Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function
Abstract
1. Introduction
2. Materials and Methods
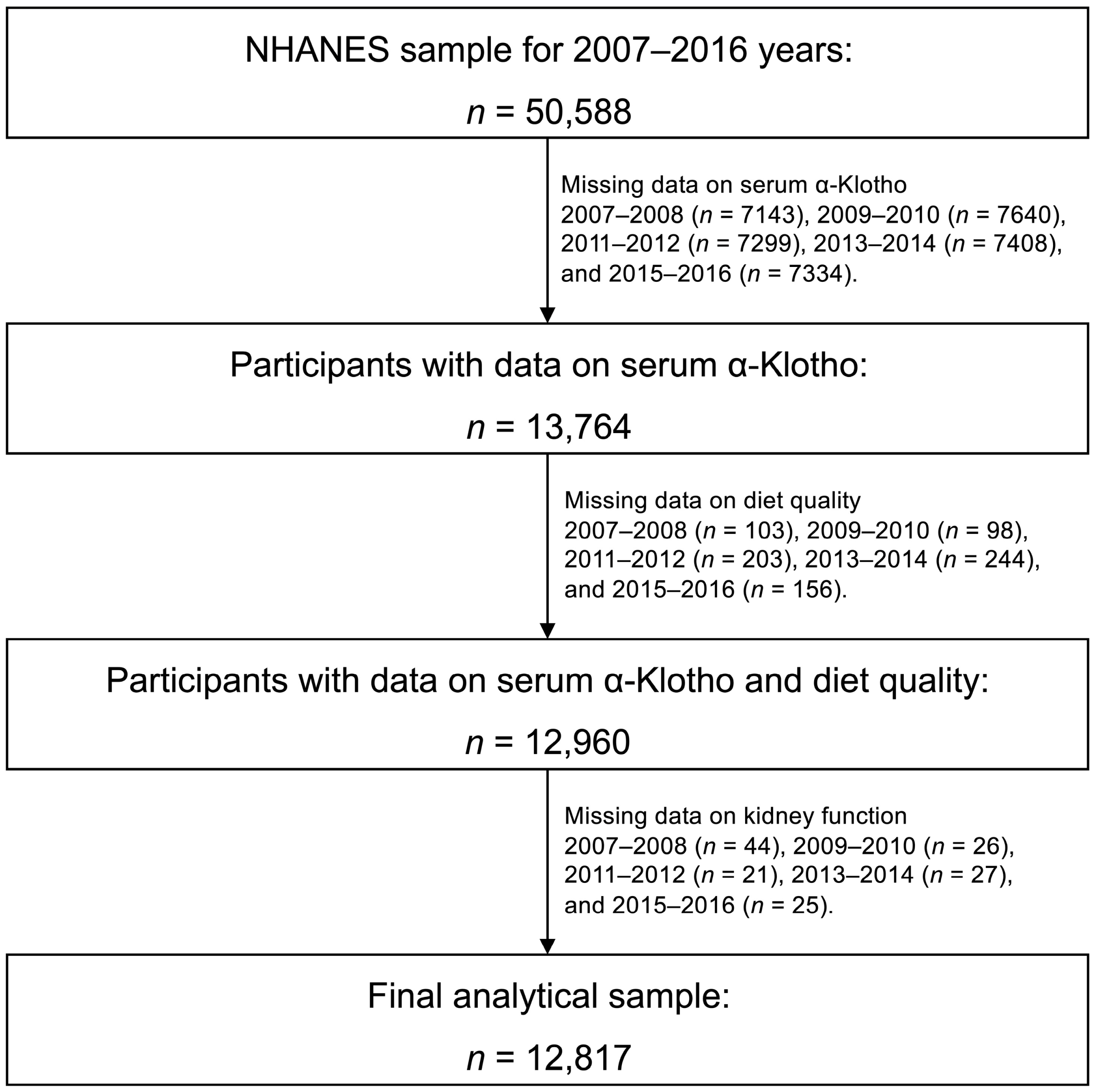
2.1. Data Source and Study Participants
2.2. Assessment of Diet Quality
2.3. Assessment of Kidney Function
2.4. Quantitation of Serum α-Klotho
2.5. Identification of the Covariates
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Association between HEI-2015 Score and Kidney Function
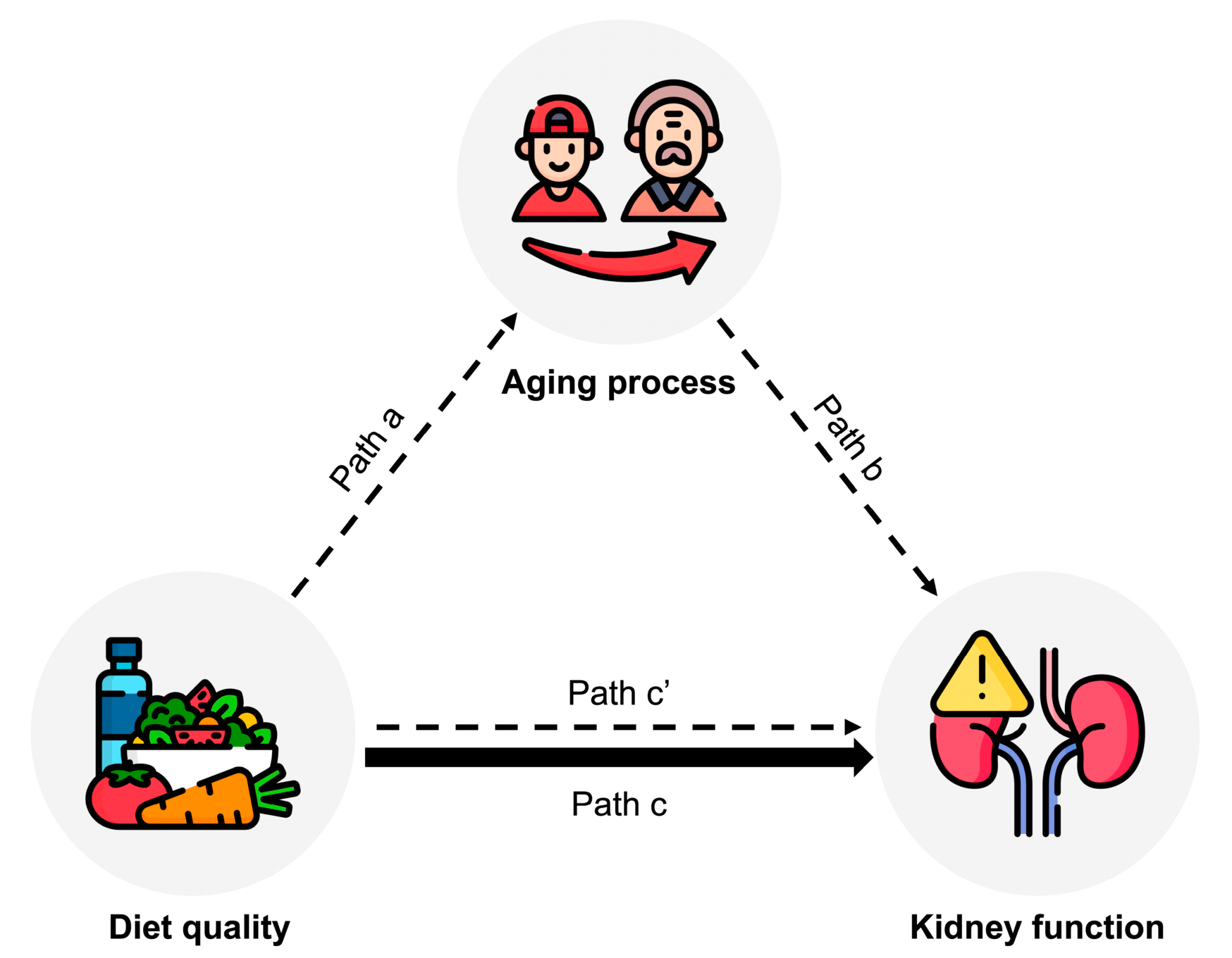
3.3. Mediating Role of Serum α-Klotho
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Kalache, A.; de Hoogh, A.I.; Howlett, S.E.; Kennedy, B.; Eggersdorfer, M.; Marsman, D.S.; Shao, A.; Griffiths, J.C. Nutrition interventions for healthy ageing across the lifespan: A conference report. Eur. J. Nutr. 2019, 58, 1–11. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Raubenheimer, D.; Solon-Biet, S.; de Cabo, R.; Simpson, S.J. Does diet influence aging? Evidence from animal studies. J. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Grams, M.E.; Crews, D.C.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Dietary patterns and risk of incident chronic kidney disease: The Atherosclerosis Risk in Communities study. Am. J. Clin. Nutr. 2019, 110, 713–721. [Google Scholar] [CrossRef]
- Khatri, M.; Moon, Y.P.; Scarmeas, N.; Gu, Y.; Gardener, H.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Nickolas, T.L.; Elkind, M.S. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin. J. Am. Soc. Nephrol. 2014, 9, 1868–1875. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R., 3rd; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Lindberg, K.; Amin, R.; Moe, O.W.; Hu, M.C.; Erben, R.G.; Östman Wernerson, A.; Lanske, B.; Olauson, H.; Larsson, T.E. The kidney is the principal organ mediating klotho effects. J. Am. Soc. Nephrol. 2014, 25, 2169–2175. [Google Scholar] [CrossRef]
- Shi, M.; Flores, B.; Gillings, N.; Bian, A.; Cho, H.J.; Yan, S.; Liu, Y.; Levine, B.; Moe, O.W.; Hu, M.C. αKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J. Am. Soc. Nephrol. 2016, 27, 2331–2345. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating αKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Haruna, Y.; Kashihara, N.; Satoh, M.; Tomita, N.; Namikoshi, T.; Sasaki, T.; Fujimori, T.; Xie, P.; Kanwar, Y.S. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl. Acad. Sci. USA 2007, 104, 2331–2336. [Google Scholar] [CrossRef]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef]
