The Mixture of Natural Products SH003 Exerts Anti-Melanoma Effects through the Modulation of PD-L1 in B16F10 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. SH003 and FMN
2.2. Cell Lines
2.3. Melanin Content and Tyrosinase Activity Assay
2.4. MTT Assay
2.5. Western Blot Analysis
2.6. Cell Cycle Analysis
2.7. Cell Apoptosis Assay
2.8. qPCR
2.9. Immunofluorescent Staining
2.10. T Cell-Mediated Cytotoxicity Assay
2.11. Statistical Analysis
3. Results
3.1. Regulatory Effect of SH003 and FMN on the Melanogenesis of B16F10 Cells
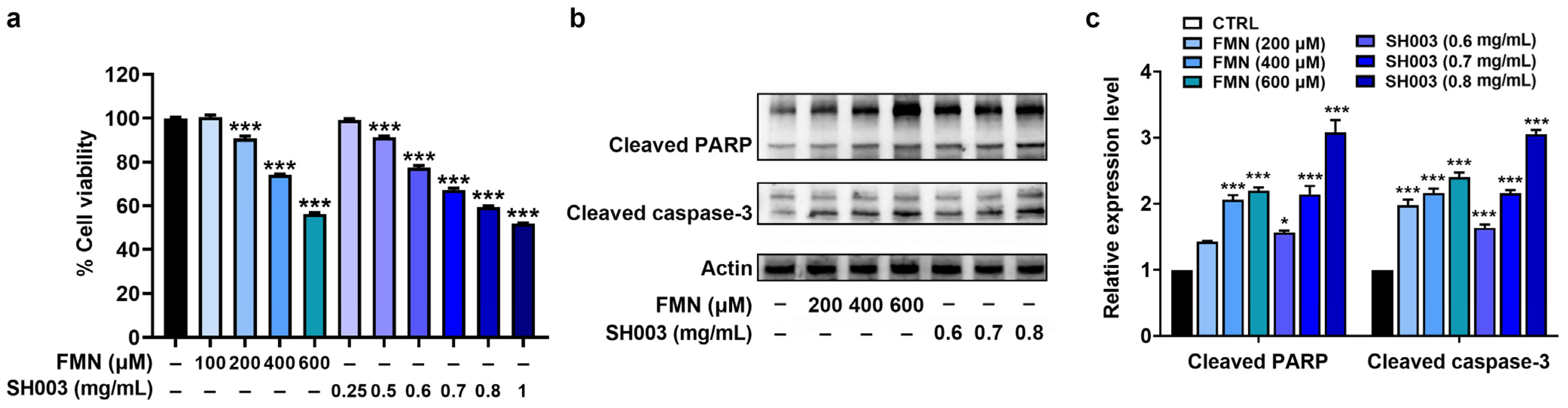
3.2. Regulatory Effect of SH003 and FMN on the Proliferation and Apoptosis of B16F10 Cells
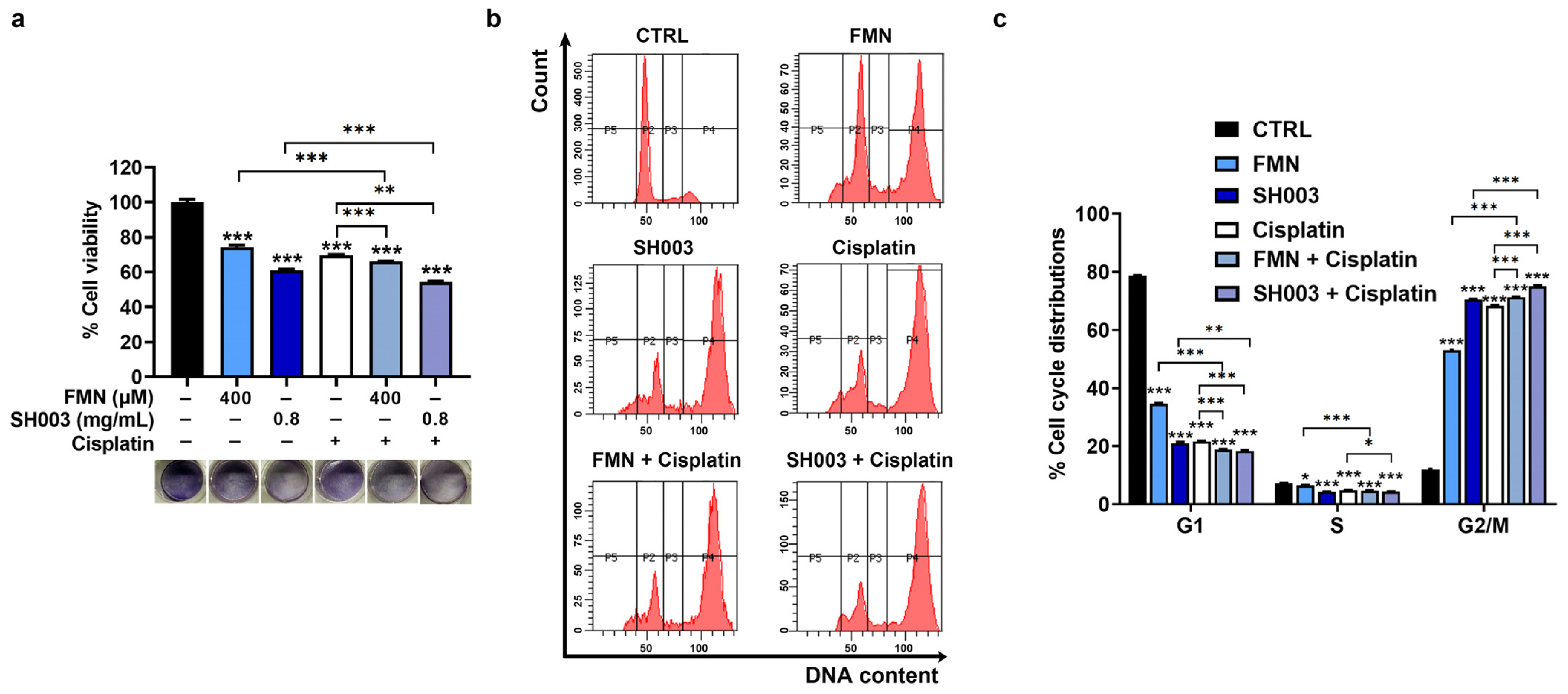
3.3. Synergistic Effect of Cisplatin Combined with SH003 and FMN on Cell Cycle Arrest of B16F10 Cells
3.4. Synergistic Effect of Cisplatin Combined with SH003 and FMN on Apoptosis of B16F10 Cells
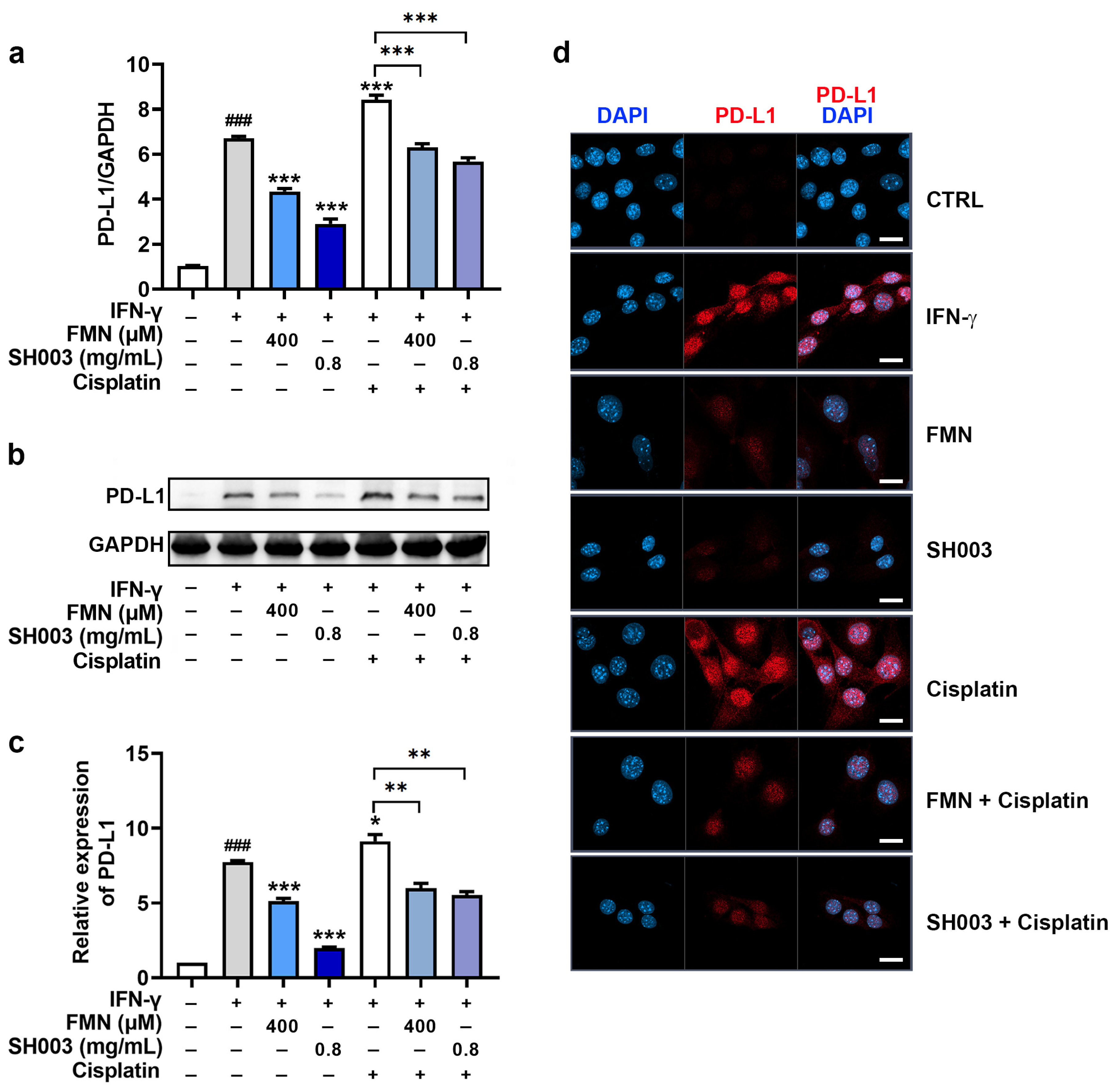
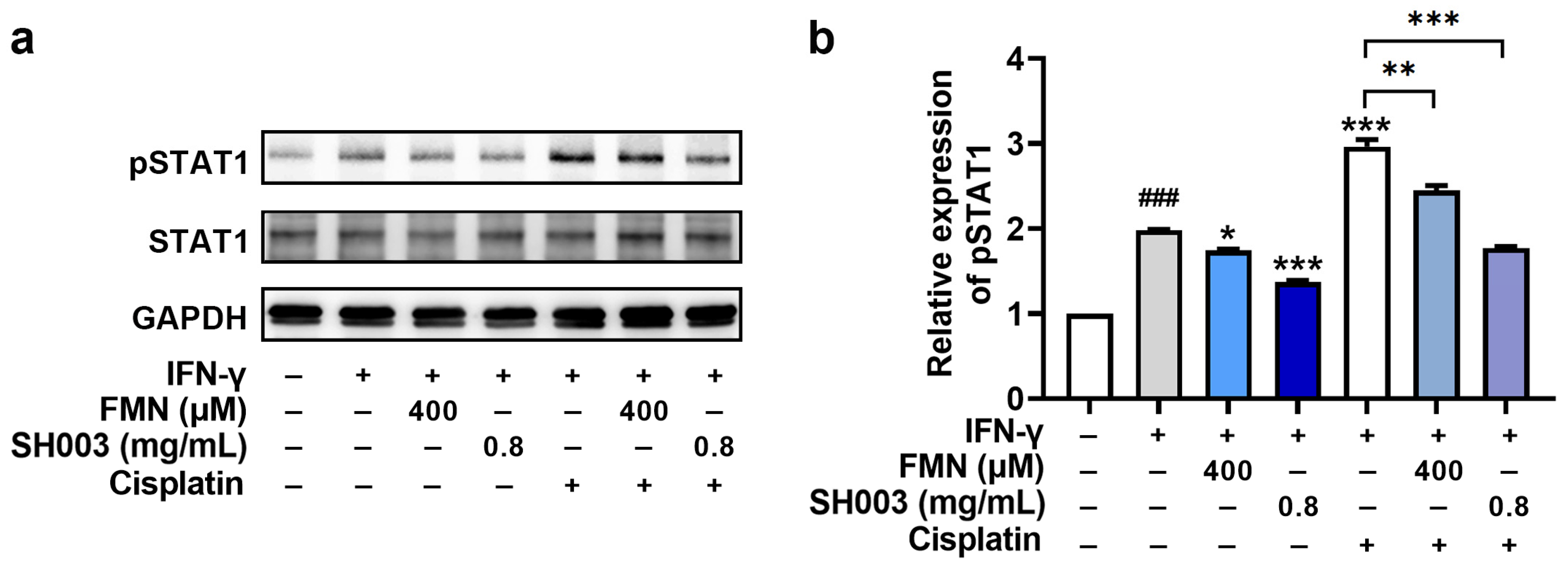
3.5. Regulatory Effect of SH003 and FMN on IFN-γ-induced PD-L1 Expression in B16F10 Cells
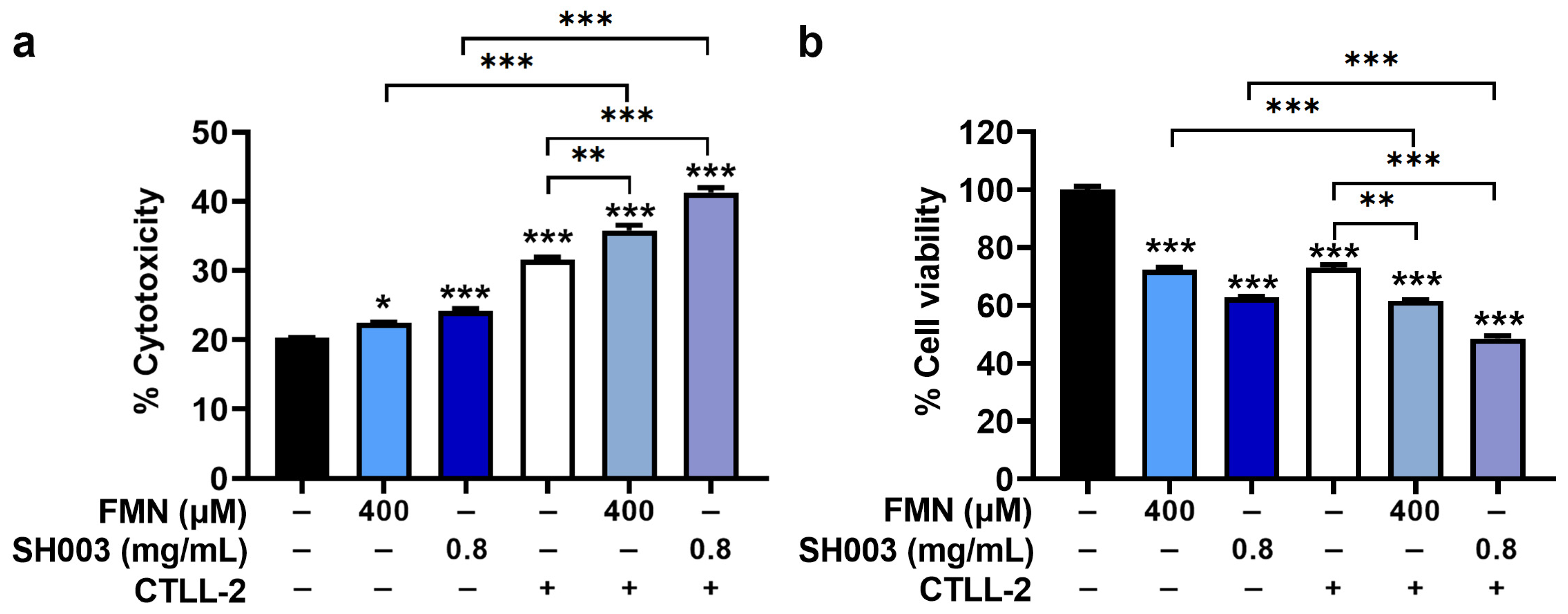
3.6. Regulatory Effect of SH003 and FMN on Cytotoxicity of CTLL-2 Cells against B16F10 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dermani, F.K.; Samadi, P.; Rahmani, G.; Kohlan, A.K.; Najafi, R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J. Cell. Physiol. 2019, 234, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Thomas, D.A.; Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zha, H.; Wu, W.; Yuan, T.; Xie, S.; Jin, Z.; Long, H.; Yang, F.; Wang, Z.; Zhang, A.; et al. CD200+ cytotoxic T lymphocytes in the tumor microenvironment are crucial for efficacious anti-PD-1/PD-L1 therapy. Sci. Transl. Med. 2023, 15, eabn5029. [Google Scholar] [CrossRef]
