Diet in Different Calcium Oxalate Kidney Stones
Abstract
1. Introduction
2. Materials and Methods
3. Results
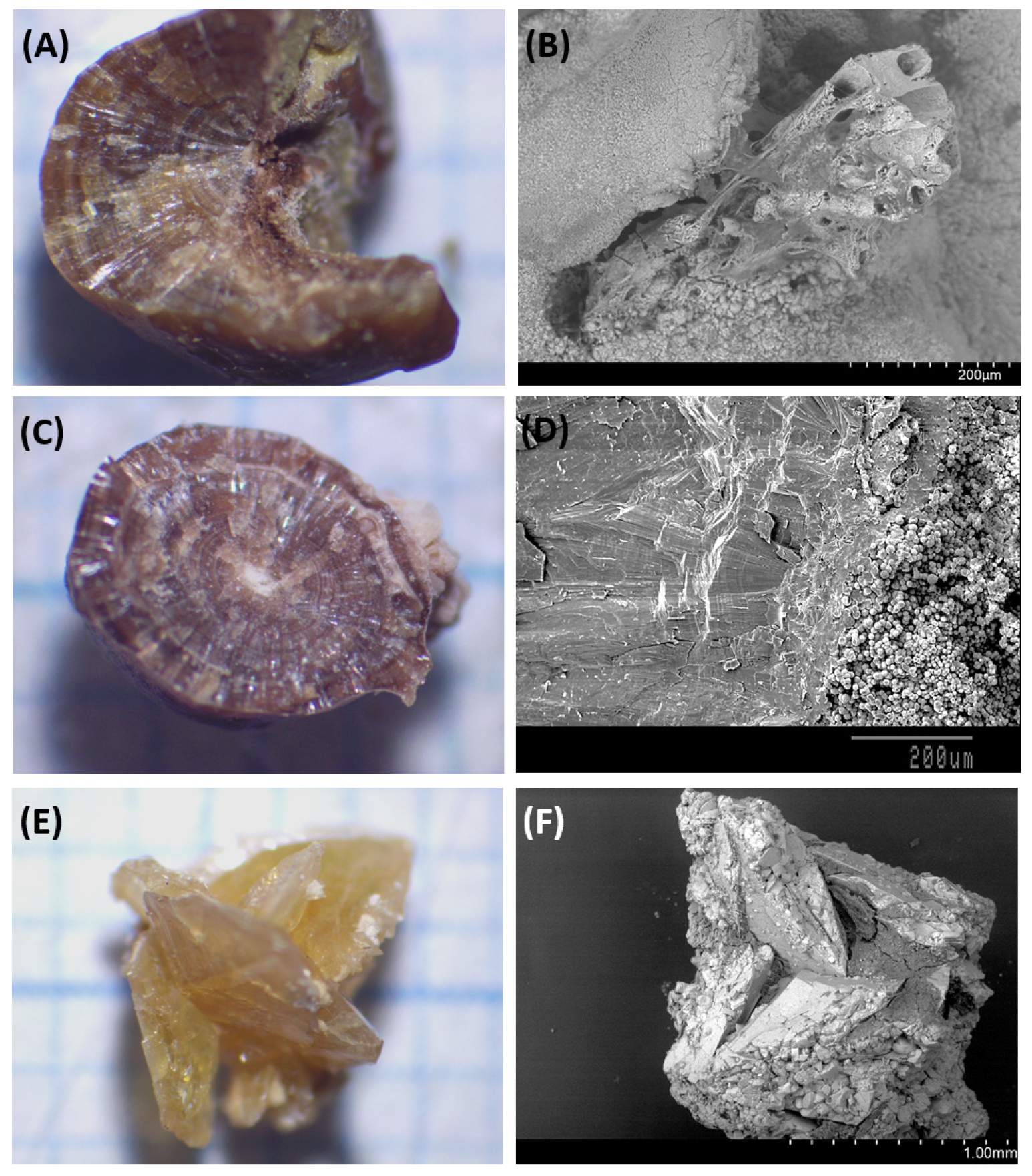
3.1. Papillary COM Stones
3.2. Non-Papillary COM Stones
3.3. COD Stones
4. Discussion
4.1. Papillary COM Stones
4.2. Non-Papillary COM Stones
4.3. COD Stones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prochaska, M.L.; Taylor, E.N.; Curhan, G.C. Insights Into Nephrolithiasis From the Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, O.; Terai, A.; Ohkawa, T.; Okada, Y. National trend of the incidence of urolithiasis in Japan from 1965 to 1995. Kidney Int. 1999, 56, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauzá, A.; Ramis, M.; Montesinos, V.; Conte, A. Simple classification of renal calculi closely related to their micromorphology and etiology. Clin. Chim. Acta 2002, 322, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Costa-Bauzá, A.; Grases, F.; Julià, F. The power of desktop scanning electron microscopy with elemental analysis for analyzing urinary stones. Urolitihiasis 2023, 51, 50. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A.; Mandressi, A.; Luongo, P.; Longo, G.; Pisani, E. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. Br. J. Urol. 1991, 67, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A.; Nespoli, R.; Ostini, F.; Rovera, F.; Zanetti, G.; Pisani, E. A study of dietary calcium and other nutrients in idiopathic renal calcium stone formers with low bone mineral content. J. Urol. 1998, 159, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Damasio, P.C.; Amaro, C.R.; Cunha, N.B.; Pichutte, A.; Goldberg, J.; Padovani, C. The role of salt abuse on risk for hypercalciuria. Nutr. J. 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-B.; Lin, M.-E.; Huang, R.-H.; Hong, Y.-K.; Lin, B.-L.; He, X.-J. Dietary and lifestyle factors for primary prevention of nephrolithiasis: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Neal, N.L.; Bradbury, K.E.; Heers, H.; Allen, N.E.; Turney, B.W. Fluid Intake and Dietary Factors and the Risk of Incident Kidney Stones in UK Biobank: A Population-based Prospective Cohort Study. Eur. Urol. Focus 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Ferraro, P.M.; Capasso, G. Calcium nephrolithiasis, metabolic syndrome and the cardiovascular risk. Nephrol. Dial. Transpl. 2012, 27, 3008–3010. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Cook, H.M.; Milne, D.B.; Gallagher, S.; Clayman, R.V. Oxalate degradation by gastrointestinal bacteria from humans. J. Nutr. 1986, 116, 455–460. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 50) | COM Papillar (n = 13) | COM Non-Papillar (n = 27) | COD (n = 50) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | (n/N)/SD | Mean | (n/N)/SD | Mean | (n/N)/SD | Mean | (n/N)/SD | |
