Comparative Effects of Different Nutritional Supplements on Inflammation, Nutritional Status, and Clinical Outcomes in Colorectal Cancer Patients: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Design and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Search Methods
2.4. Study Selection
2.5. Data Extraction and Quality Assessment
2.6. Data Synthesis and Statistical Analysis
3. Results
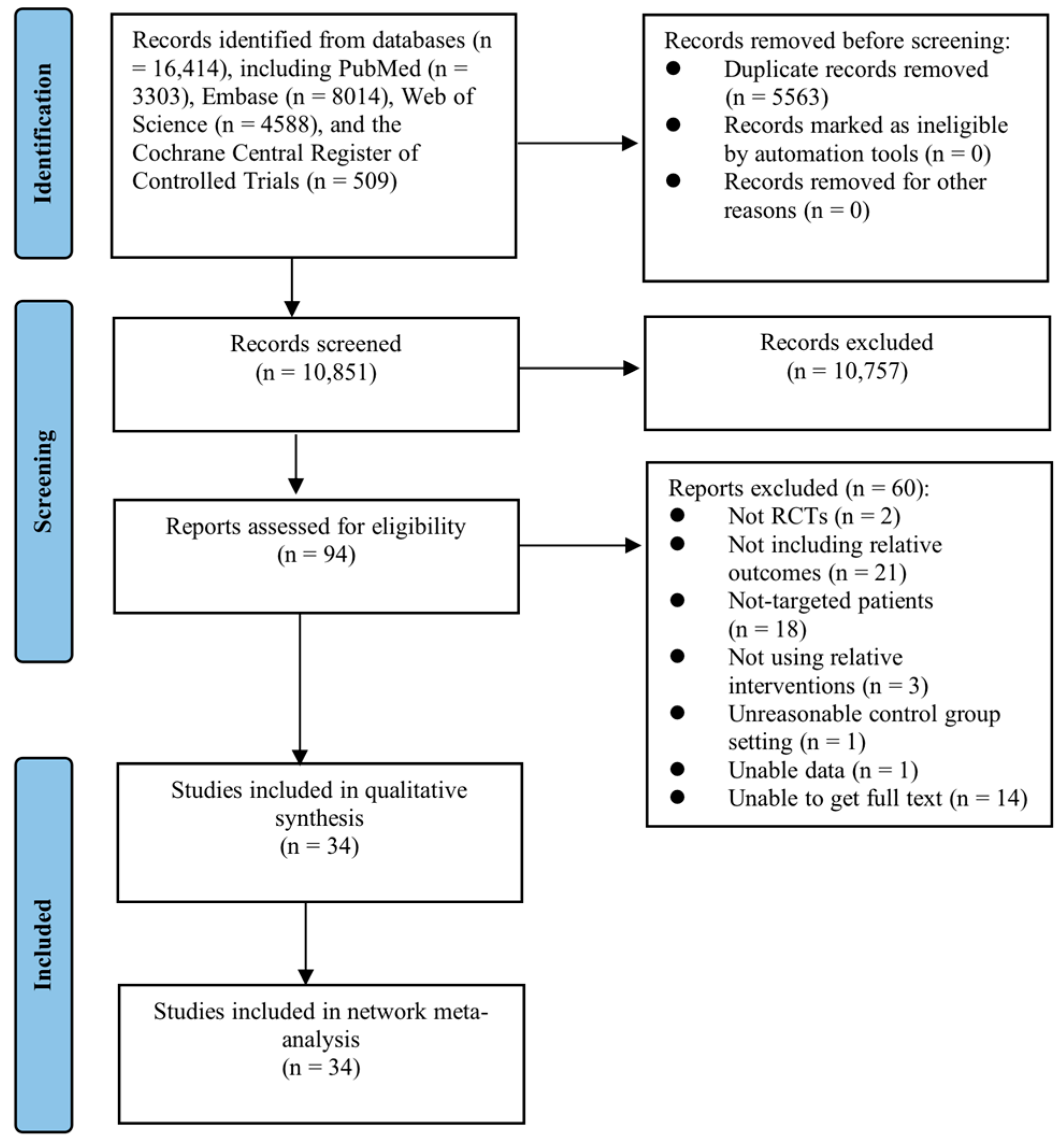
3.1. Search Outcomes
3.2. Characteristics of Included Studies
3.3. Quality Assessment
3.4. Network Meta-Analysis
3.4.1. Inflammatory Indicators
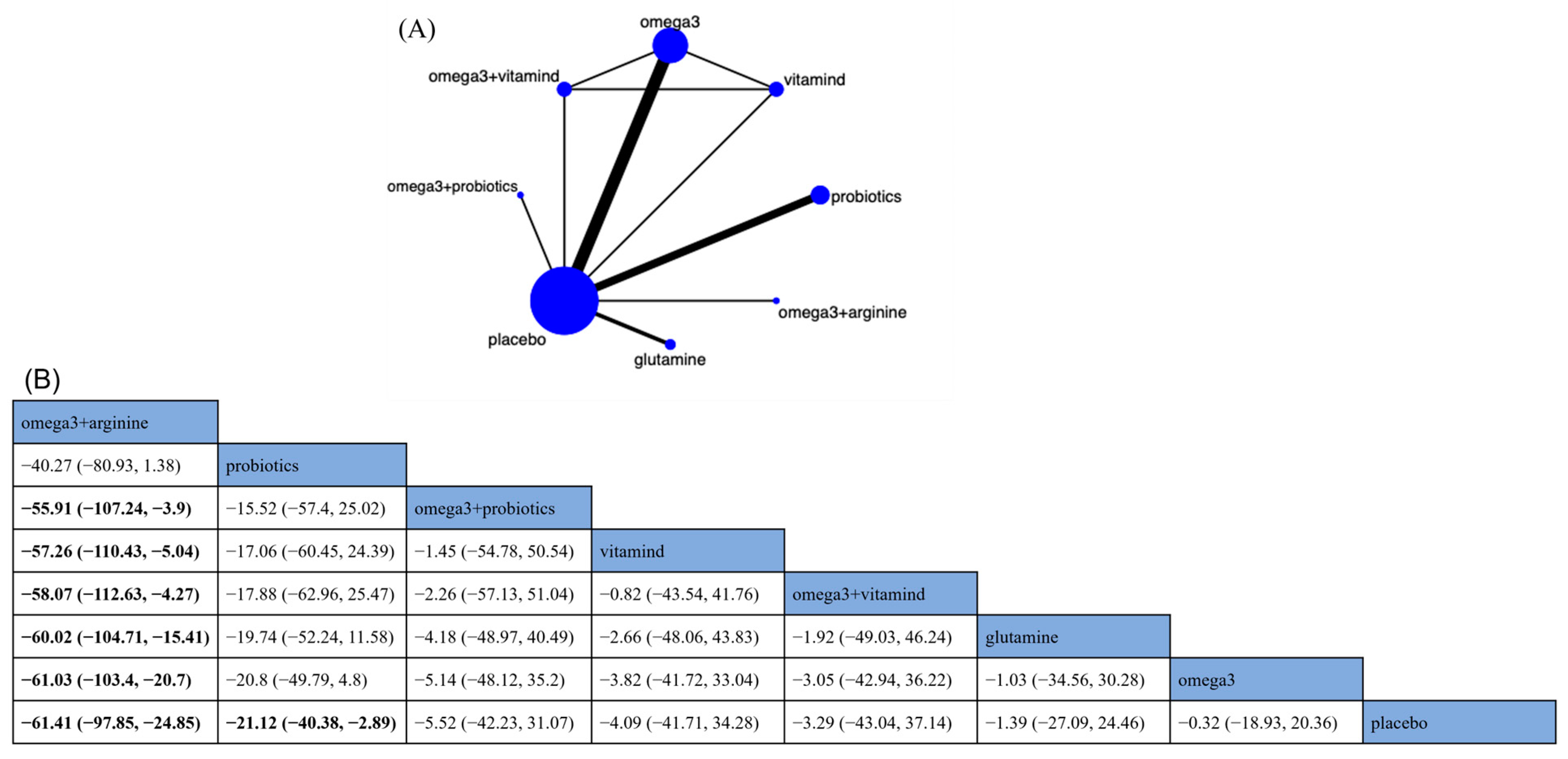
Tumor Necrosis Factor-α
Interleukin-6
C-reactive Protein
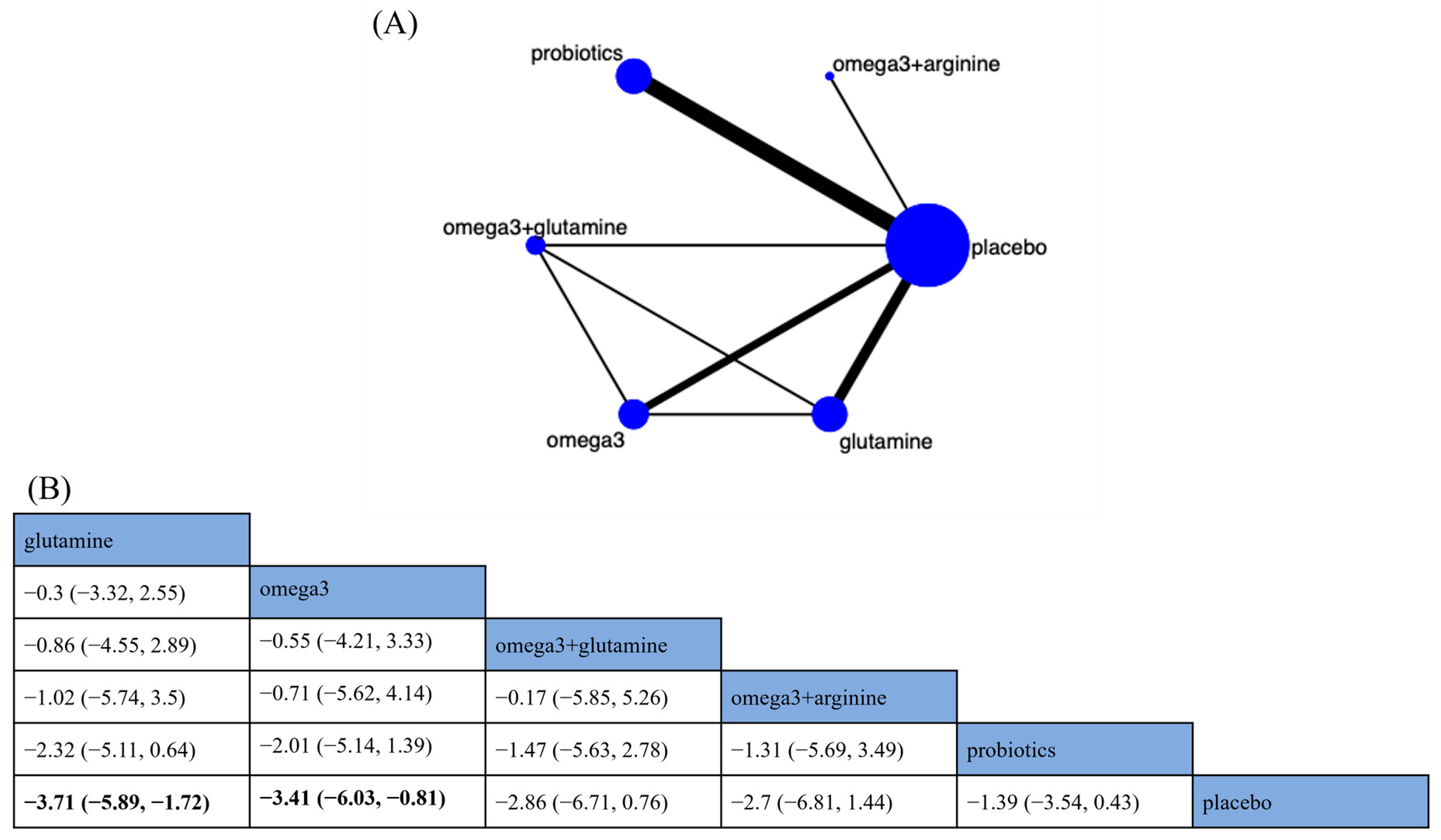
3.4.2. Nutritional Indicators
3.4.3. Clinical Outcomes
Length of Hospital Stay
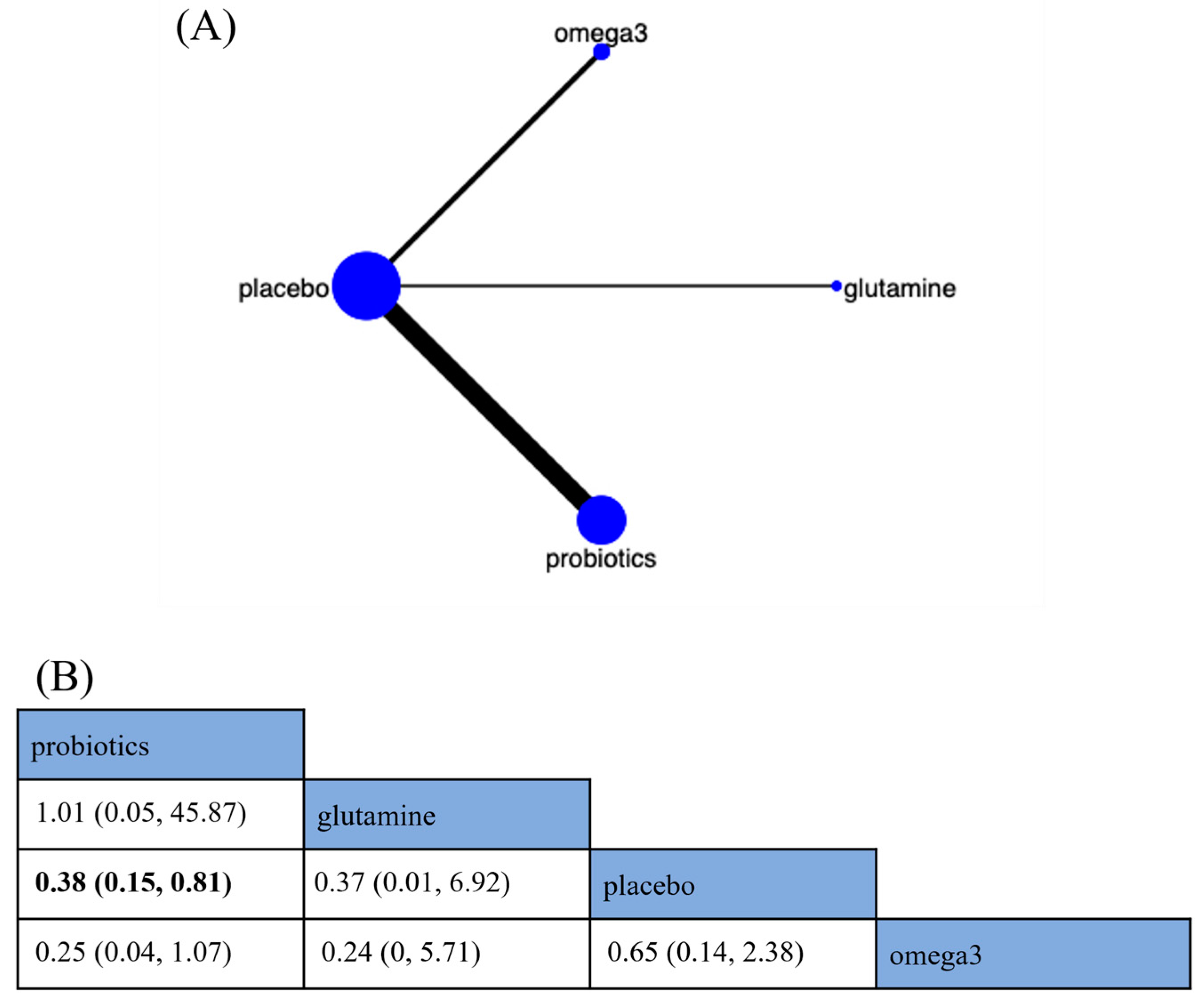
Urinary Tract Infections
Wound Infections
Anastomotic Leaks
Pneumonia
3.5. Consistency and Publication Bias Assessment
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25 (Suppl. S3), iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Mendes, M.C.; Pimentel, G.D.; Costa, F.O.; Carvalheira, J.B. Molecular and neuroendocrine mechanisms of cancer cachexia. J. Endocrinol. 2015, 226, R29–R43. [Google Scholar] [CrossRef]
- Yue, T.; Xiong, K.; Deng, J.; Hu, W.; Tan, T.; Li, S.; Yang, T.; Xiao, T. Meta-analysis of omega-3 polyunsaturated fatty acids on immune functions and nutritional status of patients with colorectal cancer. Front. Nutr. 2022, 9, 945590. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.N.; Watt, A.E.; Isenring, E.A.; de van der Schueren, M.A.E.; van der Meij, B.S. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3815–3826. [Google Scholar] [CrossRef]
- Moore, M.M.; Chua, W.; Charles, K.A.; Clarke, S.J. Inflammation and cancer: Causes and consequences. Clin. Pharmacol. Ther. 2010, 87, 504–508. [Google Scholar] [CrossRef]
- Burden, S.T.; Hill, J.; Shaffer, J.L.; Todd, C. Nutritional status of preoperative colorectal cancer patients. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2010, 23, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2017, 104, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, M.C.; Camargo, C.Q.; Nunes, E.A.; Fiates, G.M.R.; Trindade, E. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin. Nutr. 2016, 35, 359–369. [Google Scholar] [CrossRef]
- Song, H.N.; Wang, W.B.; Luo, X.; Huang, D.D.; Ruan, X.J.; Xing, C.G.; Chen, W.Z.; Dong, Q.T.; Chen, X.L. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn. J. Clin. Oncol. 2022, 52, 466–474. [Google Scholar] [CrossRef]
- Tahiri, M.; Sikder, T.; Maimon, G.; Teasdale, D.; Hamadani, F.; Sourial, N.; Feldman, L.S.; Guralnick, J.; Fraser, S.A.; Demyttenaere, S.; et al. The impact of postoperative complications on the recovery of elderly surgical patients. Surg. Endosc. 2016, 30, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Pradarelli, J.C.; Healy, M.A.; Osborne, N.H.; Ghaferi, A.A.; Dimick, J.B.; Nathan, H. Variation in Medicare Expenditures for Treating Perioperative Complications: The Cost of Rescue. JAMA Surg. 2016, 151, e163340. [Google Scholar] [CrossRef]