- Greil, R.; Hutterer, E.; Hartmann, T.N.; Pleyer, L. Reactivation of dormant anti-tumor immunity—A clinical perspective of therapeutic immune checkpoint modulation. Cell Commun. Signal. 2017, 15, 5. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Moan, J.; Dahlback, A.; Setlow, R.B. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem. Photobiol. 1999, 70, 243–247. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, T.H.; Choi, W.G.; Chung, Y.H.; Ko, S.G.; Cheon, C.; Cho, S.G. SH003 Causes ER Stress-mediated Apoptosis of Breast Cancer Cells via Intracellular ROS Production. Cancer Genom. Proteom. 2023, 20, 88–116. [Google Scholar] [CrossRef]
- Jeong, M.S.; Lee, K.W.; Choi, Y.J.; Kim, Y.G.; Hwang, H.H.; Lee, S.Y.; Jung, S.E.; Park, S.A.; Lee, J.H.; Joo, Y.J.; et al. Synergistic Antitumor Activity of SH003 and Docetaxel via EGFR Signaling Inhibition in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 8405. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Cheon, C.; Ko, S.G. SH003 activates autophagic cell death by activating ATF4 and inhibiting G9a under hypoxia in gastric cancer cells. Cell Death Dis. 2020, 11, 717. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, K.; Choi, Y.K.; Choi, Y.J.; Seo, H.S.; Ko, S.G. SH003-induced G1 phase cell cycle arrest induces apoptosis in HeLa cervical cancer cells. Mol. Med. Rep. 2017, 16, 8237–8244. [Google Scholar] [CrossRef]
