |
| 7-(van der Watt et al., 2015) [19] | Prospective, observational study | HIV-infected individuals before or after antiretroviral therapy (ART), n = 159 (31% male) | South Africa | Low B6 | Distal sensory neuropathy (DSP) | The Brief Peripheral Neuropathy Screen (BPNS) and the modified version of the Total Neuropathy Score (TNSr) | No association between pyridoxine deficiency and overall DSP |
| 8-(Centner et al., 2014) [20] | Prospective, observational study | HIV-infected patients receiving TB therapy and prophylactic B6 supplementation (25 mg daily n = 88, 50–150 mg daily n = 28), n = 116 (45% male) | South Africa | Low B6 | Sensory neuropathy | The Brief Peripheral Neuropathy Screen (BPNS);Neuropathic symptoms of pain, paresthesia or numbness were quantified using a visual numerical rating scale (NRS) | HIV-infected patients receiving TB treatment developed sensory polyneuropathy (SPN) at a high rate, despite receiving pyridoxine and having normal plasma B6. The SPN group did not show significant changes in plasma B6 compared with the SPN-free group |
| 9-(Trippe et al., 2016) [21] | Prospective, observational study | Patients with diabetic PN receiving B6 supplementation (35 mg daily), n = 544 (46% male) | USA | Management of PN | Sensory neuropathy | Neuropathy Total Symptom Score-6 (NTSS-6) questionnaire | Improvement of PN symptoms with B6 supplementation |
| 10-(Visser et al., 2014) [22] | Prospective, case-control study | Patients with CIAP (chronic idiopathic axonal polyneuropathy) n = 381 (70% males) | Netherlands | Excess B6 | Sensory motor neuropathy | The sensory sum score, which ranges from 0 to 28, and a Medical Research Council (MRC) scale, resulting in a motor sum score from 0 to 40 for both lower limbs + NCS | No association between CIAP and elevated vitamin B6 serum levels |
| 11-(Fonseca et al., 2013) [23] | Randomized, double-blind, placebo-controlled trial (Jadad score 4) | Patients with type 2 diabetes and PN receiving B6 supplementation (35 mg daily) or placebo, n = 214 (69% male) | USA | Management of PN | Sensory neuropathy | Vibration perception threshold (VPT) and Neuropathy Total Symptom Score-6 (NTSS-6) and others (NDS, SF-36) | VPT did not differ significantly between B6 supplementation and placebo groups; NTSS-6 scores improved significantly in patients receiving supplementation |
| 12-(Scott, Zeris and Kothari, 2008) [24] | Retrospective, observational study | Patients with PN and elevated B6 levels, n = 26 | USA | Excess B6 | Sensory small fiber neuropathy (SFN) | Signs and symptoms of peripheral neuropathy + NCS and Quantitative Sensory Testing (QST) | Elevated B6 levels should be considered in the differential diagnosis of any sensory or sensorimotor PN |
| 13-(Peters et al., 2006) [25] | Randomized, double-blind, placebo-controlled trial (Jadad score 4) | Patients with alcoholic PN receiving B6 supplementation (250 mg TID) or placebo, n = 325 (75% male) | Poland and Ukraine | Management of PN | Sensory neuropathy | Vibration perception threshold (VPT), McGill’s pain questionnaire and 2-point discrimination test | Improvement of PN symptoms after B6 supplementation |
| 14-(Moriwaki et al., 2000) [26] | Prospective, observational study | Patients on chronic peritoneal dialysis with PN symptoms, n = 12 | Japan | Low B6 Management of PN | Sensory neuropathy | Symptoms were assessed, focusing specifically on burning and painful paresthesia | Lower B6 levels were associated with PN symptoms; supplementation improved sensory abnormalities in 8 of 12 patients |
| 15-(Okada et al., 2000) [27] | Prospective, case-control study | Patients with chronic renal failure on high flux hemodialysis (HD) suffering from PN receiving B6 supplementation (60 mg daily), n = 14 (43% male) versus B12 supplementation (500 μg daily), n = 12 (50% male) | Japan | Management of PN | Sensory neuropathy | Peripheral polyneuropathy (PPN) was assessed using a score of 0–4, ranging from no symptoms to painful symptoms | Improvement of PN symptoms after B6 supplementation |
| 16-(Parry and Bredesen, 1985) [28] | Observational study | Patients with PN receiving B6 supplementation (0.2–5 g daily), n = 16 (0% male) | USA | Excess B6 | Sensory neuropathy | Signs and symptoms of peripheral neuropathy + NCS and biopsy | B6 excess causes pure sensory, length-dependent, axonal neuropathy; improvement followed discontinuation of B6. |
| 17-(McCann and Davis, 1978) [29] | Prospective, case-control study | Patients with diabetic PN and controls without PN, n = 50 (48% males) | Australia | Low B6 | Sensory motor neuropathy | Signs and symptoms of peripheral neuropathy were assessed | Lower B6 concentrations in patients with PN compared with diabetic patients without PN. |
| 18-(Devadatta et al., 1960) [30] | Prospective, interventional study | Poorly nourished tuberculous patients under isoniazid treatment with PN receiving B6 supplementation (200 mg or 6 mg), n = 16 | India | Management of PN | Sensory motor neuropathy | Signs and symptoms of peripheral neuropathy were assessed | Improvement of neuropathy symptoms after B6 supplementation |
| 19-(Pauls et al., 2021) [31] | Retrospective, observational study | Patients with advanced idiopathic Parkinson’s disease (n = 19) receiving levodopa/carbidopa intestinal gel (LCIG), n = 19 (47% male) | Finland | Low B6 | Sensory neuropathy | Signs and symptoms of peripheral neuropathy + NCS | B6 supplementation (3 mg of B6) reduced the odds of developing PN |
| 20-(Chelban et al., 2019) [32] | Prospective, observational study | 5 individuals (1 male) from 2 unrelated families with primary axonal polyneuropathy and optic atrophy and biallelic mutations in PDXK | Cyprus, Scotland, and Italy | Low B6, management of PN | Sensory motor neuropathy | Signs and symptoms of peripheral neuropathy + NCS | Improvement of neuropathy symptoms after B6 supplementation (50 mg of B6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhamad, R.; Akrivaki, A.; Papagiannopoulou, G.; Zavridis, P.; Zis, P. The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review. Nutrients 2023, 15, 2823. https://doi.org/10.3390/nu15132823
Muhamad R, Akrivaki A, Papagiannopoulou G, Zavridis P, Zis P. The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review. Nutrients. 2023; 15(13):2823. https://doi.org/10.3390/nu15132823
Chicago/Turabian StyleMuhamad, Raman, Alexandra Akrivaki, Georgia Papagiannopoulou, Periklis Zavridis, and Panagiotis Zis. 2023. "The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review" Nutrients 15, no. 13: 2823. https://doi.org/10.3390/nu15132823
APA StyleMuhamad, R., Akrivaki, A., Papagiannopoulou, G., Zavridis, P., & Zis, P. (2023). The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review. Nutrients, 15(13), 2823. https://doi.org/10.3390/nu15132823






