The Impact of Plant-Based Diets on Dietary Acid Load Metrics in Venezuela: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Background
2.2. Dietary Acid Load Estimations
2.3. Statistical Analysis
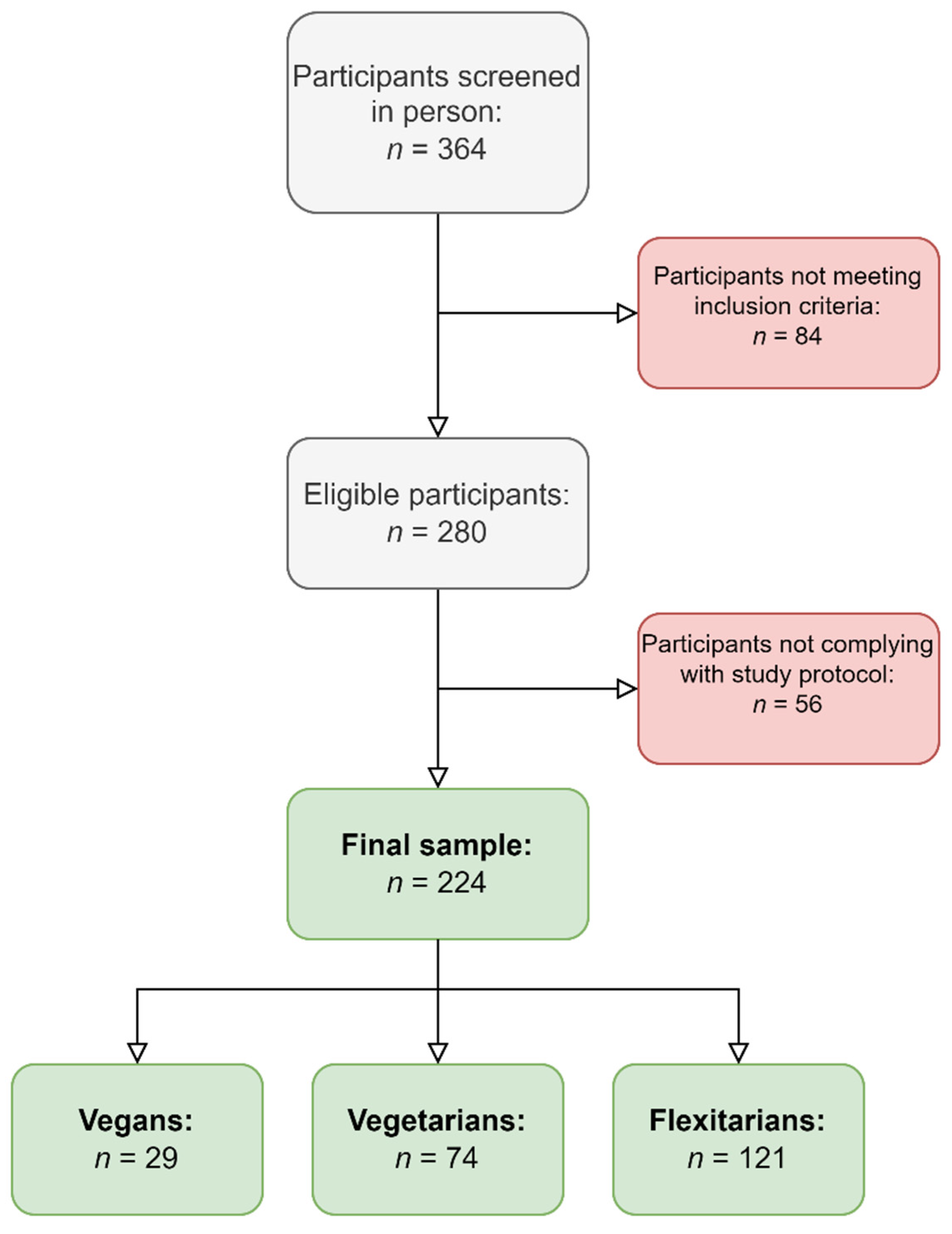
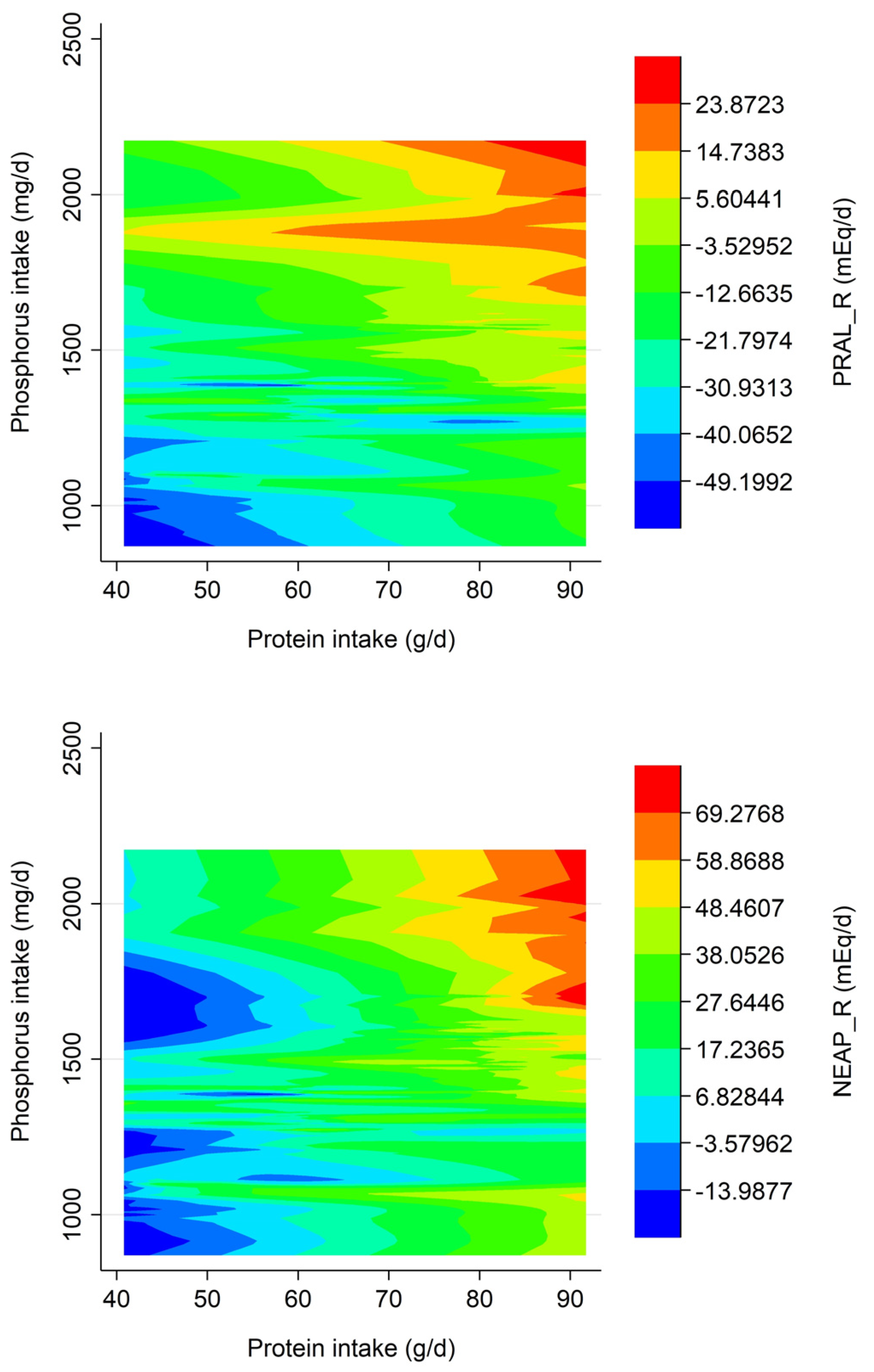
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahleova, H.; McCann, J.; Alwarith, J.; Rembert, E.; Tura, A.; Holubkov, R.; Barnard, N.D. A Plant-Based Diet in Overweight Adults in a 16-Week Randomized Clinical Trial: The Role of Dietary Acid Load. Clin. Nutr. ESPEN 2021, 44, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.V.; Nemec, K.B.; Zisman, A.L. Plant-Based Diets in Kidney Disease: Nephrology Professionals’ Perspective. J. Ren. Nutr. 2022, 32, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Asplin, J.R. Neglected Analytes in the 24-h Urine: Ammonium and Sulfate. Curr. Opin. Nephrol. Hypertens. 2022, 31, 168–174. [Google Scholar] [CrossRef]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The Role of Dietary Acid Load and Mild Metabolic Acidosis in Insulin Resistance in Humans. Biochimie 2016, 124, 171–177. [Google Scholar] [CrossRef]
- Storz, M.A.; Ronco, A.L.; Hannibal, L. Observational and Clinical Evidence That Plant-Based Nutrition Reduces Dietary Acid Load. J. Nutr. Sci. 2022, 11, e93. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-Induced Metabolic Acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Storz, M.A.; Ronco, A.L. Reduced Dietary Acid Load in U.S. Vegetarian Adults: Results from the National Health and Nutrition Examination Survey. Food Sci. Nutr. 2022, 10, 2091–2100. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hübscher, G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef]
- Robey, I.F. Examining the Relationship between Diet-Induced Acidosis and Cancer. Nutr. Metab. 2012, 9, 72. [Google Scholar] [CrossRef]
- Gannon, R.H.T.; Millward, D.J.; Brown, J.E.; Macdonald, H.M.; Lovell, D.P.; Frassetto, L.A.; Remer, T.; Lanham-New, S.A. Estimates of Daily Net Endogenous Acid Production in the Elderly UK Population: Analysis of the National Diet and Nutrition Survey (NDNS) of British Adults Aged 65 Years and Over. Br. J. Nutr. 2008, 100, 615–623. [Google Scholar] [CrossRef]
