Dietary Sources of Linoleic Acid (LA) Differ by Race/Ethnicity in Adults Participating in the National Health and Nutrition Examination Survey (NHANES) between 2017–2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Dietary Data
2.2.2. Demographic Variables
2.3. Statistical Analyses
2.3.1. Descriptive Statistics
2.3.2. Differences by Race/Ethnicity in the Contribution of Each Food Group to Overall LA Intake
3. Results
3.1. Descriptive Statistics
3.2. Contribution of Food Groups to Overall LA Intake by Race and Ethnicity
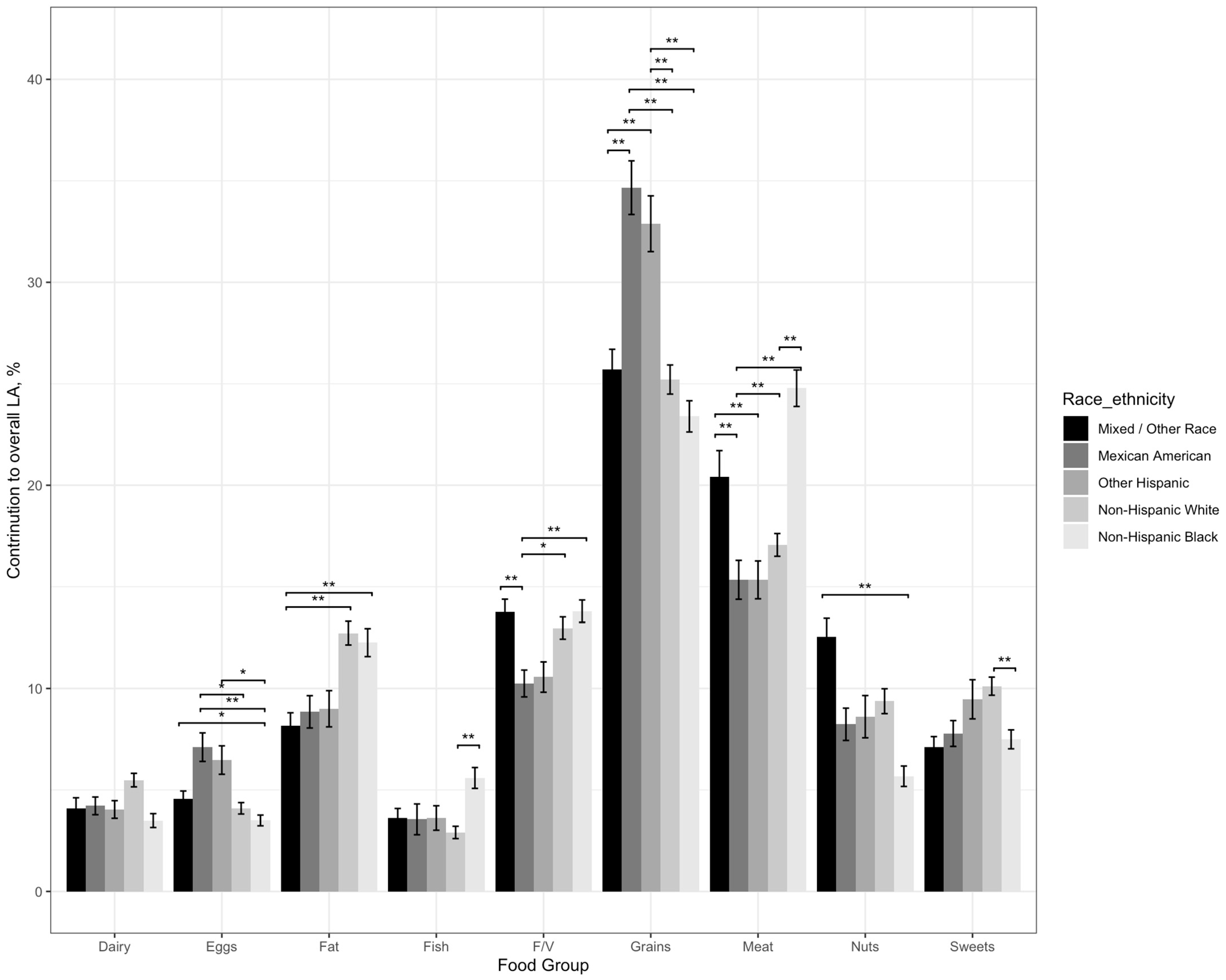
3.2.1. Differences in the Contribution of Dairy to Overall LA Intake by Race/Ethnicity
3.2.2. Differences in the Contribution of Eggs to Overall LA Intake by Race/Ethnicity
3.2.3. Differences in the Contribution of Fat to Overall LA Intake by Race/Ethnicity
3.2.4. Differences in the Contribution of Fish to Overall LA Intake by Race/Ethnicity
3.2.5. Differences in the Contribution of Fruits and Vegetables to Overall LA Intake by Race/Ethnicity
3.2.6. Differences in the Contribution of Grains to Overall LA Intake by Race/Ethnicity
3.2.7. Differences in the Contribution of Meat to Overall LA Intake by Race/Ethnicity
3.2.8. Differences in the Contribution of Nuts to Overall LA Intake by Race/Ethnicity
3.2.9. Differences in the Contribution of Sweets to Overall LA Intake by Race/Ethnicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Public Law. National Nutrition Monitoring and Related Research Act of 1990. Public Law no 101-445; 1990. Available online: https://www.congress.gov/bill/101st-congress/house-bill/1608 (accessed on 4 June 2023).