- Choi, Y.J.; Choi, Y.K.; Lee, K.M.; Cho, S.G.; Kang, S.Y.; Ko, S.G. SH003 induces apoptosis of DU145 prostate cancer cells by inhibiting ERK-involved pathway. BMC Complement. Altern. Med. 2016, 16, 507. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Kim, K.C.; Kim, J.S.; Ko, S.G.; Park, H.J.; Moon, P.D. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. J. Ethnopharmacol. 2022, 285, 114893. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Ko, S.G.; Kim, W. Analgesic Effect of SH003 and Trichosanthes kirilowii Maximowicz in Paclitaxel-Induced Neuropathic Pain in Mice. Curr. Issues Mol. Biol. 2022, 44, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ku, J.M.; Choi, Y.J.; Hwang, H.H.; Jeong, M.; Kim, Y.G.; Kim, M.J.; Ko, S.G. Herbal Prescription SH003 Alleviates Docetaxel-Induced Neuropathic Pain in C57BL/6 Mice. Evid. Based Complement. Alternat. Med. 2021, 2021, 4120334. [Google Scholar] [CrossRef]
- Chen, L.; Xing, D.; Guo, L.R.; Jin, J.; Li, S. Formononetin, an Active Component of Astragalus Membranaceus, Inhibits the Pathogenesis and Progression of Esophageal Cancer Through the COX-2/Cyclin D1 Axis. Clin. Lab. 2023, 69, 220403. [Google Scholar] [CrossRef]
- Han, N.R.; Park, H.J.; Ko, S.G.; Moon, P.D. The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways. Foods 2023, 12, 276. [Google Scholar] [CrossRef]
- Choi, Y.K.; Cho, S.G.; Woo, S.M.; Yun, Y.J.; Park, S.; Shin, Y.C.; Ko, S.G. Herbal extract SH003 suppresses tumor growth and metastasis of MDA-MB-231 breast cancer cells by inhibiting STAT3-IL-6 signaling. Mediat. Inflamm. 2014, 2014, 492173. [Google Scholar] [CrossRef]
- Choi, Y.J.; Choi, W.G.; Lee, K.; Jeong, M.; Park, S.C.; Jang, Y.P.; Ko, S.G. The effect of isoflavonoid contents in SH003 and its subfractions on breast cancer. J. Korean Med. 2022, 43, 79–93. [Google Scholar] [CrossRef]