- Lemann, J. Relationship between Urinary Calcium and Net Acid Excretion as Determined by Dietary Protein and Potassium: A Review. Nephron 1998, 81 (Suppl. 1), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, J.; Janiszewska, J.; Szostak-Węgierek, D. Dietary Acid Load and Cardiometabolic Risk Factors—A Narrative Review. Nutrients 2020, 12, 3419. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Chen, J.; Yu, B.; Boerwinkle, E.; Coresh, J.; Grams, M.E.; Rebholz, C.M. Metabolomics of Dietary Acid Load and Incident Chronic Kidney Disease. J. Ren. Nutr. 2022, 32, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Abbasi, K.; Salehi-sahlabadi, A.; Beigrezaei, S.; Bahrami, A.; Ghiasvand, R.; Pourmasoumi, M. Dietary Acid Load and Risk of Type 2 Diabetes Mellitus: A Case–Control Study. Clin. Nutr. ESPEN 2022, 48, 308–312. [Google Scholar] [CrossRef]
- Ronco, A.L.; Martínez-López, W.; Calderón, J.M.; Storz, M.A. Dietary acid load and esophageal cancer risk: A case-control study. Thorac. Cancer 2022, 13, 2759–2766. [Google Scholar] [CrossRef]
- Ronco, A.L.; Storz, M.A.; Martínez-López, W.; Calderón, J.M.; Golomar, W. High dietary acid load is associated with prostate cancer risk: An epidemiological study. World Cancer Res. J. 2021, 8, e2119. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Association Between Estimated Net Endogenous Acid Production and Subsequent Decline in Muscle Mass Over Four Years in Ambulatory Older Chinese People in Hong Kong: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 905–911. [Google Scholar] [CrossRef]
- Welch, A.A.; MacGregor, A.J.; Skinner, J.; Spector, T.D.; Moayyeri, A.; Cassidy, A. A Higher Alkaline Dietary Load Is Associated with Greater Indexes of Skeletal Muscle Mass in Women. Osteoporos. Int. 2013, 24, 1899–1908. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Leal-Escobar, G.; Garza-García, C.A.; Rodríguez-Castellanos, F.E. Dietary Acid Load: Mechanisms and Evidence of Its Health Repercussions. Nefrología 2019, 39, 343–354. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F.; Alexy, U.; Schoenau, E.; Wudy, S.A.; Shi, L. Long-Term High Urinary Potential Renal Acid Load and Low Nitrogen Excretion Predict Reduced Diaphyseal Bone Mass and Bone Size in Children. J. Clin. Endocrinol. Metab. 2011, 96, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, E.A.L.; Koromani, F.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Rivadeneira, F.; Kiefte-de Jong, J.C. Dietary Acid Load, Trabecular Bone Integrity, and Mineral Density in an Ageing Population: The Rotterdam Study. Osteoporos. Int. 2017, 28, 2357–2365. [Google Scholar] [CrossRef]
