Optimal Nutritional Support Strategy Based on the Association between Modified NUTRIC Score and 28-Day Mortality in Critically Ill Patients: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
2.2. Nutrition Screening Tools and Data Collection
2.3. Statistical Analysis
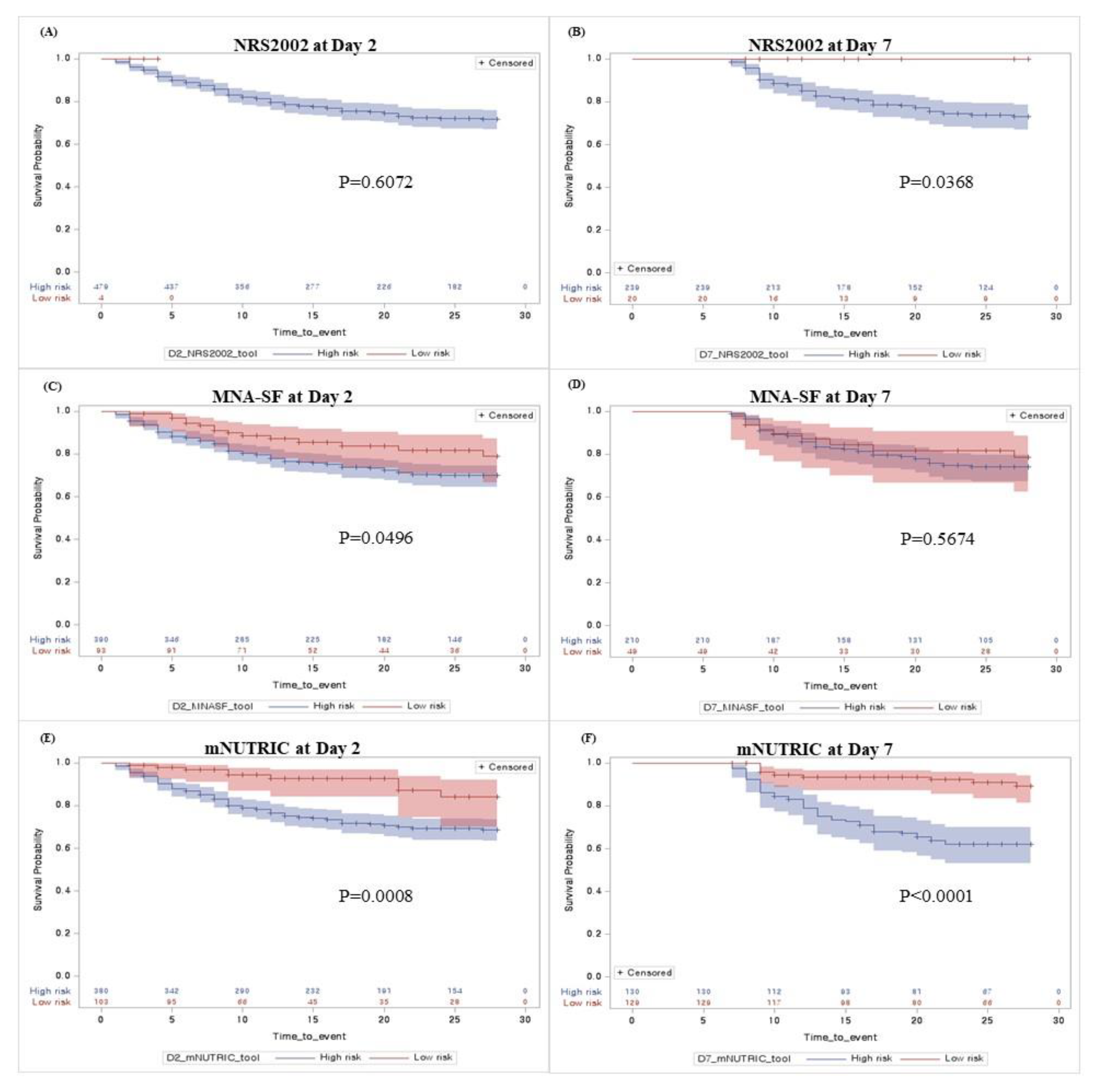
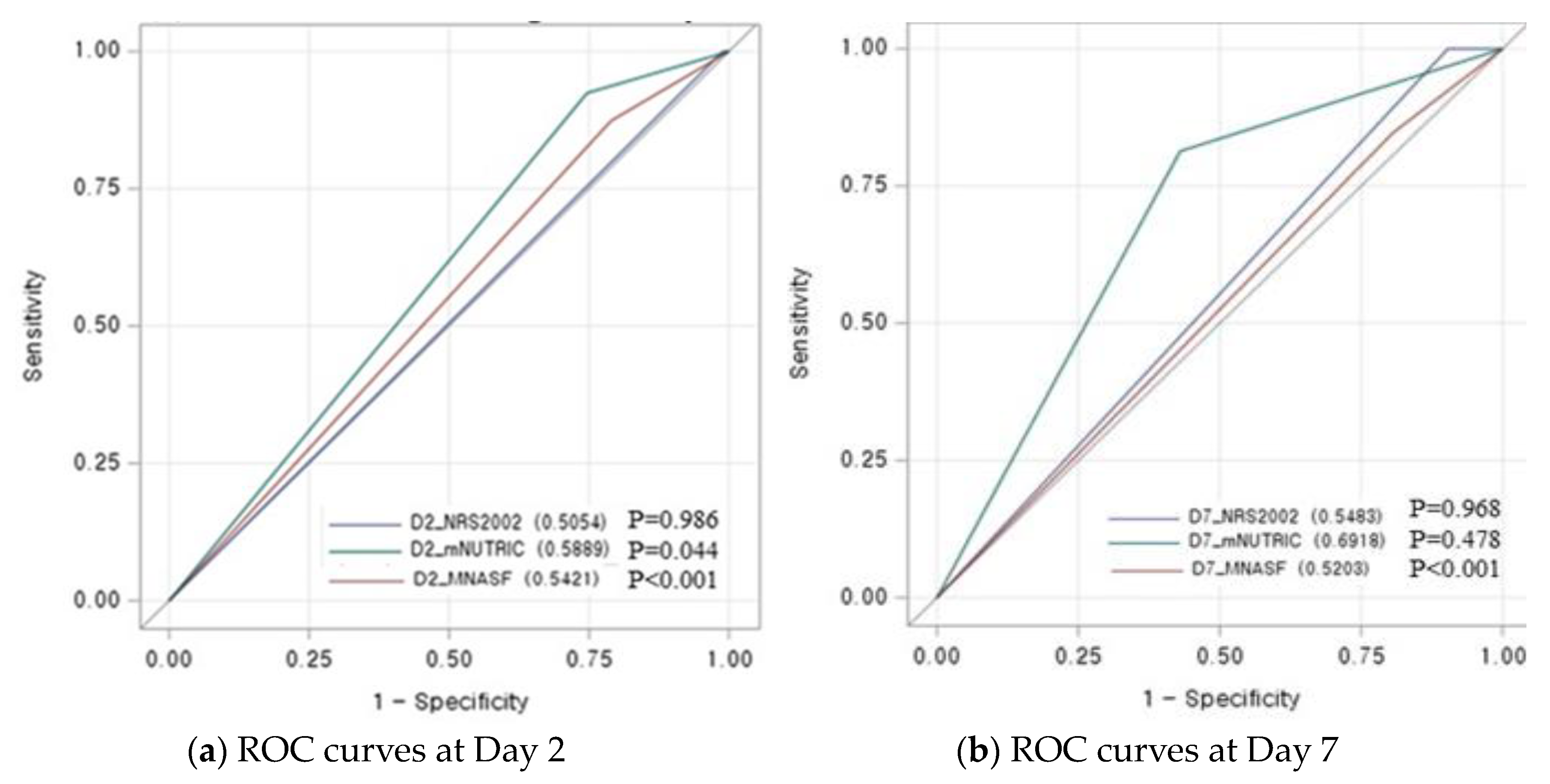
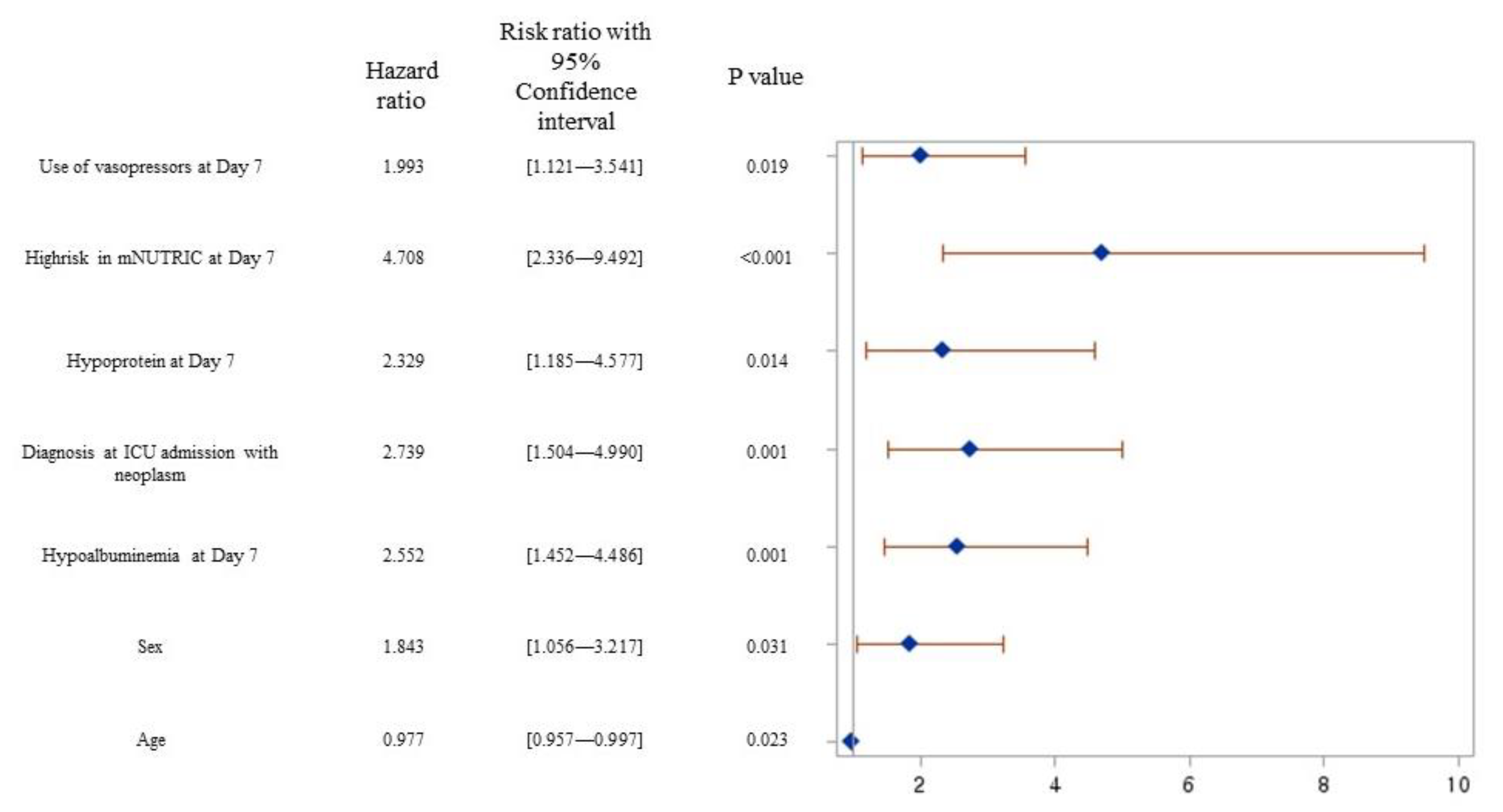
3. Results
4. Discussion
4.1. Prognostic Performance of mNUTRIC Score for 28-Day Mortality
4.2. Adequate Timing to Implement the mNUTRIC Score in Critically Ill Patients
4.3. Nutrition Support Strategy for Improving 28-Day Mortality
4.4. New Insights and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chada, R.R.; Chidrawar, S.; Goud, B.A.; Maska, A.; Medanki, R.; Nagalla, B. Association Between Nutrition Delivery, Modified Nutrition Risk In Critically III Score, and 28-Day Mortality. Nutr. Clin. Pract. 2021, 36, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.Y.; Heyland, D.K. Determination of Nutrition Risk and Status in Critically Ill Patients: What Are Our Considerations? Nutr. Clin. Pract. 2019, 34, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.M.; Malone, A.; Becker, P.; Cutrell, S.; Frank, L.; Gonzales, K.; Hudson, L.; Miller, S.; Guenter, P. Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition Consensus Malnutrition Characteristics: Usability and Association With Outcomes. Nutr. Clin. Pract. 2019, 34, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review. J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- de Vries, M.C.; Koekkoek, W.K.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional assessment of critically ill patients: Validation of the modified NUTRIC score. Eur. J. Clin. Nutr. 2018, 72, 428–435. [Google Scholar] [CrossRef]
- Mendes, R.; Policarpo, S.; Fortuna, P.; Alves, M.; Virella, D.; Heyland, D.K. Nutritional risk assessment and cultural validation of the modified NUTRIC score in critically ill patients-A multicenter prospective cohort study. J. Crit. Care 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Maday, K.R. The importance of nutrition in critically ill patients. Jaapa 2017, 30, 32–37. [Google Scholar] [CrossRef]
- Jensen, G.L. Inflammation as the key interface of the medical and nutrition universes: A provocative examination of the future of clinical nutrition and medicine. J. Parenter. Enter. Nutr. 2006, 30, 453–463. [Google Scholar] [CrossRef]
- Singer, P.; Doig, G.S.; Pichard, C. The truth about nutrition in the ICU. Intensive Care Med. 2014, 40, 252–255. [Google Scholar] [CrossRef]
- Wei, X.; Day, A.G.; Ouellette-Kuntz, H.; Heyland, D.K. The Association Between Nutritional Adequacy and Long-Term Outcomes in Critically Ill Patients Requiring Prolonged Mechanical Ventilation: A Multicenter Cohort Study. Crit. Care Med. 2015, 43, 1569–1579. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef]
- Weijs, P.J.; Stapel, S.N.; de Groot, S.D.; Driessen, R.H.; de Jong, E.; Girbes, A.R.; Strack van Schijndel, R.J.; Beishuizen, A. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: A prospective observational cohort study. J. Parenter. Enter. Nutr. 2012, 36, 60–68. [Google Scholar] [CrossRef]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.; Elsayes, A.; Yeh, D.D.; Belcher, D.; Nakayama, A.; McCarthy, C.M.; Chokengarmwong, N.; Quraishi, S.A. Nutrition Risk in Critically Ill Versus the Nutritional Risk Screening 2002: Are They Comparable for Assessing Risk of Malnutrition in Critically Ill Patients? J. Parenter. Enter. Nutr. 2019, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Basile-Filho, A.; Lago, A.F.; Menegueti, M.G.; Nicolini, E.A.; Rodrigues, L.A.B.; Nunes, R.S.; Auxiliadora-Martins, M.; Ferez, M.A. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: A retrospective cohort study. Medicine 2019, 98, e16204. [Google Scholar] [CrossRef] [PubMed]