- Haidari, F.; Abiri, B.; Iravani, M.; Ahmadi-Angali, K.; Vafa, M. Randomized Study of the Effect of Vitamin D and Omega-3 Fatty Acids Cosupplementation as Adjuvant Chemotherapy on Inflammation and Nutritional Status in Colorectal Cancer Patients. J. Diet. Suppl. 2020, 17, 384–400. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Phillips, B.E.; Doleman, B.; Lund, J.N.; Williams, J.P. A double-blind randomized controlled trial of the effects of eicosapentaenoic acid supplementation on muscle inflammation and physical function in patients undergoing colorectal cancer resection. Clin. Nutr. 2020, 39, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hu, L.; Liu, Y.J.; Wu, Y.M.; Jing, L. Intravenous alanyl-L-glutamine balances glucose-insulin homeostasis and facilitates recovery in patients undergoing colonic resection: A randomised controlled trial. Eur. J. Anaesthesiol. 2014, 31, 212–218. [Google Scholar] [CrossRef]
- Jing-Xiang, S.; Xiao-Huang, T.; Lie, W.; Chen-Jing, L. Glutamine dipeptide-supplemented parenteral nutrition in patients with colorectal cancer. Clin. Nutr. Suppl. 2004, 1, 49–53. [Google Scholar] [CrossRef]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Ligocki Campos, A.C. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Buijs, L.F.; Dunlop, M.G.; Vaughan-Shaw, P.G. Vitamin D supplementation and colorectal cancer survival: Systematic review and meta-analysis. Color. Dis. 2020, 22, 47. [Google Scholar] [CrossRef]
- Araújo, M.M.; Montalvão-Sousa, T.M.; Teixeira, P.D.C.; Figueiredo, A.; Botelho, P.B. The effect of probiotics on postsurgical complications in patients with colorectal cancer: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 493–510. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Vignali, A.; Carlo, V.D. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002, 132, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, X.; Cao, Y.; Bao, T.; Li, G.; Gu, S.; Xiong, K.; Xiao, T. Meta-analysis of Glutamine on Immune Function and Post-Operative Complications of Patients With Colorectal Cancer. Front. Nutr. 2021, 8, 765809. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health J. Int. Soc. Pharm. Outcomes Res. 2011, 14, 429–437. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Oremus, M.; Wolfson, C.; Perrault, A.; Demers, L.; Momoli, F.; Moride, Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement. Geriatr. Cogn. Disord. 2001, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Veroniki, A.A.; Straus, S.E.; Fyraridis, A.; Tricco, A.C. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J. Clin. Epidemiol. 2016, 76, 193–199. [Google Scholar] [CrossRef]