- Yuan, Q.; Ren, Q.; Li, L.; Tan, H.; Lu, M.; Tian, Y.; Huang, L.; Zhao, B.; Fu, H.; Hou, F.F.; et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat. Commun. 2022, 13, 438. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Deng, L.; Jin, K.; Xiong, X.; Su, X.; Qiu, S.; Yang, L. The association between serum soluble Klotho and chronic kidney disease among us adults ages 40 to 79 years: Cross-sectional study. Front. Public Health 2022, 10, 995314. [Google Scholar] [CrossRef]
- Ma, T.C.; Zhou, J.; Wang, C.X.; Lin, Z.Z.; Gao, F. Associations between the Healthy Eating Index-2015 and S-Klotho plasma levels: A cross-sectional analysis in middle-to-older aged adults. Front. Nutr. 2022, 9, 904745. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 1 2013, 1–37. [Google Scholar]
- National Health and Nutrition Examination Survey. NCHS Ethics Review Board (ERB) Approval. 2011. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on 22 May 2023).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Yin, J.; Huang, Y.; Liu, G.; Wang, L.; Shan, Z.; Liu, L. Trends in dietary macronutrient composition and diet quality among US adults with diagnosed and undiagnosed elevated glycemic status: A serial cross-sectional study. Am. J. Clin. Nutr. 2022, 115, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet. 2018, 118, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Du, M.; Chen, Y.; Marks, L.A.M.; Visser, A.; Xu, S.; Tjakkes, G.E. Periodontitis and cognitive impairment in older adults: The mediating role of mitochondrial dysfunction. J. Periodontol. 2022, 93, 1302–1313. [Google Scholar] [CrossRef]
- Albert, J.M.; Nelson, S. Generalized causal mediation analysis. Biometrics 2011, 67, 1028–1038. [Google Scholar] [CrossRef]
- Mera-Gaona, M.; Neumann, U.; Vargas-Canas, R.; López, D.M. Evaluating the impact of multivariate imputation by MICE in feature selection. PLoS ONE 2021, 16, e0254720. [Google Scholar] [CrossRef]