- Hu, Y.; Zhai, W.; Tan, D.; Chen, H.; Zhang, G.; Tan, X.; Zheng, Y.; Gao, W.; Wei, Y.; Wu, J.; et al. Uncovering the effects and molecular mechanism of Astragalus membranaceus (Fisch.) Bunge and its bioactive ingredients formononetin and calycosin against colon cancer: An integrated approach based on network pharmacology analysis coupled with experimental validation and molecular docking. Front. Pharmacol. 2023, 14, 1111912. [Google Scholar] [CrossRef]
- Song, X.; Li, J. Screening of Immune-Related Genes and Predicting the Immunotherapeutic Effects of Formononetin in Breast Cancer: A Bioinformatics Analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 9942373. [Google Scholar] [CrossRef]
- Mu, H.; Bai, Y.H.; Wang, S.T.; Zhu, Z.M.; Zhang, Y.W. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine 2009, 16, 314–319. [Google Scholar] [CrossRef]
- Machado Dutra, J.; Espitia, P.J.P.; Andrade Batista, R. Formononetin: Biological effects and uses—A review. Food Chem. 2021, 359, 129975. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, I.S.; So, Y.K.; Kim, H.H.; Kim, Y.H. Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens. J. Enzyme Inhib. Med. Chem. 2018, 33, 1048–1054. [Google Scholar] [CrossRef]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef]
- Bayrakçeken Güven, Z.; Saracoglu, I.; Nagatsu, A.; Yilmaz, M.A.; Basaran, A.A. Anti-tyrosinase and antimelanogenic effect of cinnamic acid derivatives from Prunus mahaleb L.: Phenolic composition, isolation, identification and inhibitory activity. J. Ethnopharmacol. 2023, 310, 116378. