Antioxidant Nutrients and Risk of Latent Autoimmune Diabetes in Adults and Type 2 Diabetes: A Swedish Case-Control Study and Mendelian Randomization Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The ESTRID Study
2.1.1. Study Population and Design
2.1.2. Laboratory Analyses and Diabetes Classification
2.1.3. Genetic Risk Assessment
2.1.4. Dietary and Covariate Assessment
2.2. Mendelian Randomization
2.3. Statistical Analysis
2.4. Sensitivity Analyses
3. Results
3.1. Participant Characteristics
3.2. Antioxidant Nutrient Intakes and Risk of LADA
3.3. Antioxidant Nutrient Intakes and Risk of Type 2 Diabetes
3.4. Antioxidant Nutrient Intakes and HOMA-B or HOMA-IR
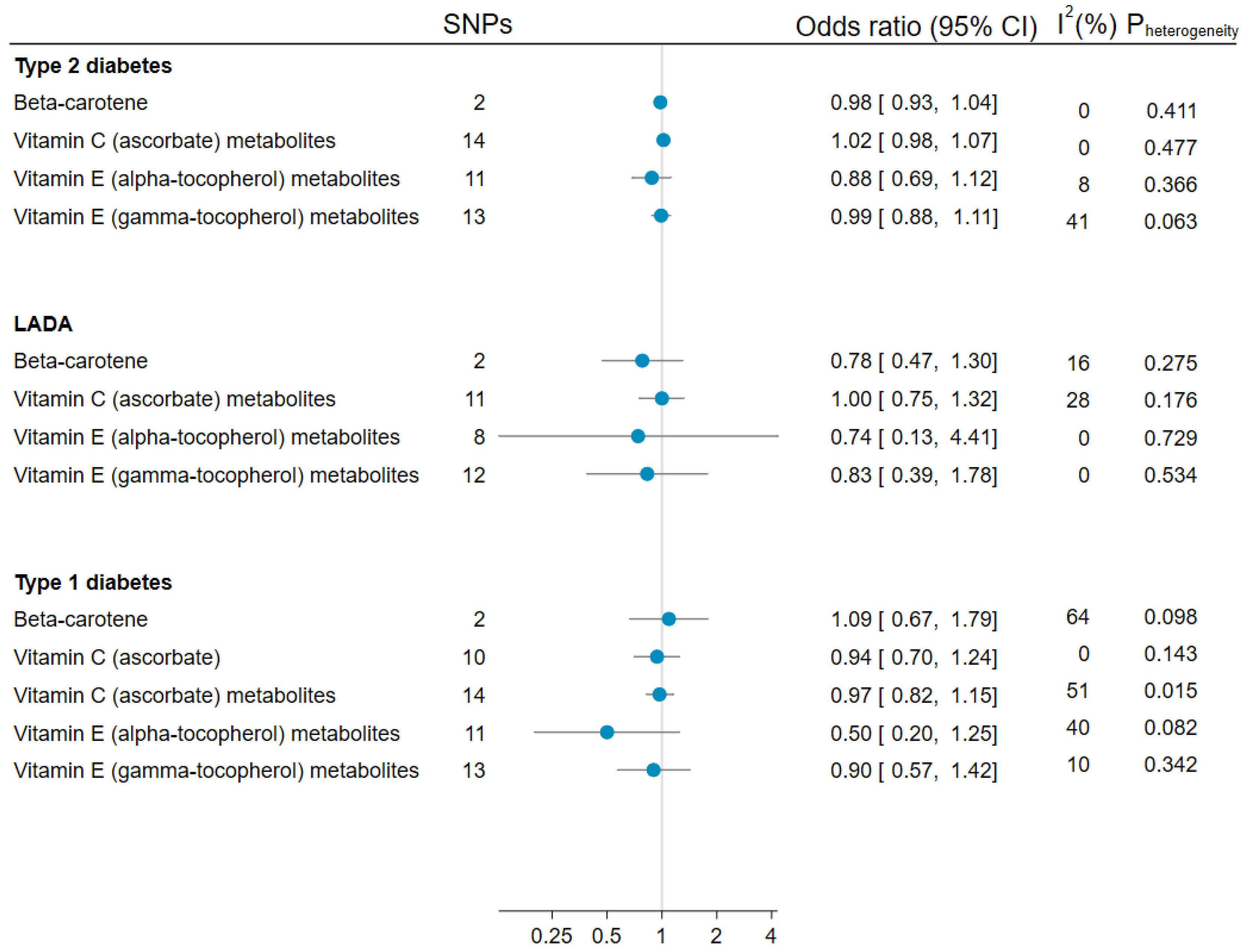
3.5. Mendelian Randomization Analyses of Antioxidants and LADA, Type 1, and Type 2 Diabetes
3.6. Sensitivity Analyses
4. Discussion
4.1. Main Findings
4.2. Findings in Relation to Previous Studies
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Genetic Instruments for Circulating Antioxidants
Appendix A.2. Statistical Analysis
References
- Mishra, R.; Hodge, K.M.; Cousminer, D.L.; Leslie, R.D.; Grant, S.F. A Global Perspective of Latent Autoimmune Diabetes in Adults. Trends Endocrinol. Metab. 2018, 29, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, T.; Santoro, N.; Caprio, S.; Cai, M.; Weng, J.; Groop, L. The Many Faces of Diabetes: A Disease with Increasing Heterogeneity. Lancet 2014, 383, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.K. New Insights into the Genetics of Latent Autoimmune Diabetes in Adults. Curr. Diab. Rep. 2020, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S. Lifestyle or Environmental Influences and Their Interaction With Genetic Susceptibility on the Risk of LADA. Front. Endocrinol. 2022, 13, 917850. [Google Scholar] [CrossRef] [PubMed]
- Lampousi, A.-M.; Carlsson, S.; Löfvenborg, J.E. Dietary Factors and Risk of Islet Autoimmunity and Type 1 Diabetes: A Systematic Review and Meta-Analysis. EBioMedicine 2021, 72, 103633. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants—An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- van der Schaft, N.; Schoufour, J.D.; Nano, J.; Jong, J.C.K.-D.; Muka, T.; Sijbrands, E.J.G.; Ikram, M.A.; Franco, O.H.; Voortman, T. Dietary Antioxidant Capacity and Risk of Type 2 Diabetes Mellitus, Prediabetes and Insulin Resistance: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 853–861. [Google Scholar] [CrossRef]