- Fleischhacker, S.E.; Ballard, R.M.; Starke-Reed, P.E.; Galuska, D.A.; Neuhouser, M.L. Developmental Process and Early Phases of Implementation for the US Interagency Committee on Human Nutrition Research National Nutrition Research Roadmap 2016–2021. J. Nutr. 2017, 147, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Monitoring Food Composition and Nutrient Intake of the Nation. Available online: https://np107.arsnet.usda.gov/post/2022/04/25/proposed-action-plan-component-2 (accessed on 5 October 2022).
- Burr, G.O.; Burr, M.M. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930, 86, 587–621. [Google Scholar] [CrossRef]
- Hansen, A.E.; Haggard, M.E.; Boelsche, A.N.; Adam, D.J.; Wiese, H.F. Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency. J. Nutr. 1958, 66, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R.; Brooks, J.; Butte, N.; Caballero, B.; Flatt, J.; Fried, S. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy Press: Washington, DC, USA, 2002; Volume 5, pp. 589–768. [Google Scholar]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef]
- Barnard, N.D. Trends in food availability, 1909–2007. Am. J. Clin. Nutr. 2010, 91, 1530S–1536S. [Google Scholar] [CrossRef]
- Wright, J.; Kennedy-Stephenson, J.; Wang, C.; McDowell, M.; Johnson, C. Trends in intake of energy and macronutrients-United States, 1971–2000. JAMA 2004, 291, 1193. [Google Scholar]
- Raatz, S.K.; Conrad, Z.; Jahns, L. Trends in linoleic acid intake in the United States adult population: NHANES 1999–2014. Prostaglandins Leukot Essent Fat. Acids 2018, 133, 23–28. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L., 3rd. Food Sources of Energy and Nutrients of Public Health Concern and Nutrients to Limit with a Focus on Milk and other Dairy Foods in Children 2 to 18 Years of Age: National Health and Nutrition Examination Survey, 2011–2014. Nutrients 2018, 10, 1050. [Google Scholar] [CrossRef]
- Xu, Z.; McClure, S.T.; Appel, L.J. Dietary cholesterol intake and sources among US adults: Results from National Health and Nutrition Examination Surveys (NHANES), 2001–2014. Nutrients 2018, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Firestone, M.J.; Popp, C.J.; Russo, R.; Yi, S.S. Age and Racial/Ethnic Differences in Dietary Sources of Protein, NHANES, 2011–2016. Front. Nutr. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Cave, C.; Hein, N.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Bisselou, K.S.; Appiah, A.K.; Kris-Etherton, P.; Skulas-Ray, A.C.; Thompson, M.; et al. Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014. Nutrients 2020, 12, 2045. [Google Scholar] [CrossRef] [PubMed]
- National Center for Farmworker Health Statistics. NHANES—About the National Health and Nutrition Examination Survey 2011; National Center for Farmworker Health: Buda, TX, USA, 2013. [Google Scholar]
- National Center for Farmworker Health Statistics. NHANES—About the National Health and Nutrition Examination Survey. 1999–2002 Sample. 2011. Available online: https://www.cdc.govlnchs/nhanes.htm (accessed on 5 July 2005).
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture: Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2015–2016; Food Surveys Research Group: Beltsville, MD, USA, 2018.
