Habitual Dietary Patterns, Nutrient Intakes, and Adherence to the Mediterranean Diet among New Zealand Adults: The NZ MED Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
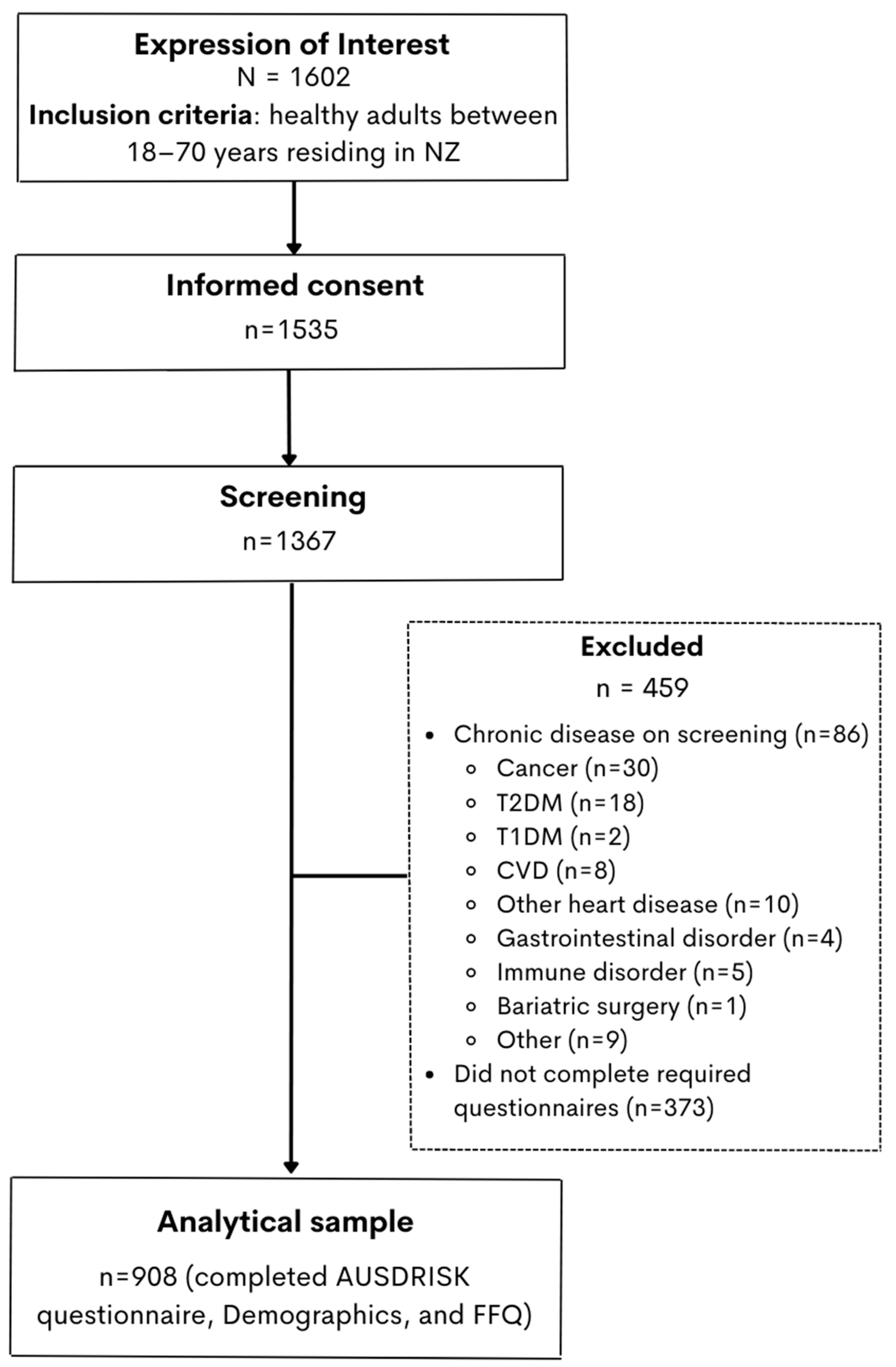
2.1. Study Design and Population
2.2. Questionnaires and Data Collection
2.3. Study Variables
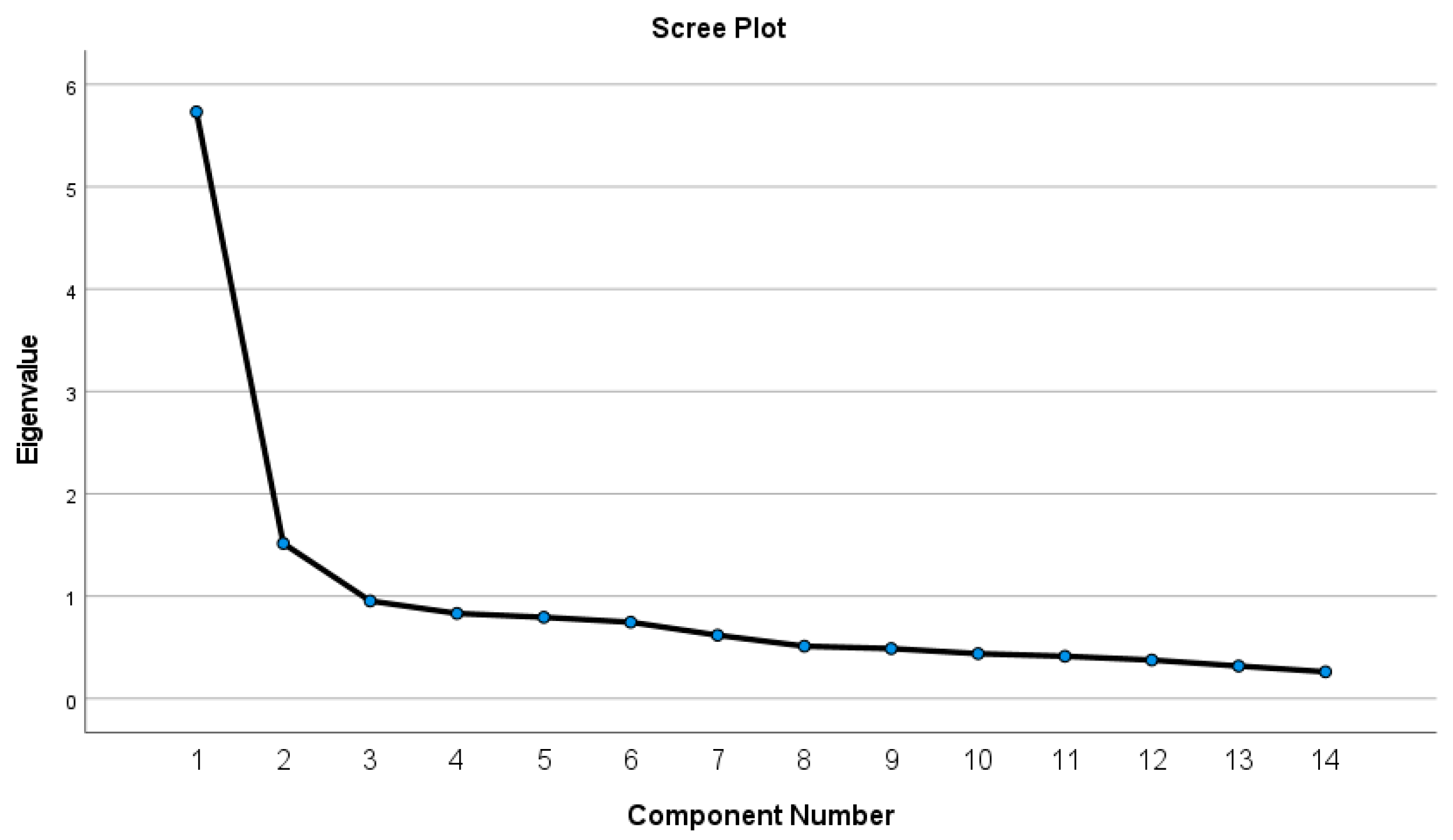
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Nutrient Intakes
3.3. Dietary Patterns
3.4. Diet Quality
4. Discussion
4.1. Limitations
4.2. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. The Mediterranean diet: Science and practice. Public Health Nutr. 2006, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Kontogianni, M.D.; Yiannakouris, N. Mediterranean diet and diabetes: Prevention and treatment. Nutrients 2014, 6, 1406–1423. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; Predimed Investigators. Benefits of the Mediterranean diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch. Intern. Med. 2012, 172, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Rumawas, M.E.; Dwyer, J.T.; Mckeown, N.M.; Meigs, J.B.; Rogers, G.; Jacques, P.F. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J. Nutr. 2009, 139, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.; Lamarche, B.; Nadeau, G.; Lemieux, S. Effect of a nutritional intervention promoting the Mediterranean food pattern on plasma lipids, lipoproteins and body weight in healthy French-Canadian women. Atherosclerosis 2003, 170, 115–124. [Google Scholar] [CrossRef]
