Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology in Adolescent Females
Abstract
1. Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology
2. Materials and Methods
2.1. Participants and Procedures
2.2. Experimental Procedure
2.3. Non-fMRI Measures
2.4. Statistical Methods
2.5. Data Availability
3. Results
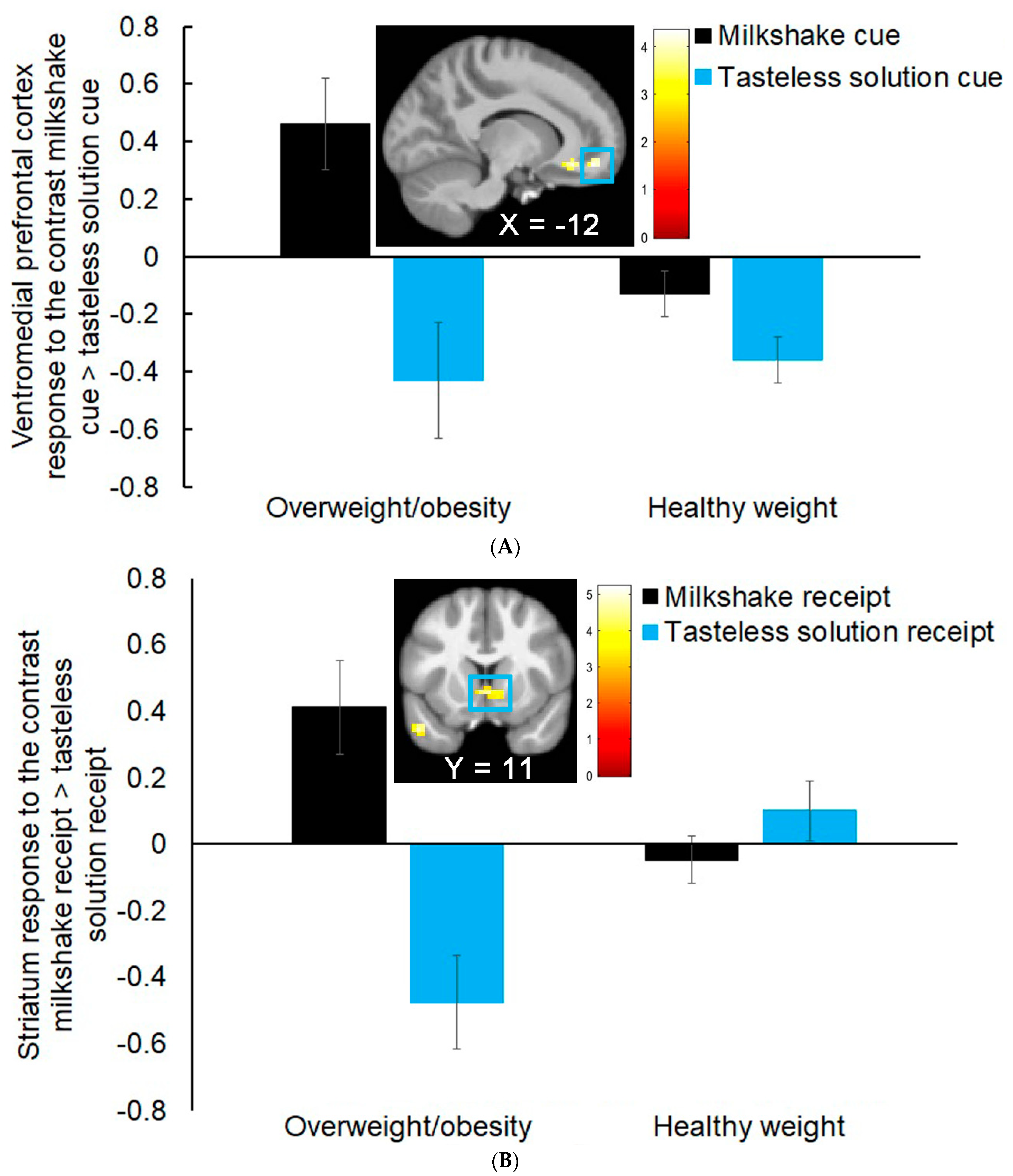
3.1. Group Differences in Neural Response to Milkshake Cues and Milkshake Receipt
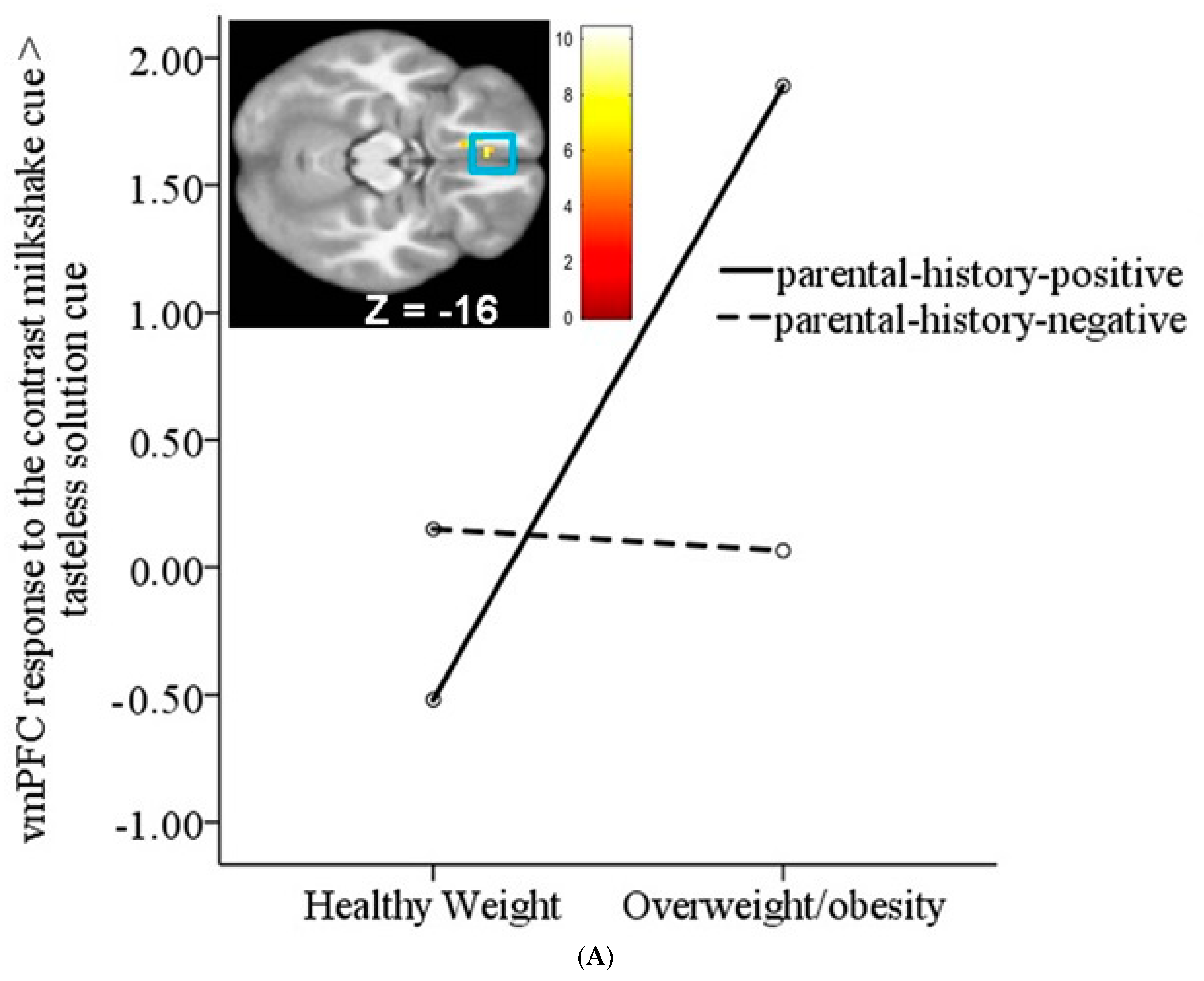
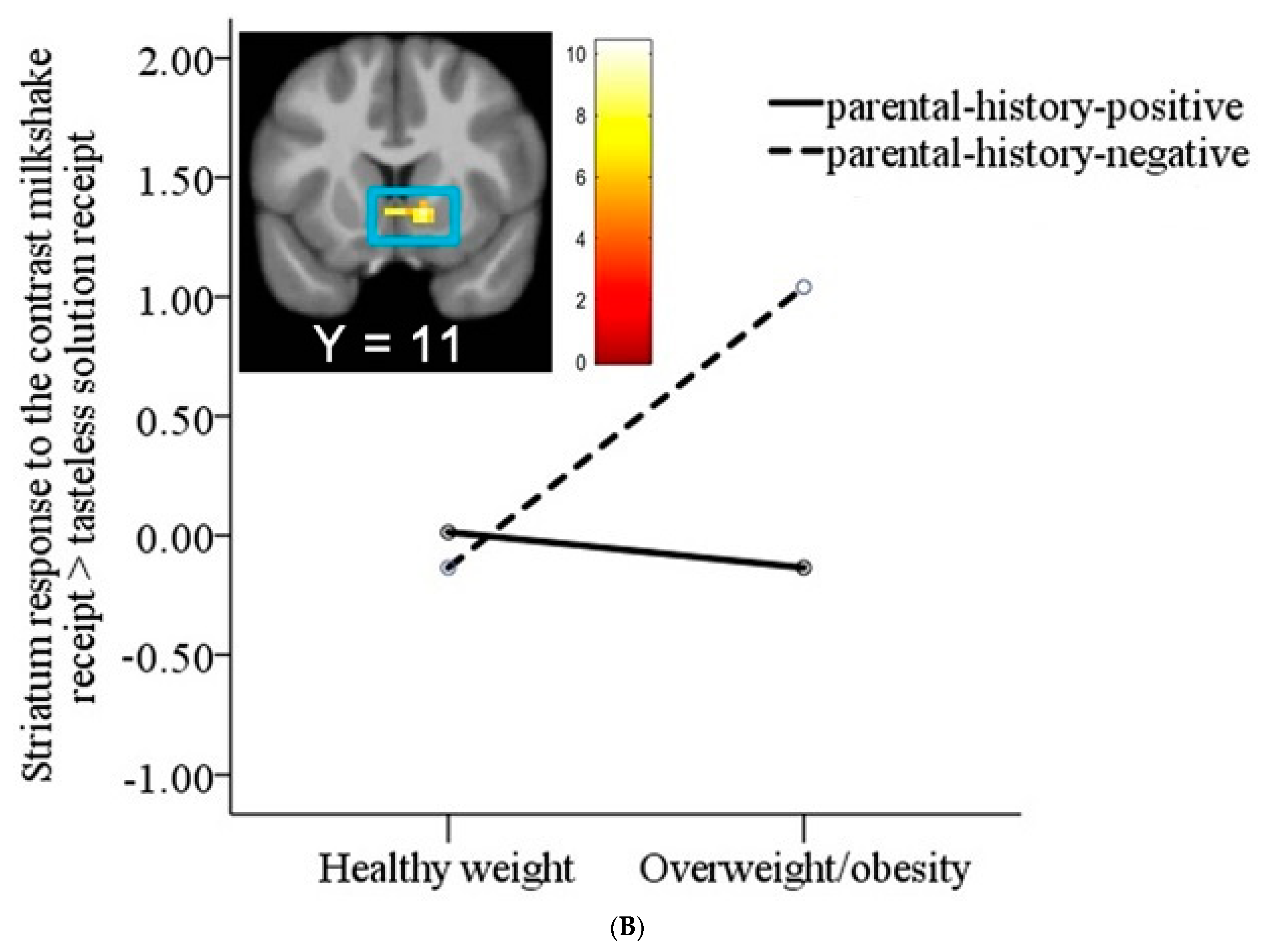
3.2. Interaction between Weight Status and Risk Status on Neural Response to Milkshake Cues and Milkshake Receipt
3.3. Associations between Sensory and Hedonic Ratings and Neural Response to Milkshake Cues and Milkshake Receipt
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Word Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/nes-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 9 June 2021).