- Zhang, L.; Curhan, G.C.; Forman, J.P. Diet-Dependent Net Acid Load and Risk of Incident Hypertension in United States Women. Hypertension 2009, 54, 751–755. [Google Scholar] [CrossRef]
- Krupp, D.; Esche, J.; Mensink, G.B.M.; Klenow, S.; Thamm, M.; Remer, T. Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population. Nutrients 2018, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Galchenko, A.; Gapparova, K.; Sidorova, E. The Influence of Vegetarian and Vegan Diets on the State of Bone Mineral Density in Humans. Crit. Rev. Food Sci. Nutr. 2023, 63, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Ronco, A.L. Carbohydrate Intake and Its Association with Dietary Acid Load in U.S. Adults: Results from a Cross-Sectional Study. Am. J. Lifestyle Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Estimation of the Renal Net Acid Excretion by Adults Consuming Diets Containing Variable Amounts of Protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Ekmeiro-Salvador, J.E.; Arévalo-Vera, C.R. Vegetarianismo: Una caracterización antropométrica, dietética y motivacional en adultos venezolanos. RESPYN Rev. Salud Pública Nutr. 2021, 20, 57–72. [Google Scholar] [CrossRef]
- Goodman, D.; González-Rivas, J.P.; Jaacks, L.M.; Duran, M.; Marulanda, M.I.; Ugel, E.; Mattei, J.; Chavarro, J.E.; Nieto-Martinez, R. Dietary Intake and Cardiometabolic Risk Factors among Venezuelan Adults: A Nationally Representative Analysis. BMC Nutr. 2020, 6, 61. [Google Scholar] [CrossRef]
- Food Processor—Nutrition Analysis Software for Dietitians|ESHA. ESHA Research. Available online: https://esha.com/products/food-processor/ (accessed on 14 April 2023).
- Remer, T.; Manz, F. Potential Renal Acid Load of Foods and Its Influence on Urine PH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of Net Endogenous Noncarbonic Acid Production in Humans from Diet Potassium and Protein Contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Dymock, M.; Banerjee, T.; Sebastian, A.; Slater, G.J.; Frassetto, L.A. Performance of Predictive Equations and Biochemical Measures