- Kouris-Blazos, A.; Gnardellis, C.; Wahlqvist, M.L.; Trichopoulos, D.; Lukito, W.; Trichopoulou, A. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br. J. Nutr. 1999, 82, 57–61. [Google Scholar] [CrossRef]
- Ministry of Health. Eating and Activity Guidelines for New Zealand Adults; Ministry of Health: Wellington, New Zealand, 2020.
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; Ngo de la Cruz, J.; Bach-Faig, A.; Donini, L.M.; Medina, F.-X.; Belahsen, R.; et al. Updating the mediterranean diet pyramid towards sustainability: Focus on environmental concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- University of Otago; Ministry of Health. A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011.
- Tucker, C. Using environmental imperatives to reduce meat consumption: Perspectives from New Zealand. Kōtuitui N. Z. J. Soc. Sci. Online 2018, 13, 99–110. [Google Scholar] [CrossRef]
- Mumme, K.D.; Conlon, C.A.; von Hurst, P.R.; Jones, B.; de Seymour, J.V.; Stonehouse, W.; Heath, A.-L.M.; Coad, J.; Haskell-Ramsay, C.F.; Mugridge, O.; et al. Associations between dietary patterns and the metabolic syndrome in older adults in New Zealand: The REACH study. Br. J. Nutr. 2022, 128, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Keegan, R.; Smith, M.F.; Alkhatib, A.; Klonizakis, M. Implementing a Mediterranean diet intervention into a RCT: Lessons learned from a non-Mediterranean based country. J. Nutr. Health Aging 2015, 19, 1019–1022. [Google Scholar] [CrossRef]