- Fernández-Cao, J.C.; Warthon-Medina, M.; Moran, V.H.; Arija, V.; Doepking, C.; Serra-Majem, L.; Lowe, N.M. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1027. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Imamura, F.; Stewart, I.D.; Day, F.R.; Pietzner, M.; Wheeler, E.; Lotta, L.A.; Gundersen, T.E.; et al. Plasma Vitamin C and Type 2 Diabetes: Genome-Wide Association Study and Mendelian Randomization Analysis in European Populations. Diabetes Care 2021, 44, 98–106. [Google Scholar] [CrossRef]
- Tuomi, T.; Carlsson, A.; Li, H.; Isomaa, B.; Miettinen, A.; Nilsson, A.; Nissén, M.; Ehrnström, B.O.; Forsén, B.; Snickars, B.; et al. Clinical and Genetic Characteristics of Type 2 Diabetes with and without GAD Antibodies. Diabetes 1999, 48, 150–157. [Google Scholar] [CrossRef]
- Buzzetti, R.; Di Pietro, S.; Giaccari, A.; Petrone, A.; Locatelli, M.; Suraci, C.; Capizzi, M.; Arpi, M.L.; Bazzigaluppi, E.; Dotta, F.; et al. High Titer of Autoantibodies to GAD Identifies a Specific Phenotype of Adult-Onset Autoimmune Diabetes. Diabetes Care 2007, 30, 932–938. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Xiang, Y.; Huang, G.; Lin, J.; Yang, L.; Zhao, Y.; Yang, Z.; Hou, C.; Li, Y.; et al. Latent Autoimmune Diabetes in Adults with Low-Titer GAD Antibodies: Similar Disease Progression with Type 2 Diabetes: A Nationwide, Multicenter Prospective Study (LADA China Study 3). Diabetes Care 2015, 38, 16–21. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel Subgroups of Adult-Onset Diabetes and Their Association with Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Rahmati, K.; Lernmark, A.; Becker, C.; Foltyn-Zadura, A.; Larsson, K.; Ivarsson, S.-A.; Törn, C. A Comparison of Serum and EDTA Plasma in the Measurement of Glutamic Acid Decarboxylase Autoantibodies (GADA) and Autoantibodies to Islet Antigen-2 (IA-2A) Using the RSR Radioimmunoassay (RIA) and Enzyme Linked Immunosorbent Assay (ELISA) Kits. Clin. Lab. 2008, 54, 227–235. [Google Scholar]
- Fourlanos, S.; Dotta, F.; Greenbaum, C.J.; Palmer, J.P.; Rolandsson, O.; Colman, P.G.; Harrison, L.C. Latent Autoimmune Diabetes in Adults (LADA) Should Be Less Latent. Diabetologia 2005, 48, 2206–2212. [Google Scholar] [CrossRef]
- The Oxford Center for Diabetes. Endocrinology & Metabolism. Diabetes Trial Unit. HOMA Calculator. Available online: http://www.dtu.ox.ac.uk/homacalculator/index.php (accessed on 20 March 2006).
