Faecalibacterium prausnitzii in Differentiated Thyroid Cancer Patients Treated with Radioiodine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Treatment
2.2. Sample Collection
2.3. DNA Extraction and Sequencing
2.4. Metagenomic Profiling and Statiscal Analysis
3. Results
3.1. Study Population
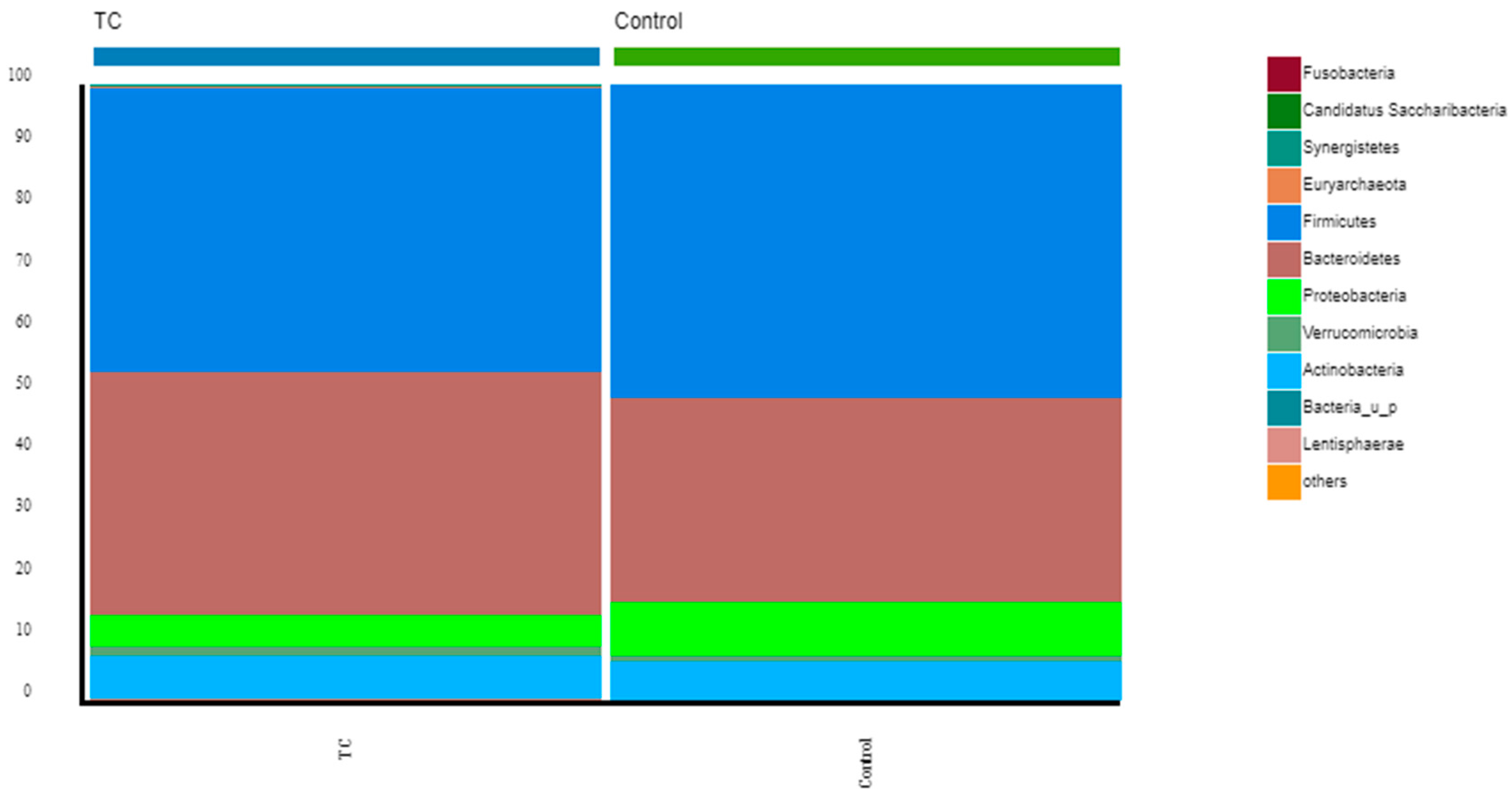
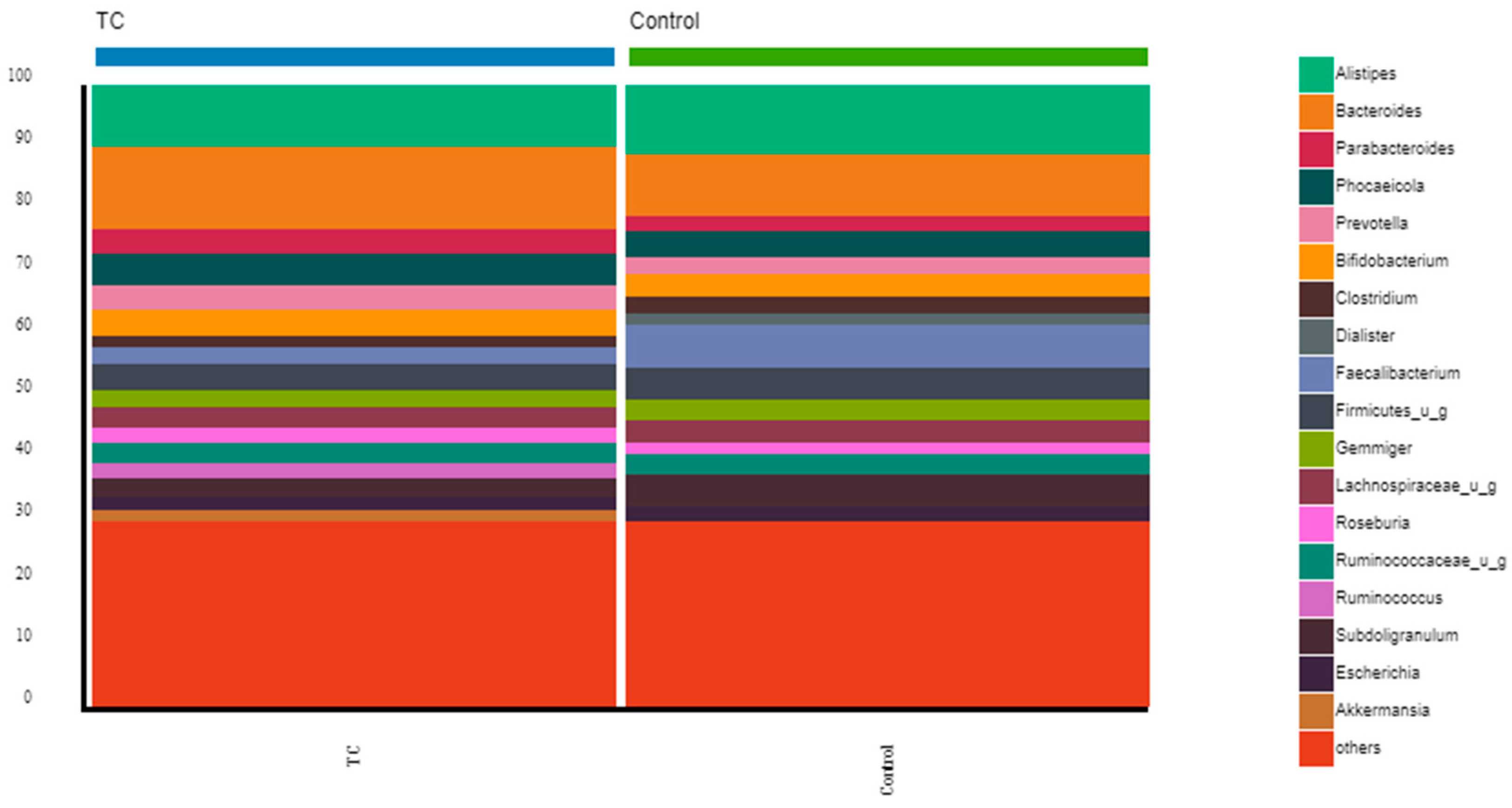
3.2. Gut Microbiota Diversity, Richness, and Taxonomic Distribution from Thyroid Cancer Patients before Treatment Compared with Healthy Controls
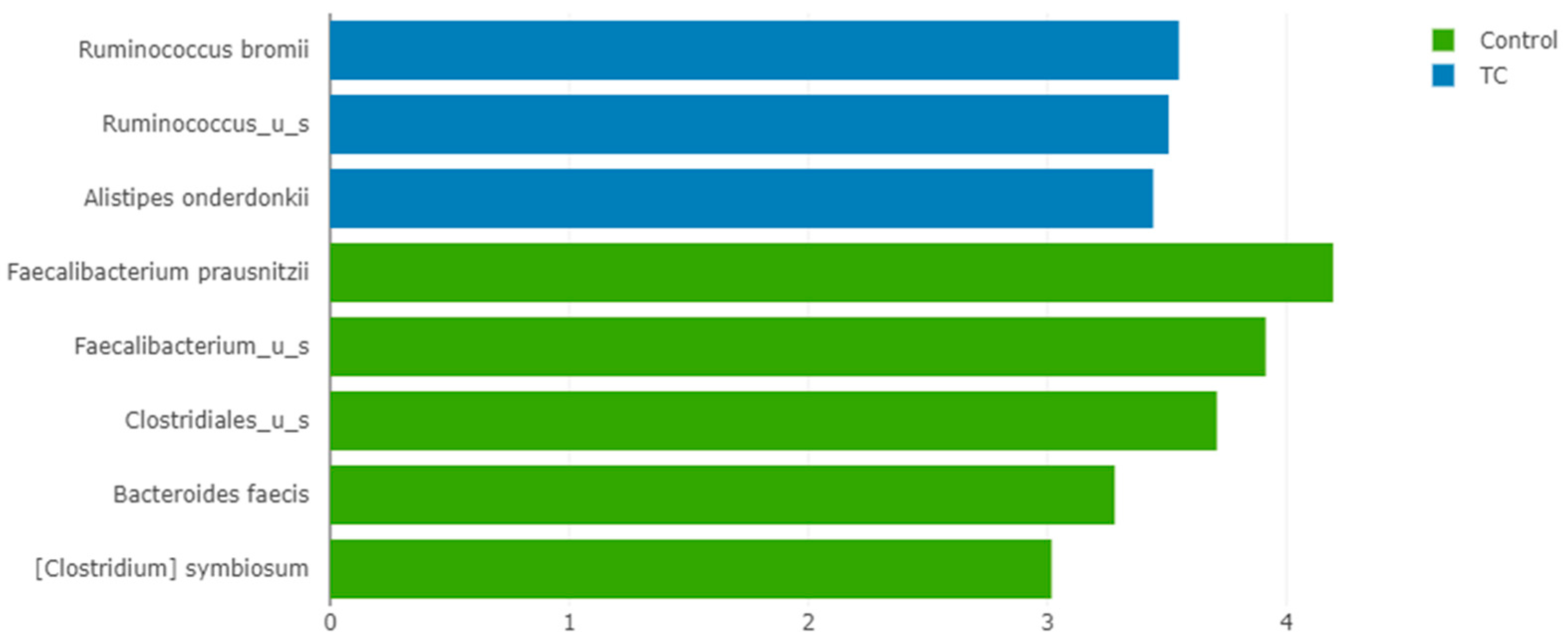
3.3. The Relative Abundance of F. prausnitzii in Differentiated Thyroid Cancer Patients Is Decreased Compared with Controls