- Ministry of Health. Custom Requested Mortality Dataset Provided to Heart Foundation from the NZ Mortality Collection; Ministry of Health: Wellington, New Zealand, 2021.
- Holder-Pearson, L.; Chase, J.G. Socio-Economic Inequity: Diabetes in New Zealand. Front. Med. 2022, 9, 756223. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Balkau, B.; Colagiuri, S.; Zimmet, P.Z.; Tonkin, A.M.; Mitchell, P.; Phillips, P.J.; Shaw, J.E.; Chen, M.L.; et al. AUSDRISK: An Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med. J. Aust. 2010, 192, 197–202. [Google Scholar] [CrossRef]
- Malo, J.A.; Versace, V.L.; Janus, E.D.; Laatikainen, T.; Peltonen, M.; Vartiainen, E.; Coates, M.J.; Dunbar, J.A. Evaluation of AUSDRISK as a screening tool for lifestyle modification programs: International implications for policy and cost-effectiveness. BMJ Open Diabetes Res. Care 2015, 3, e000125. [Google Scholar] [CrossRef]
- Sam, C.H.; Skeaff, S.; Skidmore, P.M. A comprehensive FFQ developed for use in New Zealand adults: Reliability and validity for nutrient intakes. Public Health Nutr. 2014, 17, 287–296. [Google Scholar] [CrossRef]
- Sam, C.H.Y.; Skidmore, P.; Skeaff, S.; Parackal, S.; Wall, C.; Bradbury, K.E. Relative Validity and Reproducibility of a Short Food Frequency Questionnaire to Assess Nutrient Intakes of New Zealand Adults. Nutrients 2020, 12, 619. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2006.
