Serum Essential Elements and Survival after Cancer Diagnosis
Abstract
1. Introduction
1.1. Selenium
1.2. Zinc
1.3. Copper
2. Materials and Methods
2.1. Study Group
2.2. Sample Collection, and Storage and Measurement of Elements
2.3. Statistical Analysis
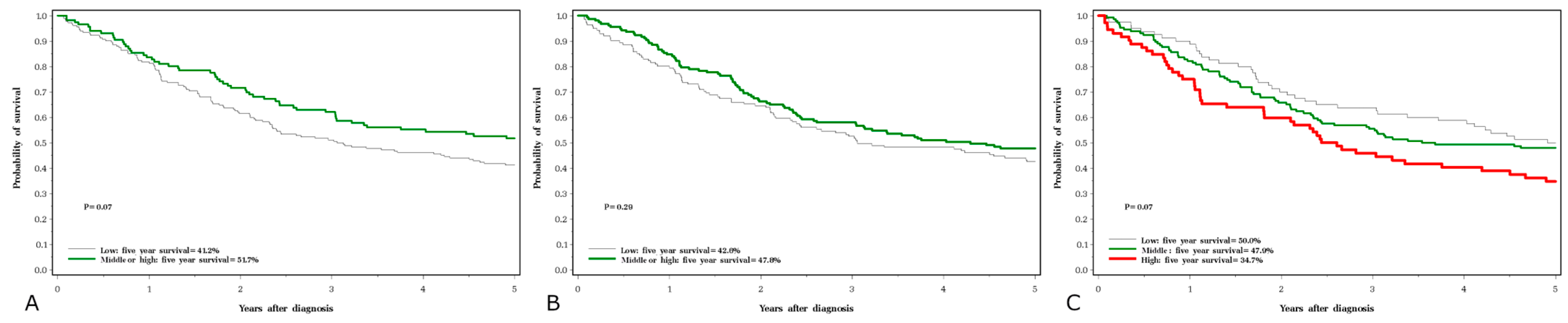
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vella, V.; Malaguarnera, R.; Lappano, R.; Maggiolini, M.; Belfiore, A. Recent views of heavy metals as possible risk factors and potential preventive and therapeutic agents in prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 57–72. [Google Scholar] [CrossRef]
- Feunteun, J.; Ostyn, P.; Delaloge, S. Tumor cell malignancy: A complex trait built through reciprocal interactions between tumors and tissue-body system. iScience 2022, 25, 104217. [Google Scholar] [CrossRef] [PubMed]
- de Jong, V.M.; Wang, Y.; ter Hoeve, N.D.; Opdam, M.; Stathonikos, N.; Jóźwiak, K.; Hauptmann, M.; Cornelissen, S.; Vreuls, W.; Rosenberg, E.H.; et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J. Clin. Oncol. 2022, 40, 2361–2374. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, 6536. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Kotsopoulos, J.; Cheung, M.C.; Rosella, L.; Brooks, J.D.; Lipscombe, L.; Akbari, M.R.; Austin, P.C.; Narod, S.A. Analysis of Platelet Count and New Cancer Diagnosis Over a 10-Year Period. JAMA Netw. Open 2022, 5, e2141633. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Jaworska-Bieniek, K.; Sukiennicki, G.; Durda, K.; Gromowski, T.; Prajzendanc, K.; et al. Association of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish population. PLoS ONE 2018, 13, e0201065. [Google Scholar] [CrossRef]
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef]
- Baszuk, P.; Marciniak, W.; Derkacz, R.; Jakubowska, A.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Huzarski, T.; Białkowska, K.; Pietrzak, S.; et al. Blood Copper Levels and the Occurrence of Colorectal Cancer in Poland. Biomedicines 2021, 9, 1628. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the selenium level on overall survival in lung cancer. J. Trace Elem. Med. Biol. 2019, 56, 46–51. [Google Scholar] [CrossRef]
- Habib, F.; Dembinski, T.; Stitch, S. The zinc and copper content of blood leucocytes and plasma from patients with benign and malignant prostates. Clin. Chim. Acta 1980, 104, 329–335. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper Signaling Axis as a Target for Prostate Cancer Therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Combs, G.F.; Clark, L.C.; Turnbull, B.W. An analysis of cancer prevention by selenium. Biofactors 2001, 14, 153–159. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, M.; Sun, H.; Yang, J.-C.; Huang, Y.-X.; Huang, J.-Q.; Lei, X.; Sun, L.-H. Selenium deficiency-induced multiple tissue damage with dysregulation of immune and redox homeostasis in broiler chicks under heat stress. Sci. China Life Sci. 2023. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Liu, M.; Niu, Q.-J.; Huang, Y.-X.; Zhao, L.; Lei, X.G.; Sun, L.-H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3247–3255. [Google Scholar]