- Dempster, A.P. The direct use of likelihood for significance testing. Stat. Comput. 1997, 7, 247–252. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Liang, B.; Wang, S.; Ye, Y.J.; Yang, X.D.; Wang, Y.L.; Qu, J.; Xie, Q.W.; Yin, M.J. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J. Gastroenterol. 2008, 14, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Aliyazicioglu, T.; Cantürk, N.; Şimşek, T.; Kolayli, F.; Çekmen, M. Effects of Standard and/or Glutamine Dipeptide and/or Omega-3 Fatty Ascid-Supplemented Parenteral Nutrition on Neutrophil Functions, Interleukin-8 Level and Length of Stay-A Double Blind, Controlled, Randomised Study. East Afr. Med. J. 2013, 90, 59–66. [Google Scholar]
- Esfahani, A.; Somi, M.H.; Ayromlou, H.; Nikanfar, A.; Jafarabadi, M.A.; Sadat, B.E.; Ghoreishi, Z. The effect of n-3 polyunsaturated fatty acids on incidence and severity of oxaliplatin induced peripheral neuropathy: A randomized controlled trial. Biomark. Res. 2016, 4, 13. [Google Scholar] [CrossRef]
- Golkhalkhali, B.; Rajandram, R.; Paliany, A.S.; Ho, G.F.; Wan Ishak, W.Z.; Johari, C.S.; Chin, K.F. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: A randomized controlled trial. Asia-Pac. J. Clin. Oncol. 2018, 14, 179–191. [Google Scholar] [CrossRef]
- Tan, C.K.; Said, S.; Rajandram, R.; Wang, Z.; Roslani, A.C.; Chin, K.F. Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: A Randomized Controlled Trial. World J. Surg. 2016, 40, 1985–1992. [Google Scholar] [CrossRef]
- Zhang, J.W.; Du, P.; Gao, J.; Yang, B.R.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, P.; Cen, Y.; Li, W. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncol. Lett. 2019, 18, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef]
- Komatsu, S.; Sakamoto, E.; Norimizu, S.; Shingu, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: A randomized controlled trial. Surg. Today 2016, 46, 479–490. [Google Scholar] [CrossRef]
- Farshi Radvar, F.; Mohammad-Zadeh, M.; Mahdavi, R.; Andersen, V.; Nasirimotlagh, B.; Faramarzi, E.; Lotfi Yagin, N. Effect of synbiotic supplementation on matrix metalloproteinase enzymes, quality of life and dietary intake and weight changes in rectal cancer patients undergoing neoadjuvant chemoradiotherapy. Mediterr. J. Nutr. Metab. 2020, 13, 225–235. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—A double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef]
- Oguz, M.; Kerem, M.; Bedirli, A.; Mentes, B.B.; Sakrak, O.; Salman, B.; Bostanci, H. L-alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2007, 9, 515–520. [Google Scholar] [CrossRef]
- Zhu, M.W.; Tang, D.N.; Hou, J.; Wei, J.M.; Hua, B.; Sun, J.H.; Cui, H.Y. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin. Med. J. 2012, 125, 178–181. [Google Scholar] [PubMed]
- Bakker, N.; van den Helder, R.S.; Stoutjesdijk, E.; van Pelt, J.; Houdijk, A.P.J. Effects of perioperative intravenous ω-3 fatty acids in colon cancer patients: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2020, 111, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Schmidt, E.B.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.C.; Lindorff-Larsen, K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br. J. Surg. 2014, 101, 33–42. [Google Scholar] [CrossRef]