- Nguyen, C.; Varney, M.D.; Harrison, L.C.; Morahan, G. Definition of High-Risk Type 1 Diabetes HLA-DR and HLA-DQ Types Using Only Three Single Nucleotide Polymorphisms. Diabetes 2013, 62, 2135–2140. [Google Scholar] [CrossRef]
- Willett, W.; Stampfer, M.J. Total Energy Intake: Implications for Epidemiologic Analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Messerer, M.; Wolk, A.; Johansson, S.-E. The Validity of Questionnaire-Based Micronutrient Intake Estimates Is Increased by Including Dietary Supplement Use in Swedish Men. J. Nutr. 2004, 134, 1800–1805. [Google Scholar] [CrossRef]
- Cousminer, D.L.; Ahlqvist, E.; Mishra, R.; Andersen, M.K.; Chesi, A.; Hawa, M.I.; Davis, A.; Hodge, K.M.; Bradfield, J.P.; Zhou, K.; et al. First Genome-Wide Association Study of Latent Autoimmune Diabetes in Adults Reveals Novel Insights Linking Immune and Metabolic Diabetes. Diabetes Care 2018, 41, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Forgetta, V.; Manousaki, D.; Istomine, R.; Ross, S.; Tessier, M.-C.; Marchand, L.; Li, M.; Qu, H.-Q.; Bradfield, J.P.; Grant, S.F.; et al. Rare Genetic Variants of Large Effect Influence Risk of Type 1 Diabetes. Diabetes 2020, 69, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Spracklen, C.N.; Zhang, W.; Ng, M.C.Y.; Petty, L.E.; Kitajima, H.; Yu, G.Z.; Rüeger, S.; Speidel, L.; Kim, Y.J.; et al. Multi-Ancestry Genetic Study of Type 2 Diabetes Highlights the Power of Diverse Populations for Discovery and Translation. Nat. Genet. 2022, 54, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Mattila, M.; Erlund, I.; Lee, H.-S.; Niinistö, S.; Uusitalo, U.; Aronsson, C.A.; Hummel, S.; Parikh, H.; Rich, S.S.; Hagopian, W.; et al. Plasma Ascorbic Acid and the Risk of Islet Autoimmunity and Type 1 Diabetes: The TEDDY Study. Diabetologia 2020, 63, 278–286. [Google Scholar] [CrossRef]
- Benson, V.S.; VanLeeuwen, J.A.; Taylor, J.; Somers, G.S.; McKinney, P.A.; Van Til, L. Type 1 Diabetes Mellitus and Components in Drinking Water and Diet: A Population-Based, Case-Control Study in Prince Edward Island, Canada. J. Am. Coll. Nutr. 2010, 29, 612–624. [Google Scholar] [CrossRef]
- Uusitalo, L.; Knip, M.; Kenward, M.G.; Alfthan, G.; Sundvall, J.; Aro, A.; Reunanen, A.; Akerblom, H.K.; Virtanen, S.M.; Childhood Diabetes in Finland Study Group. Serum Alpha-Tocopherol Concentrations and Risk of Type 1 Diabetes Mellitus: A Cohort Study in Siblings of Affected Children. J. Pediatr. Endocrinol. Metab. 2005, 18, 1409–1416. [Google Scholar] [CrossRef]
- Knekt, P.; Reunanen, A.; Marniemi, J.; Leino, A.; Aromaa, A. Low Vitamin E Status Is a Potential Risk Factor for Insulin-Dependent Diabetes Mellitus. J. Intern. Med. 1999, 245, 99–102. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate Is Depleted by Smoking and Repleted by Moderate Supplementation: A Study in Male Smokers and Nonsmokers with Matched Dietary Antioxidant Intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [CrossRef]