- Gerritsen, S.; Egli, V.; Roy, R.; Haszard, J.; Backer, C.D.; Teunissen, L.; Cuykx, I.; Decorte, P.; Pabian, S.P.; Van Royen, K.; et al. Seven weeks of home-cooked meals: Changes to New Zealanders’ grocery shopping, cooking and eating during the COVID-19 lockdown. J. R. Soc. N. Z. 2021, 51 (Suppl. 1), S4–S22. [Google Scholar] [CrossRef]
- Roy, R.; de Castro, T.G.; Haszard, J.; Egli, V.; Morenga, L.T.; Teunissen, L.; Decorte, P.; Cuykx, I.; De Backer, C.; Gerritsen, S. Who we seek and what we eat? Sources of food choice inspirations and their associations with adult dietary patterns before and during the COVID-19 lockdown in New Zealand. Nutrients 2021, 13, 3917. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, I.; Rathmanner, T.; Kunze, M. Eating and dieting differences in men and women. J. Men’s Health Gend. 2005, 2, 194–201. [Google Scholar] [CrossRef]
- Wardle, J.; Haase, A.M.; Steptoe, A.; Nillapun, M.; Jonwutiwes, K.; Bellisie, F. Gender differences in food choice: The contribution of health beliefs and dieting. Ann. Behav. Med. 2004, 27, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Jones, B.; Ullah, I.; McNaughton, S.A.; Haslett, S.J.; Stonehouse, W. Associations between dietary patterns, socio-demographic factors and anthropometric measurements in adult New Zealanders: An analysis of data from the 2008/09 New Zealand Adult Nutrition Survey. Eur. J. Nutr. 2018, 57, 1421–1433. [Google Scholar] [CrossRef]
- JaJayasinghe, S.N.; Breier, B.H.; McNaughton, S.A.; Russell, A.P.; Della Gatta, P.A.; Mason, S.; Stonehouse, W.; Walsh, D.C.; Kruger, R. Dietary patterns in New Zealand women: Evaluating differences in body composition and metabolic biomarkers. Nutrients 2019, 11, 1643. [Google Scholar] [CrossRef]
- Wall, C.R.; Gammon, C.S.; Bandara, D.K.; Grant, C.C.; Atatoa Carr, P.E.; Morton, S. Dietary patterns in pregnancy in New Zealand—Influence of maternal socio-demographic, health and lifestyle factors. Nutrients 2016, 8, 300. [Google Scholar] [CrossRef]
- Ministry of Health. Socioeconomic Indicators. Available online: https://www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-awe-o-te-hauora-socioeconomic-determinants-health/socioeconomic-indicators (accessed on 28 March 2023).
- Ministry of Health. Tagata Pasifika in New Zealand. Available online: https://www.health.govt.nz/our-work/populations/pacific-health/tagata-pasifika-new-zealand (accessed on 28 March 2023).
- Blakely, T.; Tobias, M.; Atkinson, J.; Yeh, L.C.; Huang, K. Tracking Disparity: Trends in Ethnic and Socioeconomic Inequalities in Mortality, 1981–2004; Ministry of Health: Wellington, New Zealand, 2007.