- Bajramagic, S.; Hodzic, E.; Mulabdic, A.; Holjan, S.; Smajlovic, S.V.; Rovcanin, A. Usage of Probiotics and its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Med. Arch. 2019, 73, 316–320. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Szefel, J.; Ślebioda, T.; Walczak, J.; Kruszewski, W.J.; Szajewski, M.; Ciesielski, M.; Stanisławowski, M.; Buczek, T.; Małgorzewicz, S.; Owczarzak, A.; et al. The effect of l-arginine supplementation and surgical trauma on the frequency of myeloid-derived suppressor cells and T lymphocytes in tumour and blood of colorectal cancer patients. Adv. Med. Sci. 2022, 67, 66–78. [Google Scholar] [CrossRef]
- Rotovnik Kozjek, N.; Kompan, L.; Soeters, P.; Oblak, I.; Mlakar Mastnak, D.; Možina, B.; Zadnik, V.; Anderluh, F.; Velenik, V. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: A randomised double blinded, placebo controlled pilot study. Clin. Nutr. 2011, 30, 567–570. [Google Scholar] [CrossRef]
- Rotovnik Kozjek, N.; Kompan, L.; Žagar, T.; Mrevlje, Ž. Influence of enteral glutamine on inflammatory and hormonal response in patients with rectal cancer during preoperative radiochemotherapy. Eur. J. Clin. Nutr. 2017, 71, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Decker-Baumann, C.; Buhl, K.; Frohmüller, S.; von Herbay, A.; Dueck, M.; Schlag, P.M. Reduction of chemotherapy-induced side-effects by parenteral glutamine supplementation in patients with metastatic colorectal cancer. Eur. J. Cancer 1999, 35, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.J.; Stehle, P.; Wachtler, P.; Siedhoff, H.P.; Köller, M.; König, W.; Fürst, P.; Puchstein, C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: A randomized, double-blind, controlled study. Ann. Surg. 1998, 227, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, M.C.; Pastore e Silva Jde, A.; Camargo Cde, Q.; Fabre, M.E.; Gevaerd, S.; Naliwaiko, K.; Moreno, Y.M.; Nunes, E.A.; Trindade, E.B. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 2013, 48, 879–888. [Google Scholar] [CrossRef]
- Flesch, A.T.; Tonial, S.T.; Contu, P.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Do Col. Bras. De Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef]
- Silva Jde, A.; Trindade, E.B.; Fabre, M.E.; Menegotto, V.M.; Gevaerd, S.; Buss Zda, S.; Frode, T.S. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutr. Cancer 2012, 64, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Eid, E.E.M.; Hussin, S.; Alshawsh, M.A. Probiotics supplementation in patients with colorectal cancer: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 22–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, B.; Li, X.; Hui, H.; Zhou, Y.; Li, N.; Xie, X. Omega-3 Polyunsaturated Fatty Acids can Reduce IL-6 and TNF Levels in Patients with Cancer. Br. J. Nutr. 2022, 7, 1–34. [Google Scholar] [CrossRef]