- Team, R.C. R: A Language and Environment for Statistical Computing; 887 R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Jiang, L.; Wang, J.; Xiong, K.; Xu, L.; Zhang, B.; Ma, A. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutrients 2021, 13, 2342. [Google Scholar] [CrossRef] [PubMed]
- Giosuè, A.; Calabrese, I.; Lupoli, R.; Riccardi, G.; Vaccaro, O.; Vitale, M. Relations between the consumption of fatty or lean fish and risk of cardiovascular disease and all-cause mortality: A systematic review and meta-analysis. Adv. Nutr. 2022, 13, 1554–1556. [Google Scholar] [CrossRef]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef]
- Gholami, F.; Khoramdad, M.; Esmailnasab, N.; Moradi, G.; Nouri, B.; Safiri, S.; Alimohamadi, Y. The effect of dairy consumption on the prevention of cardiovascular diseases: A meta-analysis of prospective studies. J. Cardiovasc. Thorac. Res. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.-F.; Laguzzi, F. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Mendelian Randomization of Dairy Consumption Working Group; CHARGE Consortium. Dairy intake and body composition and cardiometabolic traits among adults: Mendelian randomization analysis of 182041 individuals from 18 studies. Clin. Chem. 2019, 65, 751–760. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Brzostek, T.; Toledo, E.; Iacoviello, L.; Astrup, A.; Franco, O.H.; Galvano, F.; Martinez-Gonzalez, M.A.; Grosso, G. Egg consumption and cardiovascular risk: A dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2021, 60, 1833–1862. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Zurbau, A.; Au-Yeung, F.; Blanco Mejia, S.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.; Jenkins, D.J.; Sievenpiper, J.L. Relation of different fruit and vegetable sources with incident cardiovascular outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The effect of replacing refined grains with whole grains on cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled trials with GRADE clinical recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef]
- Mayhew, A.J.; de Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Paz-Graniel, I.; WC Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A. Refined Grain Intake and Cardiovascular Disease: Meta-analyses of Prospective Cohort Studies. Trends Cardiovasc. Med. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, G.C.B.S.; Mesquita, G.X.B.; Lima, S.C.V.C.; Silva, D.F.d.O.; de Azevedo, K.P.M.; Pimenta, I.D.S.F.; de Oliveira, A.K.d.S.G.; Lyra, C.d.O.; Martínez, D.G.; Piuvezam, G. Associations of the consumption of unprocessed red meat and processed meat with the incidence of cardiovascular disease and mortality, and the dose-response relationship: A systematic review and meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
| MA (n = 474) | OH (n = 341) | NHW (n = 1408) | NHB (n = 929) | Other (n = 663) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) a,b,e,f,g,h,i | 42.35 (0.89) | 45.16 (1.13) | 51.46 (0.63) | 46.02 (0.68) | 44.90 (0.87) |
| Gender, Female (n, %) | 252 (52%) | 179 (49%) | 703 (51%) | 473 (51%) | 325 (50%) |