- Hartmann-Boyce, J.; Johns, D.J.; Jebb, S.A.; Summerbell, C.; Aveyard, P.; Behavioural Weight Management Review Group. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: Systematic review and meta-analysis. Obes. Rev. 2014, 15, 920–932. [Google Scholar] [CrossRef]
- Plotnikoff, R.C.; Costigan, S.A.; Williams, R.L.; Hutchesson, M.J.; Kennedy, S.G.; Robards, S.L.; Allen, J.; Collins, C.E.; Callister, R.; Germov, J. Effectiveness of interventions targeting physical activity, nutrition and healthy weight for university and college students: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L.; O’Doherty, J.; Rolls, E.T.; Andrews, C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex. 2003, 13, 1064–1071. [Google Scholar] [CrossRef]
- Small, D.M.; Jones-Gotman, M.; Dagher, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K.S.; Yokum, S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 2013, 98, 1377–1384. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Burger, K.S.; Epstein, L.H.; Small, D.M. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 2011, 31, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.S.; Holsen, L.M.; Chambers, R.J.; Martin, L.E.; Brooks, W.M.; Zarcone, J.R.; Butler, M.G.; Savage, C.R. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int. J. Obes. 2010, 34, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Roitzsch, C.; Horstmann, A.; Possel, M.; Hummel, T. Increased Brain Reward Responsivity to Food-Related Odors in Obesity. Obesity 2021, 29, 1138–1145. [Google Scholar] [CrossRef]
- Martin, L.E.; Holsen, L.M.; Chambers, R.J.; Bruce, A.S.; Brooks, W.M.; Zarcone, J.R.; Butler, M.G.; Savage, C.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010, 18, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924–935. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Weller, R.E.; Cook, E.W., 3rd; Twieg, D.B.; Knowlton, R.C.; Cox, J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008, 41, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Babbs, R.K.; Sun, X.; Felsted, J.; Chouinard-Decorte, F.; Veldhuizen, M.G.; Small, D.M. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav. 2013, 121, 103–111. [Google Scholar] [CrossRef]
- Chen, E.Y.; Zeffiro, T.A. Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int. J. Obes. 2020, 44, 1636–1652. [Google Scholar] [CrossRef]
- Nelson, T.D.; Brock, R.L.; Yokum, S.; Tomaso, C.C.; Savage, C.R.; Stice, E. Much Ado About Missingness: A Demonstration of Full Information Maximum Likelihood Estimation to Address Missingness in Functional Magnetic Resonance Imaging Data. Front. Neurosci. 2021, 15, 746424. [Google Scholar] [CrossRef]
- Davids, S.; Lauffer, H.; Thoms, K.; Jagdhuhn, M.; Hirschfeld, H.; Domin, M.; Hamm, A.; Lotze, M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int. J. Obes. 2010, 34, 94–104. [Google Scholar] [CrossRef]
- Jacobson, A.; Green, E.; Haase, L.; Szajer, J.; Murphy, C. Differential Effects of BMI on Brain Response to Odor in Olfactory, Reward and Memory Regions: Evidence from fMRI. Nutrients 2019, 11, 926. [Google Scholar] [CrossRef]
- Pimpini, L.; Kochs, S.; Franssen, S.; van den Hurk, J.; Valente, G.; Roebroeck, A.; Jansen, A.; Roefs, A. More complex than you might think: Neural representations of food reward value in obesity. Appetite 2022, 178, 106164. [Google Scholar] [CrossRef] [PubMed]