- Cai, Q.; Dekker, L.H.; Vinke, P.C.; Corpeleijn, E.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Diet quality and incident chronic kidney disease in the general population: The Lifelines Cohort Study. Clin. Nutr. 2021, 40, 5099–5105. [Google Scholar] [CrossRef]
- Lin, J.; Fung, T.T.; Hu, F.B.; Curhan, G.C. Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am. J. Kidney Dis. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Perry, I.J.; Phillips, C.M. Dietary Quality Determined by the Healthy Eating Index-2015 and Biomarkers of Chronic Low-Grade Inflammation: A Cross-Sectional Analysis in Middle-to-Older Aged Adults. Nutrients 2021, 13, 222. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, C.; Wang, Y.; Huang, H.; Liu, W.; Zhang, J.F.; Zhao, H.; Feng, B.; Leung, P.S.; Xia, Y. IL-1β inhibits β-Klotho expression and FGF19 signaling in hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E289–E300. [Google Scholar] [CrossRef]
- Li, X.; Cao, X.; Zhang, J.; Fu, J.; Mohedaner, M.; Danzengzhuoga; Sun, X.; Yang, G.; Yang, Z.; Kuo, C.-L.; et al. Accelerated Aging Mediates the Associations of Unhealthy Lifestyles with Cardiovascular Disease, Cancer, and Mortality: Two Large Prospective Cohort Studies. medRxiv 2022. [Google Scholar] [CrossRef]
| Whole Population | |
|---|---|
| Number | 12,817 |
| HEI-2015 | 55.2 ± 13.4 |
| eGFR (mL/min per 1.73 m2) | 86.8 ± 19.8 |
| UACR (mg/g) | 7.6 (4.9–15.1) |
| α-Klotho (pg/mL) | 855.4 ± 310.6 |
| Age (years) | 57.7 ± 10.8 |
| Sex (Female, %) | 51.5 |
| Race (%) | |
| Non-Hispanic white | 44.1 |
| Non-Hispanic Black | 19.7 |
| Hispanic | 27.4 |
| Others | 8.7 |
| Body weight | |
| Normal weight | 23.7 |
| Overweight | 34.7 |
| Obesity | 41.6 |
| Self-reported hypertension (yes, %) | 46.5 |
| Self-reported diabetes (yes, %) | 18.3 |
| Self-reported cardiovascular disease (yes, %) | 10.6 |
| Smoking status (%) | |
| Current | 19.5 |
| Former | 29.4 |
| Never | 51.1 |
| Physical activity (%) | |
| Vigorous | 13.6 |
| Moderate | 21.0 |
| Low | 65.4 |
| Energy intake (kcal/day) | 3720 ± 1597 |
| Alcohol intake (%) | |
| Heavy | 16.2 |
| Moderate | 35.2 |