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Chung, S.; Lim, G.J.; Lee, J.Y. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci. Rep. 2019, 9, 780. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Burr, M.L.; Sparbier, C.E.; Chan, Y.C.; Williamson, J.C.; Woods, K.; Beavis, P.A.; Lam, E.Y.N.; Henderson, M.A.; Bell, C.C.; Stolzenburg, S.; et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017, 549, 101–105. [Google Scholar] [CrossRef]
- Wangpaichitr, M.; Kandemir, H.; Li, Y.Y.; Wu, C.; Nguyen, D.; Feun, L.G.; Kuo, M.T.; Savaraj, N. Relationship of Metabolic Alterations and PD-L1 Expression in Cisplatin Resistant Lung Cancer. Cell Dev. Biol. 2017, 6, 183. [Google Scholar] [CrossRef]
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Valdés-Ramos, R.; Benítez-Arciniega, A.D. Nutrition and immunity in cancer. Br. J. Nutr. 2007, 98 (Suppl. 1), S127–S132. [Google Scholar] [CrossRef]
- Che, D.; Adams, S.; Wei, C.; Gui-Xin, Q.; Atiba, E.M.; Hailong, J. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiol. Open 2019, 8, e00712. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.S.; Lee, J.; Hong, S.J.; Cho, J.J.; Cho, K.M.; Shin, E.C. Comprehensive changes in volatile/nonvolatile compounds and flavor and physicochemical characteristics in Angelica gigas Nakai roots by thermal processing. J. Food Biochem. 2019, 43, e12842. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, C.; Liu, T.; Karrar, E.; Ouyang, Y.; Li, D. Effect of microwave heating on lipid composition, chemical properties and antioxidant activity of oils from Trichosanthes kirilowii seed. Food Res. Int. 2022, 159, 111643. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Liu, P.; Duan, J.A.; Dong, L.; Shang, E.X.; Qian, D.W.; Zhu, Z.H.; Li, H.W.; Li, W.W. Comparative Analysis of Carbohydrates, Nucleosides and Amino Acids in Different Parts of Trichosanthes kirilowii Maxim. by (Ultra) High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry and Evaporative Light Scattering Detector Methods. Molecules 2019, 24, 1440. [Google Scholar] [CrossRef]
- Lee, K.; Youn, B.Y.; Choi, Y.J.; Moon, S.; Im, J.; Cho, K.; Ko, S.G.; Cheon, C. State of the Art and Future Implications of SH003: Acting as a Therapeutic Anticancer Agent. Cancers 2022, 14, 1089. [Google Scholar] [CrossRef]
- Tayier, N.; Qin, N.Y.; Zhao, L.N.; Zeng, Y.; Wang, Y.; Hu, G.; Wang, Y.Q. Theoretical Exploring of a Molecular Mechanism for Melanin Inhibitory Activity of Calycosin in Zebrafish. Molecules 2021, 26, 6998. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Kim, B.S.; Seo, H.; Kim, H.J.; Bae, S.M.; Son, H.N.; Lee, Y.J.; Ryu, S.; Park, R.W.; Nam, J.O. Decursin from Angelica gigas Nakai Inhibits B16F10 Melanoma Growth Through Induction of Apoptosis. J. Med. Food 2015, 18, 1121–1127. [Google Scholar] [CrossRef]
- Yoon, Y.; Bae, S.; Kim, T.J.; An, S.; Lee, J.H. Nodakenin Inhibits Melanogenesis Via the ERK/MSK1 Signaling Pathway. Pharmazie 2023, 78, 6–12. [Google Scholar] [CrossRef]
- Oh, H.; Mun, Y.J.; Im, S.J.; Lee, S.Y.; Song, H.J.; Lee, H.S.; Woo, W.H. Cucurbitacins from Trichosanthes kirilowii as the inhibitory components on tyrosinase activity and melanin synthesis of B16/F10 melanoma cells. Planta Med. 2002, 68, 832–833. [Google Scholar] [CrossRef]