- Bohon, C. Brain response to taste in overweight children: A pilot feasibility study. PLoS ONE 2017, 12, e0172604. [Google Scholar] [CrossRef]
- Boutelle, K.N.; Wierenga, C.E.; Bischoff-Grethe, A.; Melrose, A.J.; Grenesko-Stevens, E.; Paulus, M.P.; Kaye, W.H. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int. J. Obes. 2015, 39, 620–628. [Google Scholar] [CrossRef]
- Doornweerd, S.; De Geus, E.J.; Barkhof, F.; Van Bloemendaal, L.; Boomsma, D.I.; Van Dongen, J.; Drent, M.L.; Willemsen, G.; Veltman, D.J.; RG, I.J. Brain reward responses to food stimuli among female monozygotic twins discordant for BMI. Brain Imaging Behav. 2018, 12, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.M.; Dagher, A.; Hare, T.A.; Kullmann, S.; van der Laan, L.N.; Poldrack, R.A.; Preissl, H.; Small, D.; Stice, E.; Veldhuizen, M.G. Good practice in food-related neuroimaging. Am. J. Clin. Nutr. 2019, 109, 491–503. [Google Scholar] [CrossRef]
- Jebeile, H.; Lister, N.B.; Baur, L.A.; Garnett, S.P.; Paxton, S.J. Eating disorder risk in adolescents with obesity. Obes. Rev. 2021, 22, e13173. [Google Scholar] [CrossRef]
- Villano, I.; Ilardi, C.R.; Arena, S.; Scuotto, C.; Gleijeses, M.G.; Messina, G.; Messina, A.; Monda, V.; Monda, M.; Iavarone, A.; et al. Obese Subjects without Eating Disorders Experience Binge Episodes Also Independently of Emotional Eating and Personality Traits among University Students of Southern Italy. Brain Sci. 2021, 11, 1145. [Google Scholar] [CrossRef]
- Rancourt, D.; McCullough, M.B. Overlap in Eating Disorders and Obesity in Adolescence. Curr. Diab. Rep. 2015, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Schienle, A.; Schafer, A.; Hermann, A.; Vaitl, D. Binge-eating disorder: Reward sensitivity and brain activation to images of food. Biol. Psychiatry 2009, 65, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Skunde, M.; Walther, S.; Bendszus, M.; Herzog, W.; Friederich, H.C. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect Neurosci. 2016, 11, 1393–1401. [Google Scholar] [CrossRef]
- Uher, R.; Murphy, T.; Brammer, M.J.; Dalgleish, T.; Phillips, M.L.; Ng, V.W.; Andrew, C.M.; Williams, S.C.; Campbell, I.C.; Treasure, J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am. J. Psychiatry 2004, 161, 1238–1246. [Google Scholar] [CrossRef]
- Goldschmidt, A.B.; Dickstein, D.P.; MacNamara, A.E.; Phan, K.L.; O’Brien, S.; Le Grange, D.; Fisher, J.O.; Keedy, S. A Pilot Study of Neural Correlates of Loss of Control Eating in Children With Overweight/Obesity: Probing Intermittent Access to Food as a Means of Eliciting Disinhibited Eating. J. Pediatr. Psychol. 2018, 43, 846–855. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Rohde, P.; Cloud, K.; Desjardins, C.D. Comparing healthy adolescent females with and without parental history of eating pathology on neural responsivity to food and thin models and other potential risk factors. J. Abnorm. Psychol. 2021, 130, 608–619. [Google Scholar] [CrossRef]
- Galmiche, M.; Dechelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Orvaschel, H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version, 5th ed.; Nova Southeastern University, Center for Psychological Studies: Ft. Lauderdale, FL, USA, 1994. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Faith, M.S.; Allison, D.B.; Gallagher, D.; Chiumello, G.; Heymsfield, S.B. Body mass index as a measure of adiposity among children and adolescents: A validation study. J. Pediatr. 1998, 132, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.; Guo, S.; Wei, R.; Johnson, C. CDC Growth Charts: United States. Advance Data, no. 314; National Center for Health Statistics: Hyattsville, MD, USA, 2000. [Google Scholar]