| PIR a,d,e,f,g,h,i | |||||
| 1.3 (%) | 130 (29%) | 107 (33%) | 285 (13%) | 246 (35%) | 105 (23%) |
| >1.3–3.5 (%) | 192 (47%) | 104 (33%) | 574 (32%) | 336 (40%) | 206 (37%) |
| >3.5 (%) | 87 (24%) | 81 (34%) | 444 (55%) | 193 (25%) | 283 (40%) |
| Education a,b,c,d,e,f,h,i | |||||
| 9th grade (%) | 119 (14%) | 60 (11%) | 25 (1%) | 19 (2%) | 24 (2%) |
| Less than HS (%) | 85 (13%) | 43 (9%) | 123 (5%) | 107 (10%) | 34 (5%) |
| HS (%) | 107 (34%) | 68 (27%) | 386 (26%) | 235 (31%) | 93 (22%) |
| Some college (%) | 123 (30%) | 102 (27%) | 528 (31%) | 372 (36%) | 166 (30%) |
| College or above (%) | 39 (10%) | 66 (25%) | 346 (38%) | 194 (21%) | 345 (41%) |
| Dietary intake | |||||
| LA (kcals/day) b,c,d,e,f,g | 154 (5.23) | 144 (5.83) | 164 (3.29) | 170 (3.65) | 149 (4.90) |
| LA intake (grams) b,c,d,e,f,g | 17.11 (0.58) | 16.05 (0.65) | 18.25 (0.37) | 18.91 (0.41) | 16.53 (0.54) |
| AMDR for LA (%) a,b,c,d,e,f,g,h | 7.32 (0.15) | 6.86 (0.16) | 7.77 (0.12) | 8.19 (0.12) | 7.23 (0.13) |
| Dairy (% of LA kcals) a,e,f,g | 4.22 (0.44) | 4.04 (0.43) | 5.49 (0.33) | 3.49 (0.34) | 4.09 (0.52) |
| Eggs (% of LA kcals) b,c,e,f,i,j | 7.11 (0.7) | 6.47 (0.7) | 4.1 (0.28) | 3.5 (0.27) | 4.57 (0.38) |
| Fat (% of LA kcals) b,c,d,e,f,g | 8.85 (0.8) | 9 (0.89) | 12.72 (0.58) | 12.26 (0.69) | 8.16 (0.64) |
| Fish (% of LA kcals) a,b,c,d | 3.55 (0.76) | 3.62 (0.6) | 2.91 (0.3) | 5.59 (0.51) | 3.63 (0.46) |
| Fruits/vegetables (% of LA kcals) b,c,d,e,f,i,j | 10.25 (0.66) | 10.56 (0.75) | 12.97 (0.55) | 13.8 (0.55) | 13.77 (0.62) |
| Grains (% of LA kcals) b,c,d,e,f,i,j | 34.66 (1.32) | 32.89 (1.37) | 25.21 (0.72) | 23.39 (0.77) | 25.7 (1.0) |
| Meat (% of LA kcals) a,b,c,d,g,i,j | 15.34 (0.96) | 15.34 (0.93) | 17.06 (0.56) | 24.78 (0.9) | 20.41 (1.3) |
| Nuts (% of LA kcals) a,b,c,d,g,i,j | 8.24 (0.79) | 8.61 (1.04) | 9.38 (0.61) | 5.68 (0.5) | 12.54 (0.91) |
| Sweets (% of LA kcals) a,e,g,j | 7.78 (0.64) | 9.47 (0.96) | 10.11 (0.45) | 7.49 (0.47) | 7.1 (0.53) |
| F | df | p | |
|---|---|---|---|
| Dairy | 2258 | 4 | 2.5 × 10−4 |
| Eggs | 3983 | 4 | 3.2 × 10−6 |
| Fat | 11,944 | 4 | 2.8 × 10−7 |
| Fish | 2661 | 4 | 8.2 × 10−4 |
| Fruits/vegetables | 4201 | 4 | 0.001 |
| Grains | 39,904 | 4 | 1.2 × 10−4 |
| Meat | 27,867 | 4 | 4.0 × 10−10 |
| Nuts | 10,008 | 4 | 1.8 × 10−5 |
| Sweets | 5599 | 4 | 2.8 × 10−5 |
| Dairy | Eggs | Fat | Fish | Fruits/Vegetables | Grains | Meat | Nuts | Sweets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | ß (SE) | p * | |
| MA vs. | ||||||||||||||||||
| OH | −0.23 (0.67) | 0.10 | −1.14 (1.05) | 0.80 | −0.28 (1.32) | 0.10 | −0.22 (1.10) | 0.10 | 0.92 (1.10) | 0.92 | −1.33 (2.06) | 0.97 | 1.11 (1.47) | 0.94 | 0.13 (1.33) | 1.00 | 1.04 (1.23) | 0.91 |
| NHW | 0.60 (0.56) | 0.82 | −3.09 (0.84) | 0.002 | 2.65 (1.11) | 0.18 | −1.33 (0.88) | 0.54 | 3.71 (1.03) | 0.003 | −7.67 (1.70) | <0.001 | 4.39 (1.26) | 0.005 | −0.47 (1.15) | 0.99 | 1.14 (0.87) | 0.68 |
| NHB | −0.88 (0.6084) | 0.59 | −3.72 (0.85) | <0.001 | 2.56 (1.17) | 0.18 | 1.49 (1.06) | 0.61 | 3.94 (0.97) | <0.001 | −10.12 (1.70) | <0.001 | 10.08 (1.46) | <0.001 | −2.56 (0.97) | 0.06 | −0.80 (0.88) | 0.89 |