Quantifying Net Endogenous Acid Production and the Potential Renal Acid Load. Kidney Int. Rep. 2020, 5, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, B.H.; Slater, G.J.; Frassetto, L.A. Accuracy and Precision of Estimation Equations to Predict Net Endogenous Acid Excretion Using the Australian Food Database. Nutr. Diet. 2017, 74, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Esche, J.; Krupp, D.; Mensink, G.B.; Remer, T. Dietary Potential Renal Acid Load Is Positively Associated with Serum Uric Acid and Odds of Hyperuricemia in the German Adult Population. J. Nutr. 2018, 148, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z. Higher Dietary Acid Load Potentially Increases Serum Triglyceride and Obesity Prevalence in Adults: An Updated Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0216547. [Google Scholar] [CrossRef]
- Hejazi, E.; Emamat, H.; Sharafkhah, M.; Saidpour, A.; Poustchi, H.; Sepanlou, S.; Sotoudeh, M.; Dawsey, S.; Boffetta, P.; Abnet, C.C.; et al. Dietary Acid Load and Mortality from All Causes, CVD and Cancer: Results from the Golestan Cohort Study. Br. J. Nutr. 2022, 128, 237–243. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Adair, K.E.; Bowden, R.G. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients 2020, 12, 1007. [Google Scholar] [CrossRef]
- Cosgrove, K.; Johnston, C.S. Examining the Impact of Adherence to a Vegan Diet on Acid-Base Balance in Healthy Adults. Plant Foods Hum. Nutr. 2017, 72, 308–313. [Google Scholar] [CrossRef]
- Knurick, J.R.; Johnston, C.S.; Wherry, S.J.; Aguayo, I. Comparison of Correlates of Bone Mineral Density in Individuals Adhering to Lacto-Ovo, Vegan, or Omnivore Diets: A Cross-Sectional Investigation. Nutrients 2015, 7, 3416–3426. [Google Scholar] [CrossRef]
- Ströhle, A.; Waldmann, A.; Koschizke, J.; Leitzmann, C.; Hahn, A. Diet-Dependent Net Endogenous Acid Load of Vegan Diets in Relation to Food Groups and Bone Health-Related Nutrients: Results from the German Vegan Study. Ann. Nutr. Metab. 2011, 59, 117–126. [Google Scholar] [CrossRef]
- Müller, A.; Zimmermann-Klemd, A.M.; Lederer, A.-K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A Vegan Diet Is Associated with a Significant Reduction in Dietary Acid Load: Post Hoc Analysis of a Randomized Controlled Trial in Healthy Individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef] [PubMed]