- Miller, L.J.; Douglas, C.; McCullough, F.S.; Stanworth, S.J.; Calder, P.C. Impact of enteral immunonutrition on infectious complications and immune and inflammatory markers in cancer patients undergoing chemotherapy: A systematic review of randomised controlled trials. Clin. Nutr. 2022, 41, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2515S–2522S, discussion 2523S–2524S. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Tapia, G.; Valenzuela, R.; Espinosa, A.; Romanque, P.; Dossi, C.; Gonzalez-Mañán, D.; Videla, L.A.; D’Espessailles, A. N-3 long-chain PUFA supplementation prevents high fat diet induced mouse liver steatosis and inflammation in relation to PPAR-α upregulation and NF-κB DNA binding abrogation. Mol. Nutr. Food Res. 2014, 58, 1333–1341. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Liu, B.; Yang, L. l-Arginine prevents 4-hydroxy-2-nonenal accumulation and depresses inflammation via inhibiting NF-κB activation. J. Biochem. Mol. Toxicol. 2022, 36, e23087. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, B.; Fazeli Moghadam, E.; Asbaghi, O.; Zeinali Khosroshahi, M.; Choghakhori, R.; Abbasnezhad, A. Effect of l-arginine supplementation on C-reactive protein and other inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 47, 102226. [Google Scholar] [CrossRef]
- Wan, G.Y.; Zheng, L.Y.; Li, H.Q.; Yuan, H.; Xue, H.; Zhang, X.Y. Effects of enteral nutritional rich in n-3 polyunsaturated fatty acids on the nutritional status of gastrointestinal cancer patients: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Rosenthal, M.H.; Ma, C.; Zhang, S.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; et al. Effect of High-Dose vs Standard-Dose Vitamin D(3) Supplementation on Body Composition among Patients with Advanced or Metastatic Colorectal Cancer: A Randomized Trial. Cancers 2020, 12, 3451. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.J.; Li, Y.; Hu, C.M.; Yang, J.Y.; Sun, M.Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Soreide, K.; Berg, M.; Skudal, B.S.; Nedreboe, B.S. Advances in the understanding and treatment of colorectal cancer. Discov. Med. 2011, 12, 393–404. [Google Scholar]
- Vonlanthen, R.; Slankamenac, K.; Breitenstein, S.; Puhan, M.A.; Muller, M.K.; Hahnloser, D.; Hauri, D.; Graf, R.; Clavien, P.A. The impact of complications on costs of major surgical procedures: A cost analysis of 1200 patients. Ann. Surg. 2011, 254, 907–913. [Google Scholar] [CrossRef]
- Bai, H.; Li, Z.; Meng, Y.; Yu, Y.; Zhang, H.; Shen, D.; Chen, L. Effects of parenteral ω-3 fatty acid supplementation in postoperative gastrointestinal cancer on immune function and length of hospital stay: A systematic review and meta-analysis. Asia Pac. J. Clin. Nutr. 2018, 27, 121–128. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2003, 11, 419–423. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Wang, H.Y.; Chen, T.X. Interactions between Intestinal Microflora/Probiotics and the Immune System. BioMed Res. Int. 2019, 2019, 6764919. [Google Scholar] [CrossRef] [PubMed]


| Author (Year) | Nationality | Participant Types | Sample Size (n) | Age (Years) | Intervention | Route | Outcomes | Jadad Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |||||||
| Aliyazicioglu et al. (2013) [37] | Turkey | CRC surgery patients | 8/8/10 | 10 | 58.0 ± 15.6/ 63.9 ± 17.2/ 59.9 ± 15.1 | 56.3 ± 14.3 | glutamine/omega3 fatty acid/ glutamine + omega 3 fatty acid/glutamine:0.3–0.4 g/kg/d, omega 3 fatty acid: 0.1–0.2 g/kg/d, 2 postoperative days to 7 postoperative days | PN | g | 4 |