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit. Rev. Food Sci. Nutr. 2020, 60, 684–694. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Mejia, S.B.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Van Blarigan, E.L.; Dupre, N.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Selenium Supplementation and Prostate Cancer Mortality. J. Natl. Cancer Inst. 2014, 107, dju360. [Google Scholar] [CrossRef]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef]
- Psathakis, D.; Wedemeyer, N.; Oevermann, E.; Krug, F.; Siegers, C.-P.; Bruch, H.-P. Blood selenium and glutathione peroxidase status in patients with colorectal cancer. Dis. Colon Rectum 1998, 41, 328–335. [Google Scholar] [CrossRef]
- Rogoża-Janiszewska, E.; Malińska, K.; Baszuk, P.; Marciniak, W.; Derkacz, R.; Lener, M.; Jakubowska, A.; Cybulski, C.; Huzarski, T.; Masojć, B.; et al. Serum Selenium Level and 10-Year Survival after Melanoma. Biomedicines 2021, 9, 991. [Google Scholar] [CrossRef]
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum selenium levels and the risk of progression of laryngeal cancer. PLoS ONE 2018, 13, e0184873. [Google Scholar] [CrossRef]
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865. [Google Scholar] [CrossRef]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P Status Correlates to Cancer-Specific Mortality in Renal Cancer Patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef]
- To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef]
- Zaichick, V.Y.; Sviridova, T.V.; Zaichick, S.V. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef]
- El-Ahmady, O.; El-Maraghy, A.; Ibrahim, A.; Ramzy, S. Serum copper, zinc, and iron in patients with malignant and benign pulmonary diseases. Nutrition 1995, 11, 498–501. [Google Scholar] [PubMed]
- Pasha, Q.; Malik, S.A.; Shah, M.H. Statistical analysis of trace metals in the plasma of cancer patients versus controls. J. Hazard. Mater. 2008, 153, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Jyoti; Singh, S.; Mehrotra, P.; Singh, K.; Sarangi, R. Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions: An Indian study. Environ. Int. 2006, 32, 630–637. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Element Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary Iron, Zinc, and Calcium and the Risk of Lung Cancer. Epidemiology 2005, 16, 772–779. [Google Scholar] [CrossRef]
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A prospective study of intakes of zinc and heme iron and colorectal cancer risk in men and women. Cancer Causes Control 2011, 22, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.M.; Kasperzyk, J.L.; Andrén, O.; Giovannucci, E.L.; Wolk, A.; Håkansson, N.; Andersson, S.-O.; Johansson, J.-E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a Swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Lossow, K.; Schwarz, M.; Kipp, A.P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol. 2021, 42, 101900. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Asp. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim. Et Biophys. Acta (BBA)—Lipids Lipid Metab. 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.; Tew, K. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka-Słowińska, K.; Prescha, A.; Płaczkowska, S.; Porębska, I.; Kosacka, M.; Pawełczyk, K. Serum and Whole Blood Cu and Zn Status in Predicting Mortality in Lung Cancer Patients. Nutrients 2020, 13, 60. [Google Scholar] [CrossRef]