- Tsao, S.W.; Yan, K.T.; Yeung, H.W. Selective killing of choriocarcinoma cells in vitro by trichosanthin, a plant protein purified from root tubers of the Chinese medicinal herb Trichosanthes kirilowii. Toxicon 1986, 24, 831–840. [Google Scholar] [CrossRef]
- Cabaço, L.C.; Tomás, A.; Pojo, M.; Barral, D.C. The Dark Side of Melanin Secretion in Cutaneous Melanoma Aggressiveness. Front. Oncol. 2022, 12, 887366. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chou, T.H.; Lin, R.J.; Chan, L.P.; Wang, G.H.; Liang, C.H. Antioxidant and antimelanogenic behaviors of Paeonia suffruticosa. Plant Foods Hum. Nutr. 2011, 66, 275–284. [Google Scholar] [CrossRef]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco. Targets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Tong, Q.; Wu, Z. Curcumin inhibits colon cancer malignant progression and promotes T cell killing by regulating miR-206 expression. Clin. Anat. 2023. [Google Scholar] [CrossRef]
- Cheon, H.; Holvey-Bates, E.G.; McGrail, D.J.; Stark, G.R. PD-L1 sustains chronic, cancer cell-intrinsic responses to type I interferon, enhancing resistance to DNA damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2112258118. [Google Scholar] [CrossRef]
- Chen, J.; Chen, R.; Huang, S.; Zu, B.; Zhang, S. Atezolizumab alleviates the immunosuppression induced by PD-L1-positive neutrophils and improves the survival of mice during sepsis. Mol. Med. Rep. 2021, 23, 144. [Google Scholar] [CrossRef]