| Bakker et al. (2020) [53] | Netherlands | CRC surgery patients | 18 | 23 | 68.2475 ± 8.849 | 68.6415 ± 8.6919 | omega 3 fatty acid (fish oil)/0.2 g/kg/d, 1 preoperative day and 1 postoperative day | PN | a, c, h, i, j, k | 6 |
| Sorensen et al. (2014) [54] | Denmark | CRC surgery patient | 74 | 74 | 69 ± 11 | 71 ± 10 | omega 3 fatty acid (EPA:2.0 g/d and DHA:1.0 g/d)/400 mL/d, 7 preoperative days and 7 postoperative days | Oral | h, i, j, k | 7 |
| Liang et al. (2008) [36] | China | CRC surgery patients | 20 | 21 | 55.80 ± 10.10 | 59.19 ± 10.61 | omega 3 fatty acid/0.2 g/kg/d, 7 postoperative days | PN | b, c, g | 7 |
| Esfahani et al. (2016) [38] | Iran | CRC chemotherapy patients | 36 | 35 | 54.14 ± 10.53 | 53.40 ± 15.70 | omega 3 fatty acid (54% DHA, 10% EPA)/1920 mg/d, 1 month | Oral | b, c | 7 |
| Mocellin et al. (2013) [62] | Brazil | CRC chemotherapy patients | 6 | 5 | 55.2 ± 7.7 | 53.6 ± 12.9 | omega 3 fatty acid (90 mg EPA and 60 mg DHA)/2 g/d, 9 weeks | Oral | a, b, d, e, f | 4 |
| Haidari et al. (2020) [19] | Iran | CRC chemotherapy patients | 20/21/20 | 20 | 56.75 ± 10.60/ 56.90 ± 12.45/ 57.15 ± 10.17 | 59.90 ± 8.75 | omega 3 fatty acid (54 mg EPA, 250 mg DHA, 26 mg other omega-3 fatty acids)/ Vitamin D/ omega 3 fatty acid (54 mg EPA, 250 mg DHA, 26 mg other omega-3 fatty acids) + vitamin D/vitamin D: 50,000 IU soft gel/w, omega3 fatty acid: 660 mg/d, 8 weeks | Oral | a, b, c, d, e, f | 4 |
| Golkhalkhali et al. (2017) [39] | Malaysia | CRC chemotherapy patients | 70 | 70 | ≤56:20 57–66: 22 ≥67:23 | ≤56:28 57–66:19 ≥67:19 | omega 3 fatty acid (700 mg EPA) + probiotics (L. acidophilus, Lactobacillus lactis, Bififidobacterium bififidum, Bififidobacterium longum, Bififidobacterium infantis)/omega 3 fatty acid: 2 g/d + probiotics:2 sachets/d, 8 weeks | Oral | a, b, c, e, f | 7 |
| Bajramagic et al. (2019) [55] | Sarajevo | CRC surgery patients | 39 | 39 | NR | NR | probiotics (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium bifidum, Bifidobacterium breve, Streptococcusthermophilus)/4 capsules/d for 30 days, 1 capsule/d for 2 weeks in each month to 1 year | Oral | i, j | 4 |
| Tan et al. (2016) [40] | Malaysia | CRC surgery patients | 20 | 20 | 64.3 ± 14.5 | 68.4 ± 11.9 | probiotics (Lactobacillus acidophilus, Lactobacillus lactis, Bififidobacterium bififidum, Bififidobacterium longum, and Bififidobacterium infantis)/2 sachets/d, 7 preoperative days | Oral | g, i, j, k | 6 |
| Zhang et al. (2012) [41] | China | CRC surgery patients | 30 | 30 | 67.14 ± 10.29 | 62.09 ± 8.82 | probiotics (B longum, L acidophilus, and Enterococcus faecalis)/0.63 g/d, 5 preoperative days to 3 preoperative days | Oral | c, i, j, k | 5 |
| Flesch et al. (2017) [63] | Brazil | CRC surgery patients | 49 | 42 | 64.5 | 61.1 | probiotics (Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, and Bifidobacterium lactis)/12 g/d, 5 preoperative days and 14 postoperative days | Oral | i, k | 4 |