- Fang, A.; Chen, P.; Wang, X.; Liu, Z.; Zhang, D.; Luo, Y.; Liao, G.; Long, J.; Zhong, R.; Zhou, Z.; et al. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef]
- Jones, D.R.; Jarrett, J.M.; Tevis, D.S.; Franklin, M.; Mullinix, N.J.; Wallon, K.L.; Quarles, C.D.; Caldwell, K.L.; Jones, R.L. Analysis of whole human blood for Pb, Cd, Hg, Se, and Mn by ICP-DRC-MS for biomonitoring and acute exposures. Talanta 2017, 162, 114–122. [Google Scholar] [CrossRef]
- Clases, D.; de Vega, R.G. Facets of ICP-MS and their potential in the medical sciences—Part 1: Fundamentals, stand-alone and hyphenated techniques. Anal. Bioanal. Chem. 2022, 414, 7337–7361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, F.; Liu, W.; Liu, C.; You, J.; Tian, M.; Cao, T.; Jiang, J.; Yang, Z.; Wu, H.; et al. A simple, rapid method for simultaneous determination of multiple elements in serum by using an ICP-MS equipped with collision cell. BMC Chem. 2023, 17, 34. [Google Scholar] [CrossRef]
- Jaworska, K.; Gupta, S.; Durda, K.; Muszyńska, M.; Sukiennicki, G.; Jaworowska, E.; Grodzki, T.; Sulikowski, M.; Woloszczyk, P.; Wójcik, J.; et al. A Low Selenium Level Is Associated with Lung and Laryngeal Cancers. PLoS ONE 2013, 8, e59051. [Google Scholar] [CrossRef]
- Giamougiannis, P.; Martin-Hirsch, P.L.; Martin, F.L. The evolving role of MUC16 (CA125) in the transformation of ovarian cells and the progression of neoplasia. Carcinogen 2021, 42, 327–343. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef] [PubMed]
- Dall’era, M. Liquid biomarkers in active surveillance. World J. Urol. 2022, 40, 21–26. [Google Scholar] [CrossRef]
- Augustine, T.N. The aegis: Platelets as biomarkers of tumor progression. Biomark. Med. 2020, 14, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Campos-Carrillo, A.; Weitzel, J.N.; Sahoo, P.; Rockne, R.; Mokhnatkin, J.V.; Murtaza, M.; Gray, S.W.; Goetz, L.; Goel, A.; Schork, N.; et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol. Ther. 2020, 207, 107458. [Google Scholar] [CrossRef] [PubMed]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021, 47, 102145. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Selenius, M.; Rundlöf, A.-K.; Olm, E.; Fernandes, A.P.; Björnstedt, M. Selenium and the Selenoprotein Thioredoxin Reductase in the Prevention, Treatment and Diagnostics of Cancer. Antioxid. Redox Signal. 2010, 12, 867–880. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.J.; Snell, D.C.; Kucuk, O. Zinc in Cancer Prevention. Nutr. Cancer 2009, 61, 879–887. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]



| Cancer Site | ||||
|---|---|---|---|---|
| Breast n = 531 |
Prostate n = 347 |
Lung n = 298 |
Larynx n = 299 | |
| Age of diagnosis | ||||
| Years (mean, range) | 57.2 (26–84) | 66.0 (41–84) | 64.1 (43–84) | 61.0 (40–81) |
| Sex | ||||
| Male | 0 | 347 (100%) | 193 (65%) | 254 (85%) |
| Female | 531 (100%) | 0 | 105 (35%) | 45 (15%) |
|
Year of diagnosis (mean, range) | 2011 (2009–2016) | 2013 (2010–2015) | 2011 (2010–2012) | 2013 (2010–2018) |