- von Delwig, A.; Altmann, D.M.; Charlton, F.G.; McKie, N.; Isaacs, J.D.; Holmdahl, R.; Robinson, J.H. T cell responses to a non-glycosylated epitope predominate in type II collagen-immunised HLA-DRB1*0101 transgenic mice. Ann. Rheum. Dis. 2007, 66, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.R.; Lee, W.H.; Choi, J.W.; Park, S.O.; Paik, S.G.; Kim, Y.S. Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2. Exp. Mol. Med. 2005, 37, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Shin, S.; Kang, S.M.; Jung, I.; Ryu, S.; Noh, S.; Choi, S.J.; Jeong, J.; Lee, B.Y.; Kim, K.S.; et al. Reprogramming of T cell-derived small extracellular vesicles using IL2 surface engineering induces potent anti-cancer effects through miRNA delivery. J. Extracell. Vesicles 2022, 11, e12287. [Google Scholar] [CrossRef]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019, 38, 2380–2393. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.-R.; Park, H.-J.; Ko, S.-G.; Moon, P.-D. The Mixture of Natural Products SH003 Exerts Anti-Melanoma Effects through the Modulation of PD-L1 in B16F10 Cells. Nutrients 2023, 15, 2790. https://doi.org/10.3390/nu15122790
Han N-R, Park H-J, Ko S-G, Moon P-D. The Mixture of Natural Products SH003 Exerts Anti-Melanoma Effects through the Modulation of PD-L1 in B16F10 Cells. Nutrients. 2023; 15(12):2790. https://doi.org/10.3390/nu15122790
Chicago/Turabian StyleHan, Na-Ra, Hi-Joon Park, Seong-Gyu Ko, and Phil-Dong Moon. 2023. "The Mixture of Natural Products SH003 Exerts Anti-Melanoma Effects through the Modulation of PD-L1 in B16F10 Cells" Nutrients 15, no. 12: 2790. https://doi.org/10.3390/nu15122790
APA StyleHan, N.-R., Park, H.-J., Ko, S.-G., & Moon, P.-D. (2023). The Mixture of Natural Products SH003 Exerts Anti-Melanoma Effects through the Modulation of PD-L1 in B16F10 Cells. Nutrients, 15(12), 2790. https://doi.org/10.3390/nu15122790