| Kotzampassi et al. (2015) [56] | Greece | CRC surgery patients | 84 | 80 | 65.9 ±11.5 | 66.4 ± 11.9 | probiotics (Lactobacillus acidophilus LA-5, Lactobacillus plantarum, Bififidobacterium lactis BB-12, and Saccharomyces boulardii)/2 capsules/d, on the day of operation and 14 postoperative days | Oral/ EN | h, i, j, k | 4 |
| Sadahiro et al. (2014) [42] | Japan | CRC surgery patients | 100 | 95 | 67 ± 9 | 66 ± 12 | probiotics (Bifidobacteria)/9 tablets/d, 7 preoperative days and 5 postoperative days to 15 postoperative days | Oral | i, j | 4 |
| Xu et al. (2019) [43] | China | CRC surgery patients | 30 | 30 | 61.03 ± 15.28 | 62.35 ± 13.71 | probiotics (bifidus-triple viable preparation)/NA,7 preoperative days | Oral | a, i | 4 |
| Polakowski et al. (2019) [24] | Brazil | CRC surgery patients | 36 | 37 | 60.9 ± 6.7 | 58.9 ± 6.3 | probiotics (fructooligosaccharide, Lactobacillus acidophilus NCFM, L. rhamnosus HN001, L. casei LPC-37, and Bififidobacterium lactis HN019)/2 times/d, 7 preoperative days | Oral | a, c, g | 7 |
| Mizuta et al.(2016) [44] | Japan | CRC surgery patients | 31 | 29 | 68.9 ± 10.4 | 71.2 ± 9.5 | probiotics (B. longum)/2 g/d, 7–14 preoperative days and 14 postoperative days | Oral | c, d, g, i, j | 7 |
| Komatsu et al. (2016) [45] | Japan | CRC surgery patients | 168 | 194 | 66.7 ± 11.6 | 67.7 ± 10.7 | probiotics (Lactobacillus casei, galactooligosaccharides, and Bifidobacterium breve)/NA, 7–11 preoperative days and 2–7 postoperative days | Oral | i, j | 5 |
| Radvar et al. (2020) [46] | Iran | CRC chemoradiotherapy patients | 23 | 23 | 57.58 ± 12.78 | 62.89 ± 13.93 | probiotics (Lactobacillus casei PXN 37, Lactobacillus rhamnosus PXN 54, Streptococcus thermophilus PXN 66, Bififidobacteriumbreve PXN 25, Lactobacillus acidophilus PXN 35, Bififidobacteriumlongum PXN 30, Lactobacillus bulgaricus PXN 39, Fructooligosaccharide, magnesium stearate, and hydroxypropyl methyl- cellulose)/2 times/d, 6 weeks | Oral | e, f | 7 |
| Xie et al. (2019) [47] | China | CRC surgery patients | 66 | 69 | 62.62 ± 9.627 | 60.29 ± 9.54 | probiotics (fructooligosaccharide, xylooligosaccharide, polydextrose, and resistant dextrin)/30 g/d, 7 preoperative days | Oral | d | 6 |
| Liu et al. (2015) [48] | China | CRC surgery patients | 66 | 68 | 65.62 ± 18.18 | 60.16 ± 16.20 | probiotics (Lactobacillus plantarum, Lactobacillus acidophilus-11, and Bifido-bacterium longum-88)/2 g/d, 6 preoperative days and 10 postoperative days | Oral | g, h, i, k | 7 |
| Liu et al. (2011) [49] | China | CRC surgery patient | 50 | 50 | 65.3 ± 11.0 | 65.7 ± 9.9 | probiotics (Lactobacillus plantarum, Lactobacillus acidophilus-11, and Bifido-bacterium longum-88)/2 g/d, 6 preoperative days and 10 postoperative days | Oral | g, h, i, k | 7 |
| Yang et al. (2016) [50] | China | CRC surgery patients | 30 | 30 | 63.90 ± 12.25 | 62.17 ± 11.06 | probiotics (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis)/6 g/d, 5 preoperative days and 7 postoperative days | Oral | d, g, h, i, j, k | 6 |
| Szefel et al. (2022) [57] | Poland | CRC surgery patients | 28 | 37 | 68.9 ± 10.9 | 68.7 ± 9.3 | arginine/20 capsules/d, 9 preoperative days | Oral | a | 7 |