- Te Whatu Ora Health New Zealand. Virtual Diabetes Register and Web Tool. 2022. Available online: https://www.tewhatuora.govt.nz/our-health-system/data-and-statistics/virtual-diabetes-tool/ (accessed on 28 March 2023).
- Hobbs, M.; Ahuriri-Driscoll, A.; Marek, L.; Campbell, M.; Tomintz, M.; Kingham, S. Reducing health inequity for Māori people in New Zealand. Lancet 2019, 394, 1613–1614. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef]
- Lenighan, Y.M.; McNulty, B.A.; Roche, H.M. Dietary fat composition: Replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proc. Nutr. Soc. 2019, 78, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Milà-Villarroel, R.; Bach-Faig, A.; Puig, J.; Puchal, A.; Farran, A.; Serra-Majem, L.; Carrasco, J.L. Comparison and evaluation of the reliability of indexes of adherence to the Mediterranean diet. Public Health Nutr. 2011, 14, 2338–2345. [Google Scholar] [CrossRef]
- Metcalf, P.A.; Scragg, R.; Willoughby, P.; Finau, S.; Tipene-Leach, D. Ethnic differences in perceptions of body size in middle-aged European, Maori and Pacific people living in New Zealand. Int. J. Obes. 2000, 24, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, S.; Harré, S.; Swinburn, B.; Rees, D.; Renker-Darby, A.; Bartos, A.E.; Waterlander, W.E. Systemic barriers and equitable interventions to improve vegetable and fruit intake in children: Interviews with national food system actors. Int. J. Environ. Res. Public Health 2019, 16, 1387. [Google Scholar] [CrossRef]
| Characteristics | Total (%), or Mean ± SD |
|---|---|
| N | 908 |
| Sex | |
| Female | 794 (88) |
| Male | 114 (13) |
| Age (years) | 48 ± 17 |
| Ethnicity | |
| NZ European | 675 (74) |
| Māori | 25 (3) |
| Pacific | 12 (1) |
| Asian | 71 (8) |
| Other European | 101 (11) |
| Other | 24 (3) |
| Employment status | |
| Paid employee/employer | 663 (73) |
| Not employed and starting a new job in 4 weeks | 7 (1) |
| Not employed | 176 (19) |
| Other (retired) | 47 (5) |
| Relationship status | |
| Married | 584 (64) |
| Single/never married | 218 (24) |
| Divorced/separated | 86 (10) |
| Widowed | 20 (2) |
| Highest secondary school qualification | |
| No secondary school qualification | 13 (1) |
| National Certificate Level 1, 2, or 3 | 724 (80) |
| Overseas secondary education | 171 (19) |
| Highest qualification | |
| National Certificate Level 1–4/Trade Certificate | 49 (5) |
| University | 494 (54) |
| Post-Graduate study, including PhD | 217 (24) |