- Bruno, R.S.; Ramakrishnan, R.; Montine, T.J.; Bray, T.M.; Traber, M.G. α-Tocopherol Disappearance Is Faster in Cigarette Smokers and Is Inversely Related to Their Ascorbic Acid Status. Am. J. Clin. Nutr. 2005, 81, 95–103. [Google Scholar] [CrossRef]
- Møller, P.; Wallin, H.; Knudsen, L.E. Oxidative Stress Associated with Exercise, Psychological Stress and Life-Style Factors. Chem. Biol. Interact. 1996, 102, 17–36. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative Study on the Action of Tocopherols and Tocotrienols as Antioxidant: Chemical and Physical Effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. An Atlas on Risk Factors for Type 2 Diabetes: A Wide-Angled Mendelian Randomisation Study. Diabetologia 2020, 63, 2359–2371. [Google Scholar] [CrossRef]
- Liu, S.; Lee, I.-M.; Song, Y.; Van Denburgh, M.; Cook, N.R.; Manson, J.E.; Buring, J.E. Vitamin E and Risk of Type 2 Diabetes in the Women’s Health Study Randomized Controlled Trial. Diabetes 2006, 55, 2856–2862. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.; Sundell, J.R.; Männistö, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Effect of α-Tocopherol and β-Carotene Supplementation on the Incidence of Type 2 Diabetes. Diabetologia 2007, 51, 47–53. [Google Scholar] [CrossRef]
- Song, Y.; Cook, N.R.; Albert, C.M.; Van Denburgh, M.; Manson, J.E. Effects of Vitamins C and E and β-Carotene on the Risk of Type 2 Diabetes in Women at High Risk of Cardiovascular Disease: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2009, 90, 429–437. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Meagher, E.A.; Barry, O.P.; Lawson, J.A.; Rokach, J.; Fitzgerald, G.A. Effects of Vitamin E on Lipid Peroxidation in Healthy Persons. JAMA 2001, 285, 1178–1182. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rovner, A.J.; Mumford, S.L.; Yeung, E.; Browne, R.W.; Trevisan, M.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F.; BioCycle Study Group. Adherence to a Mediterranean Diet and Plasma Concentrations of Lipid Peroxidation in Premenopausal Women. Am. J. Clin. Nutr. 2010, 92, 1461–1467. [Google Scholar] [CrossRef]
- Bacchetti, T.; Turco, I.; Urbano, A.; Morresi, C.; Ferretti, G. Relationship of Fruit and Vegetable Intake to Dietary Antioxidant Capacity and Markers of Oxidative Stress: A Sex-Related Study. Nutrition 2019, 61, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Siljander, H.; Honkanen, J.; Knip, M. Microbiome and Type 1 Diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In Vitro and in Vivo Evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Löfvenborg, J.E.; Andersson, T.; Carlsson, P.-O.; Dorkhan, M.; Groop, L.; Martinell, M.; Tuomi, T.; Wolk, A.; Carlsson, S. Fatty Fish Consumption and Risk of Latent Autoimmune Diabetes in Adults. Nutr. Diabetes 2014, 4, e139. [Google Scholar] [CrossRef]
- Costacou, T.; Ma, B.; King, I.B.; Mayer-Davis, E.J. Plasma and Dietary Vitamin E in Relation to Insulin Secretion and Sensitivity. Diabetes Obes. Metab. 2008, 10, 223–228. [Google Scholar] [CrossRef]