| Selenium level (µg/L) (mean, range) |
86 (52–172) |
78 (42–138) |
62 (17–108) |
58 (21–105) |
| Zinc level (µg/L) (mean, range) |
867 (525–11045) |
847 (516–1340) |
718 (350–1071) |
640 (358–1318) |
| Copper level (µg/L) (mean, range) |
1153 (685–2153) |
1093 (460–2197) |
1146 (671–2866) |
1116 (436–2795) |
| Years of follow-up (mean) | 9.4 | 7.6 | 6.0 | 6.8 |
| 5- year survival | 85.9% | 83.3% | 45.3% | 63.2% |
| Men (n = 794) | Women (n = 681) | |||||
|---|---|---|---|---|---|---|
| Age of Diagnosis Frequency M/F | Selenium (µg/L) Mean, Range | Zinc (µg/L) Mean, Range | Copper (µg/L) Mean, Range | Selenium (µg/L) Mean, Range | Zinc (µg/L) Mean, Range | Copper (µg/L) Mean, Range |
| <50 20/139 | 1213 (839–1545) | 665 (377–884) | 61 (31–92) | 1150 (500–2049) | 840 (525–1254) | 83 (40–124) |
| 50–60 221/227 | 1124 (643–1391) | 729 (358–1244) | 65 (21–138) | 1158 (632–2151) | 867 (455–11045) | 83 (35–172) |
| 60–70 376/213 | 1090 (436–2197) | 761 (359–1340) | 69 (25–109) | 1172 (658–2866) | 810 (350–1389) | 80 (33–123) |
| 70+ 177/102 | 1092 (460–1842) | 745 (366–1190) | 67 (17–106) | 1161 (710–1910) | 794 (553–1128) | 76 (36–121) |
| Men (n = 794) | Women (n = 681) | |||||
|---|---|---|---|---|---|---|
| Cutoff Level | Selenium (µg/L) | Zinc (µg/L) | Copper (µg/L) | Selenium (µg/L) | Zinc (µg/L) | Copper (µg/L) |
| 25 percentile | 56 | 636 | 944 | 71 | 727 | 1011 |
| 75 percentile | 78 | 850 | 1230 | 93 | 899 | 1260 |
| Selenium | Zinc | Copper | |
|---|---|---|---|
| Selenium | - | ||
| Zinc | 0.195 p < 0.0001 | - | |
| Copper | 0.059 p = 0.13 | 0.087 p = 0.02 | - |
| Total /Deaths | Age-Adjusted Only | Adjusted | |
|---|---|---|---|
| HR (95%CI) p-Value | HR (95%CI) p-Value | ||
| Selenium | |||
| Low | 484/250 | 2.18 (1.81–2.62) < 0.0001 | 1.61 (1.28–2.02) < 0.0001 |
| Middle | 702/206 | 1.0 | 1.0 |
| High | 289/58 | 0.66 (0.49–0.88) 0.005 | 0.71 (0.52–0.96) 0.03 |
| Zinc | |||
| Low | 473/224 | 1.68 (1.40–2.01) < 0.0001 | 1.19 (0.97–1.47) 0.10 |
| Middle | 737/240 | 1.0 | 1.0 |
| High | 265/50 | 0.55 (0.41–0.75) 0.0001 | 0.72 (0.52–0.99) 0.04 |
| Copper | |||
| Low | 406/115 | 0.86 (0.69–1.33) 0.19 | 0.81 (0.64–1.01) 0.07 |
| Middle | 746/239 | 1.0 | 1.0 |
| High | 323/160 | 1.91 (1.56–2.08) < 0.0001 | 1.72 (1.41–2.11) < 0.0001 |
| Current Smoker | |||
| No | 641/183 | 1 | 1 |
| Yes | 676/278 | 1.77 (1.46–2.13) < 0.0001 | 0.97 (0.76–1.25) 0.83 |
| Missing | 158/53 | 1.11 (0.81–1.51) 0.51 | 1.46 (0.99–2.15) 0.06 |
| Sex | |||
| Female | 681/214 | 1 | 1 |
| Male | 794/300 | 1.28 (1.06–1.53) 0.009 | 1.21 (0.91–1.60) 0.18 |
| Total /Deaths | Age-Adjusted Only | Adjusted | |
|---|---|---|---|
| HR (95%CI) p-Value | HR (95%CI) p-Value | ||
| Selenium | |||
| Low | 182/109 | 1.34 (0.97–1.85) 0.08 | 1.16 (0.81–1.66) 0.43 |
| Middle | 111/55 | 1.0 | 1.0 |
| High | 5/2 | 0.68 (0.17–2.80) 0.59 | 1.14 (0.27–4.85) 0.86 |
| Zinc | |||
| Low | 141/83 | 1.25 (0.91–1.71) 0.17 | 1.17 (0.83–1.65) 0.37 |
| Middle | 145/74 | 1.0 | 1.0 |
| High | 12/9 | 1.73 (0.86–3.45) 0.12 | 1.33 (0.62–2.87) 0.47 |
| Copper | |||
| Low | 80/41 | 0.88 (0.61–1.29) 0.52 | 0.97 (0.65–1.46) 0.88 |
| Middle | 146/78 | 1.0 | 1.0 |