| Other | 25 (3) |
| Missing | 123 (14) |
| Weight (kg) | 72 ± 16 |
| BMI (kg/m2) | 26 ± 11 |
| Waist circumference (cm) | 83 ± 17 |
| Total AUSDRISK score | 11 ± 4 |
| AUSDRISK category | |
| Low | 27 (3) |
| Intermediate | 419 (46) |
| High | 462 (51) |
| MSDPS score | 20.9 ± 7.4 |
| Nutrient | Mean ± SD | |
|---|---|---|
| Energy (kJ) | 7277.1 ± 2103.5 | |
| Carbohydrate (g) | 180.1 ± 67.5 | |
| Protein (g) | 77.0 ± 26.3 | |
| Total fat (g) | 75.5 ± 26.5 | |
| Saturated fat (g) | 38.1 ± 16.5 | |
| MUFA (g) | 25.9 ± 9.2 | |
| PUFA (g) | 21.4 ± 8.6 | |
| Cholesterol (mg) | 228.5 ± 150.5 | |
| Fiber (g) | 29.6 ± 12.4 | |
| Calcium (mg) | 795.9 ± 382.9 | |
| Iron (mg) | 12.5 ± 4.4 | |
| Zinc (mg) | 10.6± 3.5 | |
| Folate (µg) | 456.4 ± 181.7 | |
| Vitamin C (mg) | 142.1 ± 76.6 | |
| Vitamin D (µg) | 2.3 ± 1.9 | |
| Contribution to Energy Intake (% Energy Intake) | ||
| AMDR | Mean ± SD | |
| Carbohydrate (%) | 45–65% EI | 41.2 ± 9.8 |
| Protein (%) | 15–25% EI | 17.9 ± 3.9 |
| Total fat (%) | 20–35% EI | 39.1 ± 8.1 |
| Saturated fat (%) | <10% EI | 19.8 ± 6.1 |
| MUFA (%) | 13.5 ± 3.1 | |
| PUFA (%) | 11.0 ± 2.9 | |
| Food Group | Discretionary | Guideline |
|---|---|---|
| Processed meat | 0.810 | 0.054 |
| Meat, poultry | 0.770 | 0.166 |
| Fast food | 0.548 | −0.099 |
| Sweet drinks | 0.474 | −0.076 |
| Sugar, sweets, baked goods | 0.302 | −0.234 |
| Vegetables | −0.030 | 0.823 |
| Eggs, beans | 0.082 | 0.748 |
| Fruits | −0.032 | 0.463 |
| Oil, spreads | −0.028 | 0.272 |
| Wholegrains | −0.219 | 0.213 |
| Dairy foods | 0.212 | −0.081 |
| Coffee, tea | −0.203 | 0.093 |
| Starches | 0.101 | 0.096 |
| Alcoholic beverages | 0.008 | 0.072 |
| Discretionary | Guideline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 227) | Q2 (n = 227) | Q3 (n = 227) | Q4 (n = 227) | p Value | Q1 (n = 227) | Q2 (n = 227) | Q3 (n = 227) | Q4 (n = 227) | p Value | |
| Demographics | ||||||||||
| Age (years) | 51 ± 16 | 51 ± 15 | 48 ± 15 | 43 ± 16 | <0.001 a | 41 ± 15 | 48 ± 17 | 51 ± 15 | 53 ± 14 | <0.001 a |
| Sex (% female) | 220 (94.0%) | 213 (91.0%) | 202 (86.7%) | 181 (78.0%) | <0.001 b | 191 (82.0%) | 209 (90.5%) | 204 (87.6%) | 212 (89.8%) | 0.023 b |
| NZ European (%) | 178 (76.1%) | 184 (78.0%) | 173 (73.6%) | 150 (64.4%) | 0.005 b | 139 (60.2%) | 193 (81.4%) | 175 (74.5%) | 178 (75.7%) | <0.001 b |
| Māori/Pacific (%) | 5 (2.1%) | 11 (4.7%) | 11 (4.7%) | 25 (10.7%) | <0.001 b | 20 (8.7%) | 10 (4.2%) | 15 (6.4%) | 7 (3.0%) | 0.039 b |