- Manning, P.J.; Sutherland, W.H.; Walker, R.J.; Williams, S.M.; de Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of High-Dose Vitamin E on Insulin Resistance and Associated Parameters in Overweight Subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef]
- Prasad, M.; Takkinen, H.-M.; Nevalainen, J.; Ovaskainen, M.-L.; Alfthan, G.; Uusitalo, L.; Kenward, M.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Are Serum α- and β-Carotene Concentrations Associated with the Development of Advanced Beta-Cell Autoimmunity in Children with Increased Genetic Susceptibility to Type 1 Diabetes? Diabetes Metab. 2011, 37, 162–167. [Google Scholar] [CrossRef]
- Kyvsgaard, J.N.; Overgaard, A.J.; Jacobsen, L.D.; Thorsen, S.U.; Pipper, C.B.; Hansen, T.H.; Husted, S.; Mortensen, H.B.; Pociot, F.; Svensson, J. Low Perinatal Zinc Status Is Not Associated with the Risk of Type 1 Diabetes in Children. Pediatr. Diabetes 2017, 18, 637–642. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Sun, Z.-H.; Tong, W.-W.; Yang, K.; Guo, K.-Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Yary, T.; Virtanen, J.; Ruusunen, A.; Tuomainen, T.-P.; Voutilainen, S. Serum Zinc and Risk of Type 2 Diabetes Incidence in Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J. Trace Elem. Med. Biol. 2016, 33, 120–124. [Google Scholar] [CrossRef]
- Li, X.T.; Yu, P.F.; Gao, Y.; Guo, W.H.; Wang, J.; Liu, X.; Gu, A.H.; Ji, G.X.; Dong, Q.; Wang, B.S.; et al. Association between Plasma Metal Levels and Diabetes Risk: A Case-Control Study in China. Biomed. Environ. Sci. 2017, 30, 482–491. [Google Scholar] [CrossRef]
- El Dib, R.; Gameiro, O.L.; Ogata, M.S.; Módolo, N.S.; Braz, L.G.; Jorge, E.C.; Junior, P.D.N.; Beletate, V. Zinc Supplementation for the Prevention of Type 2 Diabetes Mellitus in Adults with Insulin Resistance. Cochrane Database Syst. Rev. 2015, 2015, CD005525. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A Systematic Review and Dose-Response Meta-Analysis of Exposure to Environmental Selenium and the Risk of Type 2 Diabetes in Nonexperimental Studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium Exposure and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef]
- Rath, A.A.; Lam, H.S.; Schooling, C.M. Effects of Selenium on Coronary Artery Disease, Type 2 Diabetes and Their Risk Factors: A Mendelian Randomization Study. Eur. J. Clin. Nutr. 2021, 75, 1668–1678. [Google Scholar] [CrossRef]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of Insulin Resistance and Obesity in Mice Overexpressing Cellular Glutathione Peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef]
- Zeng, M.-S.; Li, X.; Liu, Y.; Zhao, H.; Zhou, J.-C.; Li, K.; Huang, J.-Q.; Sun, L.-H.; Tang, J.-Y.; Xia, X.-J.; et al. A High-Selenium Diet Induces Insulin Resistance in Gestating Rats and Their Offspring. Free Radic. Biol. Med. 2012, 52, 1335–1342. [Google Scholar] [CrossRef]