- Green, B.G.; Dalton, P.; Cowart, B.; Shaffer, G.; Rankin, K.; Higgins, J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem. Senses 1996, 21, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Aiken, L.S.; West, S.G. Multiple Regression: Testing and Interpreting Interactions; Sage: Newbury Park, CA, USA, 1991. [Google Scholar]
- Bartra, O.; McGuire, J.T.; Kable, J.W. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013, 76, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex, food reward, body weight and obesity. Soc. Cogn. Affect Neurosci. 2023, 18, nsab044. [Google Scholar] [CrossRef] [PubMed]
- Hare, T.A.; Camerer, C.F.; Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009, 324, 646–648. [Google Scholar] [CrossRef]
- Koban, L.; Wager, T.D.; Kober, H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat. Neurosci. 2023, 26, 316–325. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Ng, J.; Stice, E.; Yokum, S.; Bohon, C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011, 57, 65–72. [Google Scholar] [CrossRef]
- Rothemund, Y.; Preuschhof, C.; Bohner, G.; Bauknecht, H.C.; Klingebiel, R.; Flor, H.; Klapp, B.F. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007, 37, 410–421. [Google Scholar] [CrossRef]
- Oren, S.; Tittgemeyer, M.; Rigoux, L.; Schlamann, M.; Schonberg, T.; Kuzmanovic, B. Neural encoding of food and monetary reward delivery. Neuroimage 2022, 257, 119335. [Google Scholar] [CrossRef]
- Appelhans, B.M. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obesity 2009, 17, 640–647. [Google Scholar] [CrossRef]
- Small, D.M.; Zatorre, R.J.; Dagher, A.; Evans, A.C.; Jones-Gotman, M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 2001, 124, 1720–1733. [Google Scholar] [CrossRef]
- Charbonnier, L.; van Meer, F.; Johnstone, A.M.; Crabtree, D.; Buosi, W.; Manios, Y.; Androutsos, O.; Giannopoulou, A.; Viergever, M.A.; Smeets, P.A.M.; et al. Effects of hunger state on the brain responses to food cues across the life span. Neuroimage 2018, 171, 246–255. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Prechtl de Hernandez, C.G.; Beaver, J.D.; Muhammed, K.; Croese, C.; Bell, G.; Durighel, G.; Hughes, E.; Waldman, A.D.; Frost, G.; et al. Fasting biases brain reward systems towards high-calorie foods. Eur. J. Neurosci. 2009, 30, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Dimitropoulos, A. Influence of feeding state on neurofunctional differences between individuals who are obese and normal weight: A meta-analysis of neuroimaging studies. Appetite 2014, 75, 103–109. [Google Scholar] [CrossRef]
- Horstmann, A.; Fenske, W.K.; Hankir, M.K. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes. Rev. 2015, 16, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child 2012, 97, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef]
| Overweight/Obesity | Healthy Weight | Test Statistic | P-Value | |
|---|---|---|---|---|
| Age [Mean ± SD] | 14.5 ± 0.84 | 14.6 ± 0.93 | t(78) = −0.62 | 0.54 |
| BMI | 27.0 ± 2.8 | 20.3 ± 2.0 | t(78) = 11.7 | 0.00 |
| zBMI | 1.5 ± 0.35 | 0.01 ± 0.65 | t(78) = 12.0 | 0.00 |
| Risk status (%) | χ2(1,80) = 0.01 | 0.93 | ||