| Never | 48.6 |
| Education level (%) | |
| College or higher | 50.3 |
| High school | 22.2 |
| Less than high school | 27.5 |
| Annual household income (USD, %) | |
| >75,000 | 25.8 |
| 20,000–75,000 | 50.3 |
| <20,000 | 23.8 |
| Weighted β Coefficient (95% CI) for eGFR | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Overall HEI-2015 | 0.86 (0.57, 1.14) * | 1.11 (0.81, 1.41) * | 0.94 (0.64, 1.23) * |
| Total fruits | 0.47 (0.18, 0.76) * | 0.68 (0.39, 0.98) * | 0.55 (0.26, 0.85) * |
| Whole fruits | 0.59 (0.30, 0.88) * | 0.78 (0.48, 1.08) * | 0.69 (0.40, 0.99) * |
| Total vegetables | 0.47 (0.18, 0.76) * | 0.60 (0.31, 0.89) * | 0.59 (0.30, 0.88) * |
| Greens and beans | 0.65 (0.36, 0.94) * | 0.72 (0.43, 1.00) * | 0.63 (0.34, 0.92) * |
| Whole grain | 0.43 (0.14, 0.71) * | 0.59 (0.29, 0.88) * | 0.56 (0.27, 0.85) * |
| Refined grain | −0.06 (−0.36, 0.23) | −0.01 (−0.30, 0.29) | −0.14 (−0.43, 0.16) |
| Total dairy | 0.14 (−0.15, 0.44) | 0.21 (−0.09, 0.50) | 0.17 (−0.12, 0.46) |
| Total protein | −0.14 (−0.42, 0.15) | −0.11 (−0.39, 0.18) | −0.02 (−0.30, 0.27) |
| Seafood and plant protein | 0.62 (0.33, 0.90) * | 0.70 (0.41, 0.99) * | 0.63 (0.34, 0.92) * |
| Fatty acid | 0.26 (−0.03, 0.54) | 0.31 (0.03, 0.60) * | 0.31 (0.02, 0.59) * |
| Saturated fat | 0.40 (0.11, 0.69) * | 0.44 (0.15, 0.73) * | 0.31 (0.02, 0.60) * |
| Sodium | 0.18 (−0.11, 0.46) | 0.08 (−0.20, 0.37) | −0.17 (−0.46, 0.12) |
| Added sugar | 0.49 (0.20, 0.78) * | 0.64 (0.34, 0.93) * | 0.76 (0.47, 1.06) * |
| Weighted β Coefficient (95% CI) for eGFR | ||||
|---|---|---|---|---|
| Mediator: α-Klotho | Total Effect | Direct Effect | Indirect Effect | Proportion Mediated, % (95%CI) |
| Overall HEI-2015 | 0.94 (0.64, 1.23) * | 0.88 (0.59, 1.19) * | 0.05 (0.02, 0.08) * | 5.8 (2.6, 10.0) * |
| Total fruits | 0.55 (0.26, 0.85) * | 0.49 (0.20, 0.82) * | 0.06 (0.03, 0.09) * | 10.5 (4.3, 23.0) * |
| Whole fruits | 0.69 (0.40, 0.99) * | 0.62 (0.31, 0.90) * | 0.07 (0.04, 0.10) * | 9.6 (5.1, 19.0) * |
| Total vegetables | 0.59 (0.30, 0.88) * | 0.57 (0.27, 0.87) * | 0.02 (−0.01, 0.06) | - |
| Greens and beans | 0.63 (0.34, 0.92) * | 0.60 (0.33, 0.88) * | 0.04 (0.01, 0.07) * | 5.6 (1.2, 13.0) * |