- Hendrickson, S.J.; Hazra, A.; Chen, C.; Eliassen, A.H.; Kraft, P.; A Rosner, B.; Willett, W.C. β-Carotene 15,15’-Monooxygenase 1 Single Nucleotide Polymorphisms in Relation to Plasma Carotenoid and Retinol Concentrations in Women of European Descent. Am. J. Clin. Nutr. 2012, 96, 1379–1389. [Google Scholar] [CrossRef]
- Major, J.M.; Yu, K.; Wheeler, W.; Zhang, H.; Cornelis, M.C.; Wright, M.E.; Yeager, M.; Snyder, K.; Weinstein, S.J.; Mondul, A.; et al. Genome-Wide Association Study Identifies Common Variants Associated with Circulating Vitamin E Levels. Hum. Mol. Genet. 2011, 20, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.-P.; et al. An Atlas of Genetic Influences on Human Blood Metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for Performing Mendelian Randomization Investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Smith, G.D.; Bowden, J. Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy Assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
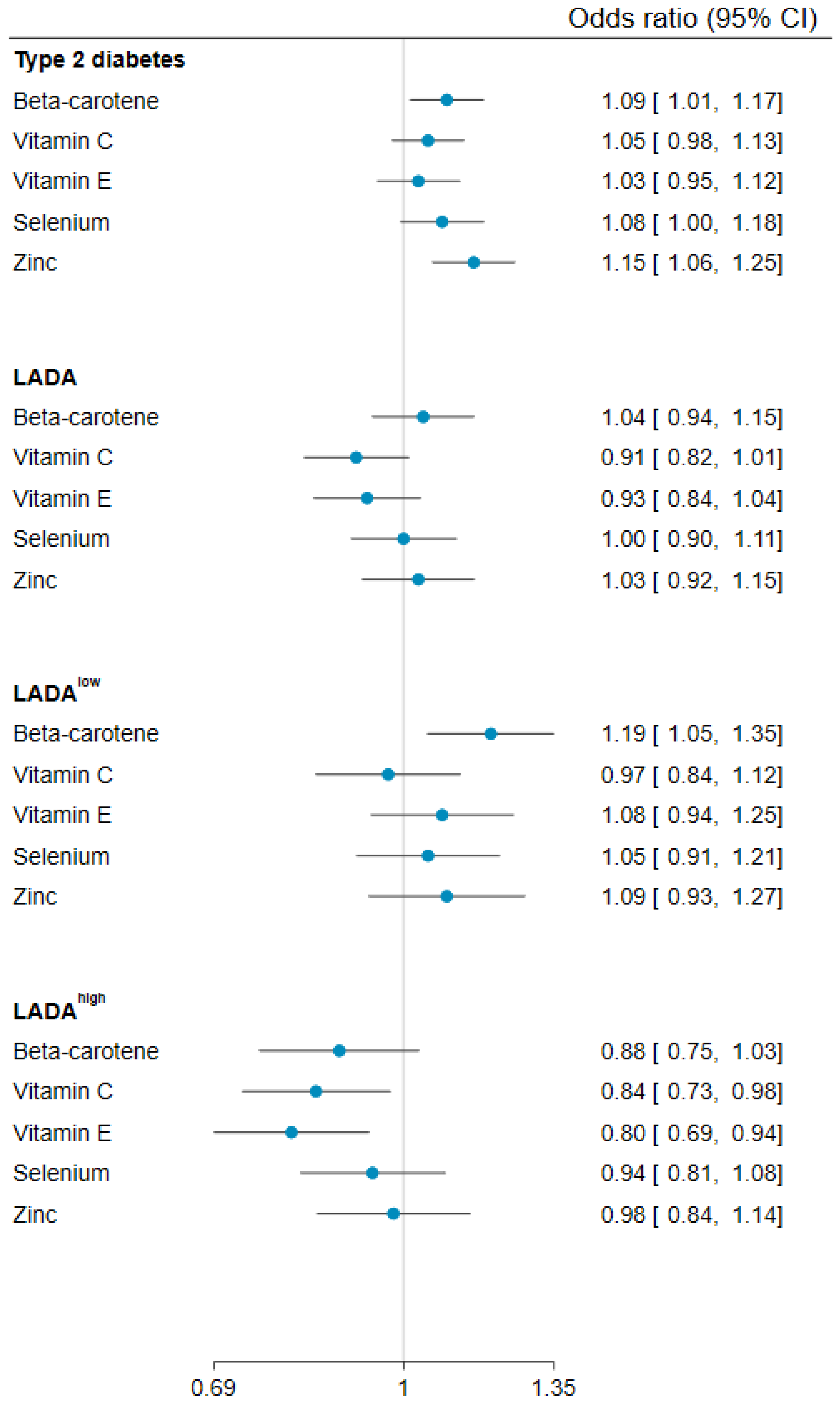
| Controls | Type 2 Diabetes | LADA | p-value 1 | LADAlow 2 | LADAhigh 2 | p-value 3 | |
|---|---|---|---|---|---|---|---|
| n | 2276 | 1989 | 584 | 262 | 313 | ||
| Age (years), mean (SD) | 59.0 (13.8) | 63.3 (10.4) | 59.1 (12.4) | <0.001 | 60 (12.1) | 58.3 (12.5) | 0.100 |
| BMI, mean (SD) | 26.0 (4.2) | 31.2 (5.4) | 28.5 (5.7) | <0.001 | 29.4 (6.0) | 27.7 (5.3) | <0.001 |
| Women, % | 52.6 | 39.9 | 47.3 | 0.002 | 43.1 | 51.1 | 0.056 |
| University education, % | 37.7 | 20.4 | 28.3 | <0.001 | 27.1 | 29.7 | 0.033 |
| Physically inactive, % | 14.1 | 25.1 | 18.3 | <0.001 | 21.8 | 16.0 | 0.162 |
| Current smoking, % | 18.5 | 21.6 | 23.0 | <0.001 | 17.9 | 26.2 | 0.029 |
| Low alcohol intake, % | 33.3 | 36.6 | 37.7 | 0.005 | 39.3 | 36.4 | 0.437 |
| High-risk HLA 4, % | - | 31.4 | 60.3 | <0.001 | 52.4 | 67.7 | <0.001 |
| Family history of diabetes, % | 25.4 | 51.0 | 45.4 | 0.016 | 45.0 | 44.7 | 0.941 |
| CVD or hypertension history, % | 26.8 | 58.9 | 42.3 | <0.001 | 45.4 | 39.9 | 0.408 |
| Insulin treatment, % | - | 5.6 | 32.1 | <0.001 | 23.0 | 39.6 | 0.001 |
| GADA (U/mL), median (IQR) | - | - | 250 (32, 250) | - | 28.5 (17, 73) | 250 (250, 250) | <0.001 |
| HOMA-B 5, median (IQR) | - | 71.2 (44.4, 96.0) | 40.5 (14.6, 70.0) | <0.001 | 50.6 (22.0, 83.6) | 29.5 (12, 59) | <0.001 |
| HOMA-IR 5, median (IQR) | - | 3.6 (2.7, 4.8) | 2.8 (1.8, 4.4) | <0.001 | 3.2 (2.2, 4.9) | 2.4 (1.7, 4.0) | <0.001 |
| Antioxidant Nutrient Intake 1 | Type 2 Diabetes | LADA | LADAlow | LADAhigh | ||||
|---|---|---|---|---|---|---|---|---|
| N Cases/Controls | OR (95% CI) | N Cases/Controls | OR (95% CI) | N Cases/Controls | OR (95% CI) | N Cases/Controls | OR (95% CI) | |
| Beta-carotene (µg/day) | ||||||||
| Q1 (<1642.4) | 556/569 | 1.00 | 156/569 | 1.00 | 67/569 | 1.00 | 85/569 | 1.00 |
| Q2 (1642.4 to < 2595.6) | 422/569 | 0.91 (0.73, 1.13) | 151/569 | 1.15 (0.87, 1.51) | 55/569 | 1.05 (0.70, 1.57) | 96/569 | 1.21 (0.86, 1.70) |
| Q3 (2595.6 to < 4072.1) | 428/569 | 0.89 (0.71, 1.11) | 121/569 | 0.86 (0.64, 1.14) | 56/569 | 1.01 (0.67, 1.51) | 62/569 | 0.73 (0.50, 1.06) |