| Parental history positive | 42.1 | 41.0 | ||
| Parental history negative | 57.9 | 59.0 | ||
| Maximum parental education (%) | t(78) = −1.19 | 0.24 | ||
| Some high school | 0 | 3.3 | ||
| High school graduate | 15.8 | 1.6 | ||
| Some college | 36.8 | 49.2 | ||
| College graduate | 36.8 | 31.2 | ||
| Advanced degree | 5.3 | 14.8 |
| Overweight/Obesity | Healthy Weight | Test Statistic | P-Value | |
|---|---|---|---|---|
| Hunger [Mean ± SD] | 7.5 ± 5.0 | 9.0 ± 3.9 | t(78) = −1.34 | 0.19 |
| Last time eaten [Mean ± SD] | 2.3 ± 1.6 | 3.1 ± 3.3 | t(78) = −1.01 | 0.32 |
| Scan time [Mean ± SD] | 3 pm ± 1.5 | 2 pm ± 2.3 | t(78) = 1.88 | 0.06 |
| Milkshake (Mean ± SD) | ||||
| Pleasantness | 14.3 ± 3.6 | 15.4 ± 2.5 | t(78) = −1.59 | 0.12 |
| Wanting | 13.7 ± 2.7 | 14.9 ± 3.0 | t(78) = −1.44 | 0.16 |
| Familiarity | 16.4 ± 3.7 | 15.6 ± 3.2 | t(78) = 0.90 | 0.37 |
| Intensity | 3.9 ± 1.3 | 5.5 ± 2.6 | t(78) = −3.39 | 0.00 |
| Tasteless solution (Mean ± SD) | ||||
| Pleasantness | 8.7 ± 2.2 | 9.5 ± 2.3 | t(78) = −1.31 | 0.19 |
| Wanting | 8.0 ± 4.0 | 8.8 ± 3.3 | t(78) = −0.92 | 0.36 |
| Familiarity | 11.7 ± 5.8 | 13.7 ± 6.2 | t(78) = −1.22 | 0.23 |
| Intensity | 2.3 ± 1.1 | 2.4 ± 2.0 | t(78) = −0.20 | 0.94 |
| Contrasts | Cluster (k) | Z-Value | MNI Coordinates | Effect Sizes (r) |
|---|---|---|---|---|
| Milkshake cue > tasteless solution cue | ||||
| Individuals with obesity/overweight > individuals with healthy weight | ||||
| Ventromedial prefrontal cortex | 33 | 4.04 | −12, 47, −13 | 0.45 |
| Ventral anterior cingulate cortex | 3.92 | −9, 32, −10 | 0.44 | |
| Milkshake receipt > tasteless solution receipt | ||||
| Individuals with obesity/overweight > individuals with healthy weight | ||||
| Ventral striatum | 37 | 4.25 | 3, 11, −4 | 0.48 |
| Subgenual anterior cingulate cortex | 4.04 | 3, 26, −10 | 0.45 | |
| Dorsomedial prefrontal cortex | 55 | 4.14 | 9, 65, 17 | 0.46 |
| Dorsomedial prefrontal cortex | 3.37 | 27, 56, 20 | 0.38 |
| Contrasts | Cluster (k) | Z-Value | MNI Coordinates | Effect Sizes (r) |
|---|---|---|---|---|
| Milkshake cue > tasteless solution cue | ||||
| Weight status X risk status | ||||
| Middle frontal gyrus | 45 | 4.53 | −24, 26, 47 | 0.51 |
| vMPFC/mOFC | 35 | 3.93 | −6, 35, −16 | 0.44 |
| vMPFC/mOFC | 3.81 | −12, 47, −13 | 0.43 | |
| vMPFC/mOFC | 3.74 | −3, 44, −10 | 0.42 | |
| Milkshake receipt > tasteless solution receipt | ||||
| Weight status X risk status | ||||
| Thalamus | 95 | 4.84 | −6, −4, −1 | 0.54 |
| Striatum | 4.68 | 12, 11, −4 | 0.52 | |
| Thalamus | 4.28 | 6, −4, −1 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokum, S.; Stice, E. Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology in Adolescent Females. Nutrients 2023, 15, 2558. https://doi.org/10.3390/nu15112558
Yokum S, Stice E. Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology in Adolescent Females. Nutrients. 2023; 15(11):2558. https://doi.org/10.3390/nu15112558
Chicago/Turabian StyleYokum, Sonja, and Eric Stice. 2023. "Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology in Adolescent Females" Nutrients 15, no. 11: 2558. https://doi.org/10.3390/nu15112558
APA StyleYokum, S., & Stice, E. (2023). Relation of Overweight/Obesity to Reward Region Response to Food Reward and the Moderating Effects of Parental History of Eating Pathology in Adolescent Females. Nutrients, 15(11), 2558. https://doi.org/10.3390/nu15112558