| Male% (n/N) | 62.0% | (31/50) | 76.9% | (10/13) | 20 | 74.1% | 66.0% | (33/50) |
| Female% (n/N) | 38.0% | (19/50) | 23.1% | (3/13) | 7 | 25.9% | 34.0% | (17/50) |
| Age (years) | 51 ± 17 | 53 ± 12 | 59.3 ± 13.1 | 50 ± 12 | ||||
| BMI (kg/cm2) | 24.3 ± 4.3 | 28.7 ± 3.4 * | 27.7 ± 6.2 | 26.8 ± 5.1 | ||||
| Charslon Index | 1.8 ± 1.9 | 1.3 ± 1.4 | 2.5 ± 2.1 a | 1.2 ± 1.3 | ||||
| DM% (n/N) | 14.3% | (7/49) | 15.4% | (2/13) | 5 | 18.5% | 4.0% | (2/50) |
| HT% (n/N) | 24.5% | (12/49) | 23.1% | (3/13) | 10 | 37.0% | 36.0% | (18/50) |
| CRD% (n/N) | 4.1% | (2/49) | 7.7% | (1/13) | 2 | 7.4% | 4.0% | (2/50) |
| Family history% (n/N) | 7.5% | (3/40) | 72.7% | (8/11) * | 5 | 25.0% a | 60.5% | (26/43) * |
| Personal history% (n/N) | 0.0% | (0/47) | 53.8% | (7/13) * | 12 | 44.4% | 65.3% | (32/49) * |
| COM Papillar (n = 13) | COM Non-Papillar (n = 27) | COD (n = 50) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| pH | 5.6 ± 0.5 | 5.5 ± 0.6 | 5.8 ± 0.7 |
| Ca 2 h (mg/dL) | 13.5 ± 8.3 | 8.6 ± 5.7 | 18.0 ± 16.0 b |
| Cr 2 h (mg/dL) | 140 ± 63 | 103 ± 38 | 136 ± 70 b |
| Cit 2 h (mg/dL) | 578 ± 313 | 361 ± 161 | 410 ± 252 |
| Urate 24 h (mg/dL) | 274 ± 392 | 141 ± 213 | 204 ± 354 |
| Urate 24 h (mg/24 h) | 431 ± 358 | 453 ± 350 | 479 ± 298 |
| Fosfate 24 h (mg/dL) | 64 ± 25 | 41 ± 17 | 50 ± 24 |
| Fosfate 24 h (mg/24 h) | 910 ± 355 | 796 ± 365 | 917 ± 305 |
| Ca 24 h (mg/dL) | 12.9 ± 6.7 | 7.3 ± 3.4 | 13.6 ± 7.6 |
| Ca 24 h (mg/24 h) | 195 ± 115 | 145 ± 68 | 248 ± 111 b |
| Mg 24 h (mg/dL) | 6.3 ± 2.6 | 4.5 ± 2.2 | 5.8 ± 3.3 |
| Mg 24 h (mg/24 h) | 92 ± 43 | 93 ± 46 | 105 ± 48 |
| Cr 24 h (mg/dL) | 113 ± 43 | 71 ± 25 a | 80 ± 35 |
| Cr 24 h (mg/24 h) | 1620 ± 592 | 1387 ± 601 | 1457 ± 407 |
| Cit 24 h (mg/dL) | 485 ± 230 | 280 ± 151 a | 341 ± 354 |
| Cit 24 h (mg/24 h) | 663 ± 299 | 574 ± 426 | 625 ± 598 |
| Ox 24 h (mg/dL) | 16.2 ± 6.2 | 16.1 ± 7.3 | 16.5 ± 8.0 |
| Ox 24 h (mg/24 h) | 25.5 ± 13.8 | 31.9 ± 18.0 | 31.4 ± 14.2 |
| Diuresis 24 h (mL) | 1546 ± 551 | 2070 ± 971 | 2012 ± 630 |
| Ca/cit 2 h | 0.026 ± 0.025 | 0.033 ± 0.034 | 0.070 ± 0.075 |
| Ca/cit 24 h | 0.031 ± 0.020 | 0.035 ± 0.033 | 0.076 ± 0.113 b |
| OR Adjusted | (95% IC of OR) | p-Value | |
|---|---|---|---|