- Deriemaeker, P.; Aerenhouts, D.; Hebbelinck, M.; Clarys, P. Nutrient Based Estimation of Acid-Base Balance in Vegetarians and Non-Vegetarians. Plant Foods Hum. Nutr. 2010, 65, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Åkesson, A.; Orsini, N.; Håkansson, N.; Wolk, A.; Carrero, J.J. Modest U-Shaped Association between Dietary Acid Load and Risk of All-Cause and Cardiovascular Mortality in Adults. J. Nutr. 2016, 146, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Penczynski, K.J.; Remer, T.; Menzel, J.; Abraham, K.; Weikert, C. Urinary Potential Renal Acid Load (UPRAL) among Vegans Versus Omnivores and Its Association with Bone Health in the Cross-Sectional Risks and Benefits of a Vegan Diet Study. Nutrients 2022, 14, 4468. [Google Scholar] [CrossRef] [PubMed]

| Dietary Pattern | Definition |
|---|---|
| Vegan | No consumption of products of animal origin. |
| Lacto-ovo-vegetarian | No consumption of meat but consumption of other products of animal origin (eggs and dairy products) that do not involve animal sacrifice. |
| Flexitarian | Diets based mainly on products of plant origin but occasionally including some products of animal origin and possibly small amounts of meat, especially marine animals. |
| Nutrient | Flexitarians (n = 121) | Lacto-Ovo-Vegetarians (n = 74) | Vegans (n = 29) | p-Value |
|---|---|---|---|---|
| Energy intake (kcal/d) | 2189.07 ± 78.79 | 2091.39 ± 109.38 | 1881.14 ± 130.45 *,**,*** | p < 0.001 |
| Protein (g/d) | 81.84 ± 4.04 | 59.68 ± 2.93 | 41.78 ± 3.39 *,**,*** | p < 0.001 |
| Carbohydrate (g/d) | 296.04 ± 15.12 | 313.30 ± 22.45 | 301.23 ± 18.56 *,*** | p < 0.001 |
| Fat (g/day) | 76.14 ± 5.91 | 67.03 ± 3.01 | 56.19 ± 6.21 *,**,*** | p < 0.001 |
| Magnesium (mg/d) | 354.98 ± 1.23 | 377.71 ± 4.87 | 390.31 ± 1.79 *,**,*** | p < 0.001 |
| Calcium (mg/d) | 876.20 ± 3.55 | 802.16 ± 10.24 | 756.89 ± 15.89 *,**,*** | p < 0.001 |
| Potassium (mg/d) | 3598.34 ± 155.61 | 3890.75 ± 257.62 | 4000.65 ± 255.03 *,**,*** | p < 0.001 |
| Phosphorus (mg/d) | 1563.41 ± 171.00 | 1310.11 ± 82.52 | 1104.8 ± 162.46 *,**,*** | p < 0.001 |
| DAL Metric | Flexitarians (n = 121) | Lacto-Ovo-Vegetarians (n = 74) | Vegans (n = 29) | p-Value |
|---|---|---|---|---|
| PRALR (mEq/d) | 1.76 ± 8.36 | −24.24 ± 6.47 | −42.65 ± 11.35 *,**,*** | p < 0.001 |