| High | 72/47 | 1.39 (0.97–2.01) 0.07 | 1.19 (0.82–1.73) 0.36 |
| Total /Deaths | Age-Adjusted Only | Adjusted | |
|---|---|---|---|
| HR (95%CI) p-Value | HR (95%CI) p-Value | ||
| Selenium | |||
| Low | 218/96 | 2.61 (1.51–4.50) 0.0006 | 2.19 (1.23–3.91) 0.008 |
| Middle | 72/15 | 1.0 | 1.0 |
| High | 9/5 | 3.32 (1.20–9.18) 0.002 | 2.10 (0.59–7.50) 0.26 |
| Zinc | |||
| Low | 212/87 | 1.46 (0.94–2.26) 0.09 | 1.03 (0.65–1.65) 0.89 |
| Middle | 82/26 | 1.0 | 1.0 |
| High | 5/3 | 2.35 (0.71–7.79) 0.16 | 1.46 (0.31–6.86) 0.63 |
| Copper | |||
| Low | 109/32 | 0.71 (0.45–1.12) 0.14 | 0.95 (0.59–1.53) 0.83 |
| Middle | 114/44 | 1.0 | 1.0 |
| High | 76/40 | 1.64 (1.06–2.52) 0.003 | 1.15 (0.74–1.79) 0.54 |
| Total /Deaths | Age-Adjusted Only | Adjusted | |
|---|---|---|---|
| HR (95%CI) p-Value | HR (95%CI) p-Value | ||
| Selenium | |||
| Low | 33/21 | 2.43 (1.49–3.97) 0.0004 | 2.55 (1.52–4.28) 0.0004 |
| Middle | 288/82 | 1.0 | 1.0 |
| High | 210/40 | 0.64 (0.44–0.94) 0.02 | 0.69 (0.46–1.03) 0.07 |
| Zinc | |||
| Low | 67/29 | 1.52 (0.99–2.34) 0.05 | 1.37 (0.87–2.18) 0.18 |
| Middle | 320/89 | 1.0 | 1.0 |
| High | 144/25 | 0.58 (0.37–0.90) 0.02 | 0.72 (0.46–1.14) 0.16 |
| Copper | |||
| Low | 103/21 | 0.83 (0.51–1.34) 0.44 | 0.87 (0.52–1.44) 0.59 |
| Middle | 306/78 | 1.0 | 1.0 |
| High | 122/44 | 1.70 (1.17–2.46) 0.005 | 1.55 (1.05–2.29) 0.03 |
| Total /Deaths | Age-Adjusted Only | Adjusted | |
|---|---|---|---|
| HR (95%CI) p-Value | HR (95%CI) p-Value | ||
| Selenium | |||
| Low | 51/24 | 1.91 (1.16–3.14) 0.01 | 1.57 (0.94–2.65) 0.09 |
| Middle | 231/54 | 1.0 | 1.0 |
| High | 65/11 | 0.84 (0.44–1.61) 0.59 | 1.02 (0.51–2.02) 0.96 |
| Zinc | |||
| Low | 53/25 | 1.52 (0.93–2.48) 0.10 | 1.30 (0.77–2.19) 0.33 |
| Middle | 190/51 | 1.0 | 1.0 |
| High | 104/13 | 0.50 (0.27–0.93) 0.03 | 0.51 (0.27–0.96) 0.04 |
| Copper | |||
| Low | 114/21 | 0.84 (0.49–1.42) 0.51 | 0.93 (0.54–1.59) 0.78 |
| Middle | 180/39 | 1.0 | 1.0 |
| High | 53/29 | 2.73 (1.67–4.44) <0.0001 | 2.71 (1.64–4.48) 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubiński, J.; Lener, M.R.; Marciniak, W.; Pietrzak, S.; Derkacz, R.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Jakubowska, A.; Huzarski, T.; et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients 2023, 15, 2611. https://doi.org/10.3390/nu15112611
Lubiński J, Lener MR, Marciniak W, Pietrzak S, Derkacz R, Cybulski C, Gronwald J, Dębniak T, Jakubowska A, Huzarski T, et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients. 2023; 15(11):2611. https://doi.org/10.3390/nu15112611
Chicago/Turabian StyleLubiński, Jan, Marcin R. Lener, Wojciech Marciniak, Sandra Pietrzak, Róża Derkacz, Cezary Cybulski, Jacek Gronwald, Tadeusz Dębniak, Anna Jakubowska, Tomasz Huzarski, and et al. 2023. "Serum Essential Elements and Survival after Cancer Diagnosis" Nutrients 15, no. 11: 2611. https://doi.org/10.3390/nu15112611
APA StyleLubiński, J., Lener, M. R., Marciniak, W., Pietrzak, S., Derkacz, R., Cybulski, C., Gronwald, J., Dębniak, T., Jakubowska, A., Huzarski, T., Matuszczak, M., Pullella, K., Sun, P., & Narod, S. A. (2023). Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients, 15(11), 2611. https://doi.org/10.3390/nu15112611