| Q4 (≥4072.1) | 583/569 | 1.15 (0.92, 1.43) | 156/569 | 1.16 (0.87, 1.54) | 84/569 | 1.62 (1.10, 2.40) | 70/569 | 0.87 (0.60, 1.26) |
| Per 1 SD (2670) | 1.09 (1.01, 1.17) | 1.04 (0.94, 1.15) | 1.19 (1.05, 1.35) | 0.88 (0.75, 1.03) | ||||
| Supplementation (ever vs. never) | 36/52 | 1.11 (0.63, 1.94) | 9/52 | 0.76 (0.36, 1.60) | 3/52 | 0.60 (0.18, 2.06) | 6/52 | 0.85 (0.35, 2.06) |
| Vitamin C (mg/day) | ||||||||
| Q1 (<73.6) | 537/569 | 1.00 | 174/569 | 1.00 | 72/569 | 1.00 | 99/569 | 1.00 |
| Q2 (73.6 to < 104.2) | 487/569 | 0.93 (0.75, 1.15) | 158/569 | 0.96 (0.74, 1.25) | 66/569 | 0.97 (0.66, 1.43) | 88/569 | 0.92 (0.66, 1.28) |
| Q3 (104.2 to < 144.6) | 439/569 | 0.82 (0.65, 1.02) | 138/569 | 0.79 (0.60, 1.04) | 65/569 | 0.90 (0.61, 1.33) | 73/569 | 0.70 (0.50, 0.99) |
| Q4 (≥144.6) | 526/569 | 0.99 (0.80, 1.24) | 114/569 | 0.66 (0.49, 0.88) | 59/569 | 0.80 (0.54, 1.20) | 53/569 | 0.51 (0.35, 0.76) |
| Per 1 SD (64.2) | 1.05 (0.98, 1.13) | 0.91 (0.82, 1.01) | 0.97 (0.84, 1.12) | 0.84 (0.73, 0.98) | ||||
| Supplementation (ever vs. never) | 180/268 | 0.85 (0.65, 1.10) | 65/268 | 1.00 (0.73, 1.36) | 29/268 | 1.01 (0.65, 1.58) | 35/268 | 0.96 (0.65, 1.43) |
| Vitamin E (mg/day) | ||||||||
| Q1 (<7.2) | 467/569 | 1.00 | 159/569 | 1.00 | 63/569 | 1.00 | 92/569 | 1.00 |
| Q2 (7.2 to < 8.7) | 393/569 | 0.87 (0.69, 1.09) | 158/569 | 1.01 (0.78, 1.33) | 63/569 | 0.94 (0.63, 1.40) | 94/569 | 1.09 (0.79, 1.52) |
| Q3 (8.7 to < 10.6) | 497/569 | 0.96 (0.77, 1.20) | 123/569 | 0.79 (0.59, 1.05) | 55/569 | 0.86 (0.57, 1.30) | 66/569 | 0.75 (0.53, 1.08) |
| Q4 (≥10.6) | 632/569 | 1.03 (0.82, 1.30) | 144/569 | 0.84 (0.63, 1.13) | 81/569 | 1.12 (0.75, 1.69) | 61/569 | 0.66 (0.45, 0.97) |
| Per 1 SD (3.0) | 1.03 (0.95, 1.12) | 0.93 (0.84, 1.04) | 1.08 (0.94, 1.25) | 0.80 (0.69, 0.94) | ||||
| Supplementation (ever vs. never) | 67/81 | 1.05 (0.67, 1.63) | 19/81 | 0.91 (0.53, 1.57) | 9/81 | 0.89 (0.42, 1.91) | 10/81 | 0.94 (0.47, 1.88) |
| Selenium (µg/day) | ||||||||
| Q1 (<33.4) | 375/569 | 1.00 | 130/569 | 1.00 | 53/569 | 1.00 | 76/569 | 1.00 |
| Q2 (33.4 to < 40.2) | 422/569 | 1.03 (0.81, 1.30) | 134/569 | 0.97 (0.73, 1.29) | 55/569 | 0.92 (0.60, 1.40) | 76/569 | 0.98 (0.69, 1.41) |
| Q3 (40.2 to < 48.7) | 512/569 | 1.11 (0.88, 1.41) | 159/569 | 1.10 (0.82, 1.46) | 71/569 | 1.10 (0.73, 1.67) | 86/569 | 1.05 (0.73, 1.50) |
| Q4 (≥48.7) | 680/569 | 1.29 (1.01, 1.64) | 161/569 | 1.10 (0.81, 1.49) | 83/569 | 1.26 (0.82, 1.93) | 75/569 | 0.93 (0.63, 1.37) |
| Per 1 SD (13.5) | 1.08 (1.00, 1.18) | 1.00 (0.90, 1.11) | 1.05 (0.91, 1.21) | 0.94 (0.81, 1.08) | ||||
| Supplementation (ever vs. never) | 48/81 | 0.91 (0.57, 1.45) | 18/81 | 1.03 (0.59, 1.79) | 11/81 | 1.48 (0.73, 3.01) | 6/81 | 0.60 (0.25, 1.42) |
| Zinc (mg/day) | ||||||||
| Q1 (<8.6) | 325/569 | 1.00 | 116/569 | 1.00 | 42/569 | 1.00 | 71/569 | 1.00 |
| Q2 (8.6 to < 9.8) | 420/569 | 1.28 (1.01, 1.63) | 147/569 | 1.24 (0.93, 1.65) | 67/569 | 1.41 (0.92, 2.16) | 79/569 | 1.15 (0.80, 1.65) |