| Whole grain | 0.56 (0.27, 0.85) * | 0.51 (0.21, 0.82) * | 0.05 (0.02, 0.08) * | 8.4 (3.3, 20.0) * |
| Refined grain | −0.14 (−0.43, 0.16) | −0.16 (−0.45, 0.12) | 0.02 (−0.01, 0.05) | - |
| Total dairy | 0.17 (−0.12, 0.46) | 0.13 (−0.15, 0.41) | 0.04 (−0.01, 0.07) | - |
| Total protein | −0.02 (−0.30, 0.27) | −0.04 (−0.34, 0.23) | 0.03 (−0.01, 0.06) | - |
| Seafood and plant protein | 0.63 (0.34, 0.92) * | 0.60 (0.31, 0.91) * | 0.03 (−0.01, 0.05) | - |
| Fatty acid | 0.31 (0.02, 0.59) * | 0.29 (−0.01, 0.60) | 0.02 (−0.01, 0.05) | - |
| Saturated fat | 0.31 (0.02, 0.60) * | 0.32 (0.02, 0.61) * | −0.01 (−0.04, 0.02) | - |
| Sodium | −0.17 (−0.46, 0.12) | −0.15 (−0.45, 0.12) | −0.02 (−0.05, 0.01) | - |
| Added sugar | 0.76 (0.47, 1.06) * | 0.79 (0.50, 1.08) * | −0.02 (−0.06, 0.01) | - |
| Weighted β Coefficient (95%CI) for eGFR | ||||
|---|---|---|---|---|
| Mediator: α-Klotho | Total Effect | Direct Effect | Indirect Effect | Proportion Mediated % (95% CI) |
| Age (years) | ||||
| 40–59 | 0.59 (0.21, 0.96) * | 0.56 (0.18, 0.93) * | 0.03 (−0.01, 0.06) | - |
| 60–79 | 1.30 (0.87, 1.77) * | 1.20 (0.76, 1.67) * | 0.10 (0.04, 0.16) * | 7.6 (3.2, 14.0) * |
| Sex | ||||
| Male | 0.92 (0.49, 1.28) * | 0.83 (0.41, 1.20) * | 0.09 (0.04, 0.14) * | 9.6 (4.5, 20.0) * |
| Female | 0.90 (0.50, 1.32) * | 0.88 (0.48, 1.29) * | 0.03 (−0.01, 0.06) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Q.; Hu, S.; Qi, C.; Yin, J.; Xu, S.; Hou, F.F.; Li, A. Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function. Nutrients 2023, 15, 2744. https://doi.org/10.3390/nu15122744
Cai Q, Hu S, Qi C, Yin J, Xu S, Hou FF, Li A. Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function. Nutrients. 2023; 15(12):2744. https://doi.org/10.3390/nu15122744
Chicago/Turabian StyleCai, Qingqing, Shixian Hu, Cancan Qi, Jiawei Yin, Shulan Xu, Fan Fan Hou, and An Li. 2023. "Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function" Nutrients 15, no. 12: 2744. https://doi.org/10.3390/nu15122744
APA StyleCai, Q., Hu, S., Qi, C., Yin, J., Xu, S., Hou, F. F., & Li, A. (2023). Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function. Nutrients, 15(12), 2744. https://doi.org/10.3390/nu15122744