| Carbohydrates (g/d) | 0.996 | (0.987–1.006) | 0.459 |
| Protein (g/d) | 1.015 | (0.982–1.049) | 0.386 |
| Lipids (g/d) | 1.014 | (0.987–1.041) | 0.309 |
| Monounsaturated fatty acids (g/d) | 1.012 | (0.972–1.053) | 0.562 |
| Polyunsaturated fatty acids (g/d) | 0.984 | (0.867–1.117) | 0.803 |
| Saturated fatty acids (g/d) | 1.060 | (0.992–1.132) | 0.085 |
| Zinc (mg/d) | 1.044 | (0.793–1.375) | 0.760 |
| Niacin (mg/d) | 0.997 | (0.934–1.063) | 0.920 |
| Cholesterol (mg/d) | 1.003 | (0.999–1.006) | 0.131 |
| Trans fatty acids (g/d) | 9.732 | (2.071–45.732) | 0.004 * |
| Legumes (g/d) | 1.003 | (0.970–1.037) | 0.876 |
| Meat and derivatives (g/d) | 1.012 | (1.003–1.021) | 0.012 * |
| Sausages (g/d) | 1.051 | (1.004–1.099) | 0.032 * |
| Glycemic load | 0.994 | (0.977–1.012) | 0.539 |
| Omega 3 fatty acids (g/d) | 1.483 | (0.931–2.361) | 0.097 |
| Caffeine and theine (mg/d) | 1.009 | (0.994–1.025) | 0.235 |
| OR Adjusted | (95% CI of OR) | p-Value | |
|---|---|---|---|
| Calcium (mg/d) | 0.997 | (0.994–0.999) | 0.002 * |
| Iodine (mcg/d) | 0.995 | (0.991–0.999) | 0.011 * |
| Legumes (g/d) | 1.012 | (0.987–1.036) | 0.355 |
| Dairy products (g/d) | 1.002 | (0.999–1.005) | 0.192 |
| Glycemic load | 1.012 | (0.998–1.026) | 0.095 |
| Caffeine and theine (mg/d) | 1.013 | (1.000–1.026) | 0.044 * |
| OR Adjusted | (95% CI of OR) | p-Value | |
|---|---|---|---|
| Legumes (g/d) | 1.022 | (0.999–1.046) | 0.067 |
| Dairy products (g/d) | 1.005 | (1.002–1.008) | <0.001 * |
| Yogurt (g/d) | 0.995 | (0.989–1.002) | 0.145 |
| Linoleic acid (g/d) | 1.067 | (0.985–1.157) | 0.112 |
| Glycemic load | 1.004 | (0.992–1.015) | 0.536 |
| Betacarotene (mcg/d) | 0.999 | (1.000–1.000) | 0.037 * |
| Fytate (mg/d) | 1.000 | (1.000–1.001) | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coello, I.; Sanchis, P.; Pieras, E.C.; Grases, F. Diet in Different Calcium Oxalate Kidney Stones. Nutrients 2023, 15, 2607. https://doi.org/10.3390/nu15112607
Coello I, Sanchis P, Pieras EC, Grases F. Diet in Different Calcium Oxalate Kidney Stones. Nutrients. 2023; 15(11):2607. https://doi.org/10.3390/nu15112607
Chicago/Turabian StyleCoello, Iris, Pilar Sanchis, Enrique C. Pieras, and Felix Grases. 2023. "Diet in Different Calcium Oxalate Kidney Stones" Nutrients 15, no. 11: 2607. https://doi.org/10.3390/nu15112607
APA StyleCoello, I., Sanchis, P., Pieras, E. C., & Grases, F. (2023). Diet in Different Calcium Oxalate Kidney Stones. Nutrients, 15(11), 2607. https://doi.org/10.3390/nu15112607