| Q3 (9.8 to < 11.5) | 515/569 | 1.27 (1.00, 1.61) | 166/569 | 1.19 (0.88, 1.60) | 71/569 | 1.15 (0.74, 1.80) | 93/569 | 1.26 (0.87, 1.83) |
| Q4 (≥11.5) | 729/569 | 1.34 (1.03, 1.74) | 155/569 | 1.00 (0.71, 1.40) | 82/569 | 1.17 (0.72, 1.91) | 70/569 | 0.88 (0.57, 1.37) |
| Per 1 SD (2.5) | 1.15 (1.06, 1.25) | 1.03 (0.92, 1.15) | 1.09 (0.93, 1.27) | 0.98 (0.84, 1.14) | ||||
| Supplementation (ever vs. never) | 61/92 | 1.01 (0.67, 1.54) | 23/92 | 1.05 (0.64, 1.73) | 9/92 | 0.93 (0.43, 1.98) | 13/92 | 0.96 (0.52, 1.79) |
| Multivitamins (ever vs. never) | 284/519 | 0.76 (0.61, 0.93) | 93/519 | 0.71 (0.54, 0.92) | 41/519 | 0.74 (0.51, 1.08) | 50/519 | 0.66 (0.47, 0.92) |
| Antioxidant Nutrient Intake (Per 1 SD) 1 | HOMA-B | HOMA-IR | ||||
|---|---|---|---|---|---|---|
| All | Type 2 Diabetes | LADA | All | Type 2 Diabetes | LADA | |
| Beta-carotene (2670 µg/day) | 0.017 (−0.009, 0.043) | 0.009 (−0.017, 0.034) | 0.018 (−0.050, 0.086) | −0.021 (−0.046, 0.004) | −0.015 (−0.041, 0.011) | −0.051 (−0.118, 0.017) |
| Vitamin C (64.2 mg/day) | 0.013 (−0.013, 0.038) | 0.002 (−0.022, 0.026) | 0.007 (−0.069, 0.083) | −0.002 (−0.027, 0.022) | −0.009 (−0.034, 0.016) | 0.007 (−0.069, 0.083) |
| Vitamin E (3.0 mg/day) | 0.043 (0.015, 0.072) | 0.027 (0.000, 0.054) | 0.100 (0.021, 0.179) | −0.042 (−0.069, −0.015) | −0.037 (−0.065, −0.009) | −0.077 (−0.156, 0.003) |
| Selenium (13.5 µg/day) | 0.010 (−0.020, 0.040) | 0.008 (−0.021, 0.036) | 0.022 (−0.066, 0.111) | −0.014 (−0.043, 0.014) | −0.003 (−0.033, 0.026) | −0.082 (−0.169, 0.005) |
| Zinc (2.5 mg/day) | 0.018 (−0.010, 0.046) | 0.011 (−0.015, 0.037) | 0.048 (−0.038, 0.134) | −0.019 (−0.046, 0.007) | −0.021 (−0.048, 0.006) | −0.022 (−0.107, 0.064) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampousi, A.-M.; Löfvenborg, J.E.; Ahlqvist, E.; Tuomi, T.; Wolk, A.; Carlsson, S. Antioxidant Nutrients and Risk of Latent Autoimmune Diabetes in Adults and Type 2 Diabetes: A Swedish Case-Control Study and Mendelian Randomization Analysis. Nutrients 2023, 15, 2546. https://doi.org/10.3390/nu15112546
Lampousi A-M, Löfvenborg JE, Ahlqvist E, Tuomi T, Wolk A, Carlsson S. Antioxidant Nutrients and Risk of Latent Autoimmune Diabetes in Adults and Type 2 Diabetes: A Swedish Case-Control Study and Mendelian Randomization Analysis. Nutrients. 2023; 15(11):2546. https://doi.org/10.3390/nu15112546
Chicago/Turabian StyleLampousi, Anna-Maria, Josefin E. Löfvenborg, Emma Ahlqvist, Tiinamaija Tuomi, Alicja Wolk, and Sofia Carlsson. 2023. "Antioxidant Nutrients and Risk of Latent Autoimmune Diabetes in Adults and Type 2 Diabetes: A Swedish Case-Control Study and Mendelian Randomization Analysis" Nutrients 15, no. 11: 2546. https://doi.org/10.3390/nu15112546
APA StyleLampousi, A.-M., Löfvenborg, J. E., Ahlqvist, E., Tuomi, T., Wolk, A., & Carlsson, S. (2023). Antioxidant Nutrients and Risk of Latent Autoimmune Diabetes in Adults and Type 2 Diabetes: A Swedish Case-Control Study and Mendelian Randomization Analysis. Nutrients, 15(11), 2546. https://doi.org/10.3390/nu15112546