| Higher Education (% yes) | 193 (85) | 191 (84) | 202 (89) | 199 (88) | 0.397 b | 191 (84) | 206 (91) | 199 (88) | 189 (83) | 0.076 b |
| Medical history | ||||||||||
| Weight (kg) | 68 ± 13 | 72 ± 15 | 72 ± 15 | 76 ± 20 | <0.001 a | 74 ± 19 | 71 ± 14 | 72 ± 16 | 71 ± 16 | 0.166 a |
| Waist circumference (cm) | 80 ± 15 | 84 ± 16 | 84 ± 16 | 84 ± 20 | <0.001 a | 84 ± 19 | 82 ± 16 | 83 ± 17 | 84 ± 16 | 0.751 a |
| BMI (kg/m2) | 24.6 ± 4.5 | 27.6 ± 20.0 | 25.9 ± 5.0 | 27.1 ± 6.6 | 0.007 a | 26.2 ± 6.0 | 26.0 ± 12.4 | 26.3 ± 5.9 | 26.6 ± 16.3 | 0.957 a |
| AUSDRISK score | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 0.392 | 10 ± 4 | 11 ± 4 | 11 ± 4 | 12 ± 4 | <0.001 |
| Dietary intake | ||||||||||
| Energy intake (kJ/day) | 7275.2 ± 2192.3 | 6728.2 ± 2047.9 | 6997.5 ± 1912.9 | 8645.7 ± 3242.7 | <0.001 a | 6110.4 ± 2730.1 | 6751.7 ± 2356.5 | 7584.2 ± 1811.1 | 9190.6 ± 1941.0 | <0.001 a |
| Fiber density (g/kJ) | 5.1 ± 1.2 | 4.1 ± 0.9 | 3.7 ± 0.9 | 3.2 ± 0.9 | <0.001 a | 3.4 ± 1.0 | 3.9 ± 1.0 | 4.2 ± 1.1 | 4.7 ± 1.3 | <0.001 a |
| Carbohydrate (%) | 42.4 ± 10.2 | 40.7 ± 9.7 | 40.5 ± 9.4 | 41.2 ± 9.8 | 0.093 a | 40.5 ± 10.4 | 40.5 ± 8.9 | 40.7 ± 10.1 | 43.0 ± 9.5 | 0.010 a |
| Protein (%) | 17.3 ± 3.7 | 18.0 ± 4.0 | 17.9 ± 3.8 | 18.4 ± 4.1 | 0.003 a | 17.9 ± 4.3 | 17.9 ± 3.7 | 17.9 ± 3.7 | 17.8 ± 3.9 | 0.953 a |
| Total fat (%) | 38.2 ± 8.3 | 39.0 ± 8.3 | 39.6 ± 8.0 | 40.7 ± 7.4 | 0.003 a | 39.3 ± 8.2 | 39.9 ± 7.6 | 39.7 ± 8.3 | 38.6 ± 8.1 | 0.309 a |
| Saturated fat (%) | 18.3 ± 5.0 | 19.5 ± 5.6 | 20.0 ± 6.2 | 21.7 ± 7.0 | <0.001 a | 20.7 ± 7.5 | 19.7 ± 6.1 | 19.4 ± 5.4 | 19.6 ± 5.3 | 0.099 a |
| MUFA (%) | 12.3 ± 3.3 | 13.2 ± 2.8 | 13.8 ± 3.1 | 14.7 ± 3.0 | <0.001 a | 14.2 ± 3.4 | 13.7 ± 3.0 | 13.5 ± 3.2 | 12.7 ± 3.0 | <0.001 a |
| PUFA (%) | 11.4 ± 3.2 | 11.3 ± 3.0 | 10.9 ± 2.6 | 10.8 ± 2.7 | 0.026 a | 10.6 ± 2.7 | 11.2 ± 2.9 | 11.4 ± 2.8 | 11.2 ± 3.0 | 0.015 a |
| MSDPS | |||||
|---|---|---|---|---|---|
| Q1 (n = 227) | Q2 (n = 228) | Q3 (n = 225) | Q4 (n = 228) | p Value | |
| MSDPS score | 12.0 ± 2.8 | 18.0 ± 1.4 | 23.1 ± 1.5 | 30.5 ± 4.4 | <0.001 |
| Demographics | |||||
| Age (years) | 42 ± 16 | 47 ± 16 | 51 ± 15 | 53 ± 15 | <0.001 b |
| Sex (% female) | 194 (82.9) | 209 (89.3%) | 202 (88.2%) | 211 (89.4%) | 0.107 b |
| NZ European (%) | 144 (61.8%) | 180 (76.3%) | 176 (75.5%) | 185 (78.4%) | <0.001 b |
| Māori/Pacific (%) | 19 (8.2%) | 8 (3.4%) | 13 (5.6%) | 12 (5.1%) | 0.156 b |