| Other | −0.62 (0.77) | 0.93 | −2.33 (0.91) | 0.07 | −1.72 (1.16) | 0.57 | −0.63 (0.10) | 0.97 | 4.26 (1.0487) | <0.001 | −7.74 (1.84) | <0.001 | 7.06 (1.81) | <0.001 | 3.03 (1.25) | 0.10 | −1.36 (0.93) | 0.58 |
| OH vs. | ||||||||||||||||||
| NHW | 0.83 (0.56) | 0.56 | −1.95 (0.78) | 0.08 | 2.94 (1.19) | 0.10 | −1.11 (0.74) | 0.55 | 2.79 (1.04) | 0.06 | −6.35 (1.66) | 0.001 | 3.28 (1.20) | 0.05 | −0.60 (1.36) | 0.99 | 0.11 (1.12) | 0.10 |
| NHB | −0.65 (0.62) | 0.83 | −2.58 (0.76) | 0.005 | 2.85 (1.24 | 0.14 | 1.71 (0.89) | 0.29 | 3.01 (1.01) | 0.02 | −8.80 (1.68) | <0.001 | 8.98 (1.41) | <0.001 | −2.69 (1.25) | 0.19 | −1.85 (1.13) | 0.46 |
| Other | −0.39 (0.75) | 0.98 | −1.19 (0.84) | 0.59 | −1.44 (1.21) | 0.76 | −0.41 (0.83) | 0.99 | 3.36 (1.06) | 0.01 | −6.41 (1.82) | 0.004 | 5.95 (1.74) | 0.006 | 2.90 (1.47) | 0.27 | −2.40 (1.16) | 0.23 |
| NHW vs. | ||||||||||||||||||
| NHB | −1.46 (0.47) | 0.02 | −0.63 (0.40) | 0.48 | −0.09 (0.10) | >0.99 | 2.82 (0.66) | <0.001 | 0.22 (0.88) | 0.10 | −2.45 (1.12) | 0.18 | 5.69 (1.15) | <0.001 | −2.08 (0.97) | 0.19 | −1.95 (0.70) | 0.04 |
| Other | −1.22 (0.62) | 0.28 | 0.76 (0.50) | 0.53 | −4.38 (0.93) | <0.001 | 0.70 (0.57) | 0.73 | 0.57 (0.89) | 0.97 | −0.06 (1.32) | 1.00 | 2.67 (1.50) | 0.38 | 3.50 (1.17) | 0.02 | −2.50 (0.73) | 0.005 |
| NHB vs. | ||||||||||||||||||
| Other | 0.25 (0.70) | 0.10 | 1.39 (0.50) | 0.04 | −4.29 (1.03) | <0.001 | −2.12 (0.75) | 0.04 | 0.35 (0.91) | 0.10 | 2.39 (1.36) | 0.39 | −3.02 (1.70) | 0.38 | 5.59 (1.10) | <0.001 | −0.55 (0.77) | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momin, S.R.; Senn, M.K.; Manichaikul, A.; Yang, C.; Mathias, R.; Phan, M.; Rich, S.S.; Sergeant, S.; Seeds, M.; Reynolds, L.; et al. Dietary Sources of Linoleic Acid (LA) Differ by Race/Ethnicity in Adults Participating in the National Health and Nutrition Examination Survey (NHANES) between 2017–2018. Nutrients 2023, 15, 2779. https://doi.org/10.3390/nu15122779
Momin SR, Senn MK, Manichaikul A, Yang C, Mathias R, Phan M, Rich SS, Sergeant S, Seeds M, Reynolds L, et al. Dietary Sources of Linoleic Acid (LA) Differ by Race/Ethnicity in Adults Participating in the National Health and Nutrition Examination Survey (NHANES) between 2017–2018. Nutrients. 2023; 15(12):2779. https://doi.org/10.3390/nu15122779
Chicago/Turabian StyleMomin, Shabnam R., Mackenzie K. Senn, Ani Manichaikul, Chaojie Yang, Rasika Mathias, Mimi Phan, Stephen S. Rich, Susan Sergeant, Michael Seeds, Lindsay Reynolds, and et al. 2023. "Dietary Sources of Linoleic Acid (LA) Differ by Race/Ethnicity in Adults Participating in the National Health and Nutrition Examination Survey (NHANES) between 2017–2018" Nutrients 15, no. 12: 2779. https://doi.org/10.3390/nu15122779
APA StyleMomin, S. R., Senn, M. K., Manichaikul, A., Yang, C., Mathias, R., Phan, M., Rich, S. S., Sergeant, S., Seeds, M., Reynolds, L., Chilton, F. H., & Wood, A. C. (2023). Dietary Sources of Linoleic Acid (LA) Differ by Race/Ethnicity in Adults Participating in the National Health and Nutrition Examination Survey (NHANES) between 2017–2018. Nutrients, 15(12), 2779. https://doi.org/10.3390/nu15122779