3.4. Impact of Radioiodine Therapy on the Richness, Diversity, and Composition
3.5. Impact of RAIT on the Relative Abundance of F. prausnitzii
4. Discussion
4.1. Composition of the Gut Microbiota of Differentiated Thyroid Cancer Patients before Treatment: The Relative Abundance of F. prausnitzii Is Decreased in Thyroid Cancer Patients Compared to Controls
4.2. Effects of Radioiodine Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 6, 2141–2146. [Google Scholar] [PubMed]
- Franks, A.H.; Harmsen, H.J.; Raangs, G.C.; Jansen, G.J.; Schut, F.; Welling, G.W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 3336–3345. [Google Scholar] [CrossRef]
- Suau, A.; Bonnet, R.; Sutren, M.; Godon, J.J.; Gibson, G.R.; Collins, M.D.; Doré, J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999, 65, 4799–4807. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. Phylogenetic analysis of Fusobacterium prausnitzii based upon the 16S rRNA gene sequence and PCR confirmation. Int. J. Syst. Bacteriol. 1996, 46, 341–343. [Google Scholar] [CrossRef]
- Stewart, L. Faecalibacterium Prausnitzii: The Gut Microbe That Prevents Inflammation. 2021. Available online: https://atlasbiomed.com/blog/faecalibacterium-prausnitzii/ (accessed on 20 February 2022).