| Braga et al. (2002) [27] | Italy | CRC surgery patients | 50 | 50 | 63.0 ± 8.1 | 62.2 ± 10.4 | arginine (12.5 g/L) + omega 3 fatty acid (3.3 g/L)/1 L/d, 5 preoperative days | Oral/ EN | c, g, h, i, j | 5 |
| Rotovnik et al. (2011) [58] | Slovenia | CRC chemoradiotherapy patients | 14 | 19 | 60.5 ± 14.2 | 63.6 ± 10.12 | glutamine/30 g/d, 5 weeks | Oral | a, c | 7 |
| Oguz et al. (2007) [51] | Turkey | CRC surgery patients | 57 | 52 | 52 ± 12 | 57 ± 17 | glutamine/1 g/kg/day, 5 preoperative days and 5 postoperative days | PN | g, h, i, j, | 4 |
| Rotovnik et al. (2017) [59] | Slovenia | CRC chemoradiotherapy patients | 33 | 40 | 60.8 ±11.9 | 61.4 ± 9.9 | glutamine/30 g/d, 5 weeks | Oral | c | 6 |
| Cui et al. (2014) [22] | China | CRC surgery patients | 20 | 20 | 55 ± 10.8 | 56 ± 10.7 | glutamine/0.5 g/kg, 1 preoperative day and the day of surgery | PN | b, g | 6 |
| Decker et al. (1999) [60] | German | CRC chemotherapy patients | 12 | 12 | 56.1 ± 9.6 | 58.4 ± 7.2 | glutamine/14 ± 22 g/d, 18 days | PN | e | 4 |
| Zaharuddin et al. (2019) [20] | Malaysia | CRC surgery patients | 27 | 25 | NR | NR | probiotics (Lactobacillus and Bifidobacteria)/2 times/d, 6 postoperative months | Oral | b, c | 5 |
| Morlion et al. (1998) [61] | German | CRC surgery patients | 15 | 13 | 67.1 ± 10.7 | 68.2.1 ± 12.5 | glutamine/0.3 g/kg/d/5 postoperative days | PN | g | 6 |
| Silva et al. (2012) [64] | Brazil | CRC chemotherapy patients | 11 | 12 | 50.1 ± 8.2 | 54.3 ± 9.3 | omega 3 fatty acid/0.6 g/d/9 weeks | Oral | a, c, d, e, f | 4 |
| Zhu et al. (2012) [52] | China | CRC surgery patients | 29 | 28 | 69.8 ± 10.5 | 70.8 ± 6.4 | omega 3 fatty acid/0.2 g/kg/d/7 postoperative days | PN | b, c, g, h, i | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Hu, Y.; Chen, X.; Chang, C.; Li, K. Comparative Effects of Different Nutritional Supplements on Inflammation, Nutritional Status, and Clinical Outcomes in Colorectal Cancer Patients: A Systematic Review and Network Meta-Analysis. Nutrients 2023, 15, 2772. https://doi.org/10.3390/nu15122772
Ye J, Hu Y, Chen X, Chang C, Li K. Comparative Effects of Different Nutritional Supplements on Inflammation, Nutritional Status, and Clinical Outcomes in Colorectal Cancer Patients: A Systematic Review and Network Meta-Analysis. Nutrients. 2023; 15(12):2772. https://doi.org/10.3390/nu15122772
Chicago/Turabian StyleYe, Jiayi, Yanjie Hu, Xinrong Chen, Chengting Chang, and Ka Li. 2023. "Comparative Effects of Different Nutritional Supplements on Inflammation, Nutritional Status, and Clinical Outcomes in Colorectal Cancer Patients: A Systematic Review and Network Meta-Analysis" Nutrients 15, no. 12: 2772. https://doi.org/10.3390/nu15122772
APA StyleYe, J., Hu, Y., Chen, X., Chang, C., & Li, K. (2023). Comparative Effects of Different Nutritional Supplements on Inflammation, Nutritional Status, and Clinical Outcomes in Colorectal Cancer Patients: A Systematic Review and Network Meta-Analysis. Nutrients, 15(12), 2772. https://doi.org/10.3390/nu15122772