| NEAPR (mEq/d) | 40.48 ± 11.45 | 14.24 ± 8.22 | −6.77 ± 13.18 *,**,*** | p < 0.001 |
| NEAPF (mEq/d) | 38.15 ± 0.29 | 22.47 ± 0.30 | 12.11 ± 0.59 *,**,*** | p < 0.001 |
| Independent Variables | β | SE | p | β | SE | p |
|---|---|---|---|---|---|---|
| PRALR | ||||||
| Model I | Model II | |||||
| Diet Category | ||||||
| Flexitarian | - | - | - | - | - | - |
| Lacto-Ovo-Vegetarian | −26.00 | 1.22 | <0.001 | −25.75 | 1.10 | <0.001 |
| Vegan | −44.42 | 1.71 | <0.001 | −42.82 | 1.60 | <0.001 |
| Sex | ||||||
| Female | −3.06 | 1.04 | 0.003 | |||
| Male | - | - | - | |||
| Body mass index | 0.89 | 0.14 | <0.001 | |||
| NEAPR | ||||||
| Model I | Model II | |||||
| Diet Category | ||||||
| Flexitarian | - | - | - | - | - | - |
| Lacto-Ovo-Vegetarian | −26.24 | 1.59 | <0.001 | −25.85 | 1.13 | <0.001 |
| Vegan | −47.25 | 2.22 | <0.001 | −42.79 | 1.65 | <0.001 |
| Sex | ||||||
| Female | −8.89 | 1.07 | <0.001 | |||
| Male | - | - | - | |||
| Body mass index | 1.58 | 0.15 | <0.001 | |||
| NEAPF | ||||||
| Model I | Model II | |||||
| Diet Category | ||||||
| Flexitarian | - | - | - | - | - | - |
| Lacto-Ovo-Vegetarian | −15.68 | 0.44 | <0.001 | −15.58 | 0.38 | <0.001 |
| Vegan | −26.04 | 0.62 | <0.001 | −24.99 | 0.56 | <0.001 |
| Sex | ||||||
| Female | −1.51 | 0.36 | <0.001 | |||
| Male | - | - | - | |||
| Body mass index | 0.36 | 0.05 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekmeiro-Salvador, J.E.; Storz, M.A. The Impact of Plant-Based Diets on Dietary Acid Load Metrics in Venezuela: A Cross-Sectional Study. Nutrients 2023, 15, 2745. https://doi.org/10.3390/nu15122745
Ekmeiro-Salvador JE, Storz MA. The Impact of Plant-Based Diets on Dietary Acid Load Metrics in Venezuela: A Cross-Sectional Study. Nutrients. 2023; 15(12):2745. https://doi.org/10.3390/nu15122745
Chicago/Turabian StyleEkmeiro-Salvador, Jesús Enrique, and Maximilian Andreas Storz. 2023. "The Impact of Plant-Based Diets on Dietary Acid Load Metrics in Venezuela: A Cross-Sectional Study" Nutrients 15, no. 12: 2745. https://doi.org/10.3390/nu15122745
APA StyleEkmeiro-Salvador, J. E., & Storz, M. A. (2023). The Impact of Plant-Based Diets on Dietary Acid Load Metrics in Venezuela: A Cross-Sectional Study. Nutrients, 15(12), 2745. https://doi.org/10.3390/nu15122745