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å.V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Miquel, S.; Leclerc, M.; Martin, R.; Chain, F.; Lenoir, M.; Raguideau, S.; Hudault, S.; Bridonneau, C.; Northen, T.; Bowen, B.; et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. MBio 2015, 6, e00300-15. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Simrén, M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig. Liver Dis. 2007, 39, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133 (Suppl. 7), 2485s–2493s. [Google Scholar] [CrossRef]
- Gibson, P.R.; Rosella, O.; Wilson, A.J.; Mariadason, J.M.; Rickard, K.; Byron, K.; Barkla, D.H. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis 1999, 20, 539–544. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Reynders, V.; Loitsch, S.; Steinhilber, D.; Stein, J.; Schröder, O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol. Immunol. 2007, 44, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L.; Huang, J.; Sasazuki, T.; Shirasawa, S.; Augenlicht, L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol. Cancer Res. 2003, 1, 855–862. [Google Scholar]
- Roediger, W.E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, S.; Li, Y. Faecalibacterium prausnitzii: A Next-Generation Probiotic in Gut Disease Improvement. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6666114. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, W.; Kosik, R.O.; Song, Y.; Luo, Q.; Qiao, T.; Tong, J.; Liu, S.; Deng, C.; Qin, S.; et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J. Adv. Res. 2021, 35, 61–70. [Google Scholar] [CrossRef]
- Cancer IAfRo. Available online: https://gco.iarc.fr/today/data/factsheets/populations/620-portugal-fact-sheets.pdf (accessed on 15 March 2022).
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 2-92. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2019, 144, 2728–2745. [Google Scholar] [CrossRef]
- Vought, R.L.; Brown, F.A.; Sibinovic, K.H.; McDaniel, E.G. Effect of changing intestinal bacterial flora on thyroid function in the rat. Horm. Metab. Res. 1972, 4, 43–47. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef] [PubMed]
- Samimi, H.; Haghpanah, V. Gut Microbiome and Radioiodine-Refractory Papillary Thyroid Carcinoma Pathophysiology. Trends Endocrinol. Metab. 2020, 31, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.; Eberlein, U.; Holm, S.; Hustinx, R.; Konijnenberg, M.; Strigari, L.; van Leeuwen, F.W.; Glatting, G.; Lassmann, M. EANM position paper on the role of radiobiology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3365–3377. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X.; et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574. [Google Scholar] [CrossRef]
- Hollingsworth, B.A.; Cassatt, D.R.; DiCarlo, A.L.; Rios, C.I.; Satyamitra, M.M.; Winters, T.A.; Taliaferro, L.P. Acute Radiation Syndrome and the Microbiome: Impact and Review. Front. Pharmacol. 2021, 12, 643283. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wang, A.Z.; Knox, S.J. The Radiobiology of Radiopharmaceuticals. Semin. Radiat. Oncol. 2021, 31, 20–27. [Google Scholar] [CrossRef]
- Terry, S.Y.A.; Nonnekens, J.; Aerts, A.; Baatout, S.; de Jong, M.; Cornelissen, B.; Pouget, J.-P. Call to arms: Need for radiobiology in molecular radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1588–1590. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A prospective, longitudinal study. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef]
- Sahly, N.; Moustafa, A.; Zaghloul, M.; Salem, T.Z. Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study. PeerJ 2019, 2019, e7683. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Shen, L.; Zou, W.; Wang, J.; Yang, J.; Wang, Y.; Liu, B.; Xie, L.; Zhu, J.; Zhang, Z. The Gut Microbiome Is Associated with Therapeutic Responses and Toxicities of Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients-A Pilot Study. Front. Cell. Infect. Microbiol. 2020, 10, 562463. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.-D.; Delzenne, N.M.; Muccioli, G.G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. [Google Scholar] [CrossRef]
- Frohlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Andoh, A.; Sugimoto, M. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Vaahtovuo, J.; Munukka, E.; Korkeamäki, M.; Luukkainen, R.; Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 1500–1505. [Google Scholar]
- Vermeiren, J.; Abbeele, P.V.D.; Laukens, D.; Vigsnaes, L.K.; De Vos, M.; Boon, N.; Van De Wiele, T. Decreased colonization of fecal Clostridium coccoides/Eubacterium rectale species from ulcerative colitis patients in an in vitro dynamic gut model with mucin environment. FEMS Microbiol. Ecol. 2012, 79, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Mohammad, I.S.; Guo, H.; Shahzad, M.; Hou, Y.J.; Ma, C.; Naseem, Z.; Wu, X.; Shi, P.; Xu, J. Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed. Pharmacother. 2017, 95, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, E.C.; Bilotta, A.L.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Laginestra, A.; Novi, M.; Sottili, S.; Serricchio, M.; Cammarota, G.; et al. Association between Hypothyroidism and Small Intestinal Bacterial Overgrowth. J. Clin. Endocrinol. Metab. 2007, 92, 4180–4184. [Google Scholar] [CrossRef] [PubMed]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.-P.; Konijnenberg, M.; Eberlein, U.; Glatting, G.; Gabina, P.M.; Herrmann, K.; Holm, S.; Strigari, L.; van Leeuwen, F.W.B.; Lassmann, M. An EANM position paper on advancing radiobiology for shaping the future of nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 242–246. [Google Scholar]
- Lapiere, A.; Geiger, M.; Robert, V.; Demarquay, C.; Auger, S.; Chadi, S.; Benadjaoud, M.; Fernandes, G.; Milliat, F.; Langella, P.; et al. Prophylactic Faecalibacterium prausnitzii treatment prevents the acute breakdown of colonic epithelial barrier in a preclinical model of pelvic radiation disease. Gut Microbes 2020, 12, 1812867. [Google Scholar] [CrossRef]




| Baseline Characteristics | n = 37 |
|---|---|
| Age (mean, SD) | 51 ± 17 |
| Gender | |
| Male | 11 |
| Female | 26 |
| BMI (mean, SD) | 28.3 ± 6.6 |
| Concomitant diseases | |
| Diabetes | 7 |
| Cardiovascular diseases | 16 |
| Other (psoriasis, chronic kidney disease, hyperuricemia, gastritis, etc.) | 8 |
| Number of chronic medications (mean, SD) | 3.1 ± 2.4 |
| Tumor Type | |
| Follicular | 1 |
| Papillar | 36 |
| Clinical T stage | |
| T1 | 16 |
| T2 | 13 |
| T3 | 6 |
| T4 | 1 |
| ND | 1 |
| Clinical N stage | |
| Nx | 25 |
| N0 | 4 |
| N1 | 8 |
| Clinical M stage | |
| M0 | 34 |
| M1 | 3 |
| R | |
| R0 | 30 |
| R1 | 5 |
| R2 | 1 |
| Age (mean, SD) | 51 ± 17 |
| Gender | |
| Male | 11 |
| Female | 26 |
| I-131 dose | |
| 1110 MBq | 7 |
| 3700 MBq | 22 |
| 5550 MBq | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Oliveira, A.; Carvalho, A.L.; Soares, R.; Barata, P. Faecalibacterium prausnitzii in Differentiated Thyroid Cancer Patients Treated with Radioiodine. Nutrients 2023, 15, 2680. https://doi.org/10.3390/nu15122680
Fernandes A, Oliveira A, Carvalho AL, Soares R, Barata P. Faecalibacterium prausnitzii in Differentiated Thyroid Cancer Patients Treated with Radioiodine. Nutrients. 2023; 15(12):2680. https://doi.org/10.3390/nu15122680
Chicago/Turabian StyleFernandes, Ana, Ana Oliveira, Ana Luísa Carvalho, Raquel Soares, and Pedro Barata. 2023. "Faecalibacterium prausnitzii in Differentiated Thyroid Cancer Patients Treated with Radioiodine" Nutrients 15, no. 12: 2680. https://doi.org/10.3390/nu15122680
APA StyleFernandes, A., Oliveira, A., Carvalho, A. L., Soares, R., & Barata, P. (2023). Faecalibacterium prausnitzii in Differentiated Thyroid Cancer Patients Treated with Radioiodine. Nutrients, 15(12), 2680. https://doi.org/10.3390/nu15122680