| Higher Education (% yes) | 188 (83) | 196 (86) | 200 (89) | 201 (88) | 0.230 b |
| Medical history | |||||
| Weight (kg) | 71 ± 18 | 71 ± 15 | 73 ± 18 | 73 ± 14 | 0.869 a |
| Waist circumference (cm) | 81 ± 19.0 | 83 ± 17 | 85 ± 16 | 84 ± 15 | 0.675 a |
| BMI (kg/m2) | 25.6 ± 6.20 | 25.6 ± 5.5 | 26.9 ± 12.8 | 27.0 ± 16.1 | 0.780 a |
| AUSDRISK score | 10 ± 4 | 11 ± 4 | 12 ± 4 | 12 ± 4 | <0.001 a |
| Dietary intake | |||||
| Energy intake (kJ/day) | 7847.0 ± 3333.9 | 7704.0 ± 2331.0 | 7039.0 ± 2216.4 | 7057.4 ± 1836.1 | 0.001 a |
| Fiber density (g/kJ) | 4.027 ± 1.518 | 3.964 ± 1.135 | 3.899 ± 1.012 | 4.271 ± 1.025 | 0.008 a |
| Carbohydrate (%) | 40.1 ± 11.0 | 41.2 ± 9.1 | 41.2 ± 9.3 | 42.2 ± 9.5 | 0.013 a |
| Protein (%) | 16.6 ± 4.0 | 17.4 ± 3.9 | 18.4 ± 3.6 | 19.1 ± 3.6 | <0.001 a |
| Total fat (%) | 40.3 ± 9.0 | 39.8 ± 7.6 | 40.1 ± 7.7 | 37.3 ± 7.4 | <0.001 a |
| Saturated fat (%) | 19.1 ± 7.9 | 20.1 ± 5.6 | 20.6 ± 19.6 | 19.6 ± 4.9 | 0.107 a |
| MUFA (%) | 13.6 ± 3.6 | 13.7 ± 3.2 | 13.8 ± 2.9 | 13.0 ± 2.8 | 0.007 a |
| PUFA (%) | 12.0 ± 3.5 | 11.3 ± 2.6 | 10.9 ± 2.5 | 10.2 ± 2.5 | <0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovell, A.L.; Roy, R.; Klein, A.; Cavadino, A.; Foster, M.; Krebs, J.D.; Braakhuis, A.; Merry, T.L. Habitual Dietary Patterns, Nutrient Intakes, and Adherence to the Mediterranean Diet among New Zealand Adults: The NZ MED Cross-Sectional Study. Nutrients 2023, 15, 2663. https://doi.org/10.3390/nu15122663
Lovell AL, Roy R, Klein A, Cavadino A, Foster M, Krebs JD, Braakhuis A, Merry TL. Habitual Dietary Patterns, Nutrient Intakes, and Adherence to the Mediterranean Diet among New Zealand Adults: The NZ MED Cross-Sectional Study. Nutrients. 2023; 15(12):2663. https://doi.org/10.3390/nu15122663
Chicago/Turabian StyleLovell, Amy L., Rajshri Roy, Alana Klein, Alana Cavadino, Meika Foster, Jeremy D. Krebs, Andrea Braakhuis, and Troy L. Merry. 2023. "Habitual Dietary Patterns, Nutrient Intakes, and Adherence to the Mediterranean Diet among New Zealand Adults: The NZ MED Cross-Sectional Study" Nutrients 15, no. 12: 2663. https://doi.org/10.3390/nu15122663
APA StyleLovell, A. L., Roy, R., Klein, A., Cavadino, A., Foster, M., Krebs, J. D., Braakhuis, A., & Merry, T. L. (2023). Habitual Dietary Patterns, Nutrient Intakes, and Adherence to the Mediterranean Diet among New Zealand Adults: The NZ MED Cross-Sectional Study. Nutrients, 15(12), 2663. https://doi.org/10.3390/nu15122663