- Petros, S.; Horbach, M.; Seidel, F.; Weidhase, L. Hypocaloric vs Normocaloric Nutrition in Critically Ill Patients: A Prospective Randomized Pilot Trial. J. Parenter. Enter. Nutr. 2016, 40, 242–249. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Donini, L.M.; Poggiogalle, E.; Molfino, A.; Rosano, A.; Lenzi, A.; Rossi Fanelli, F.; Muscaritoli, M. Mini-Nutritional Assessment, Malnutrition Universal Screening Tool, and Nutrition Risk Screening Tool for the Nutritional Evaluation of Older Nursing Home Residents. J. Am. Med. Dir. Assoc. 2016, 17, e911–e958. [Google Scholar] [CrossRef]
- Ata Ur-Rehman, H.M.; Ishtiaq, W.; Yousaf, M.; Bano, S.; Mujahid, A.M.; Akhtar, A. Modified Nutrition Risk in Critically Ill (mNUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study from the Pakistani Population. Cureus 2018, 10, e3786. [Google Scholar] [CrossRef]
- Mart, M.F.; Girard, T.D.; Thompson, J.L.; Whitten-Vile, H.; Raman, R.; Pandharipande, P.P.; Heyland, D.K.; Ely, E.W.; Brummel, N.E. Nutritional Risk at intensive care unit admission and outcomes in survivors of critical illness. Clin. Nutr. 2021, 40, 3868–3874. [Google Scholar] [CrossRef]
- Majari, K.; Imani, H.; Hosseini, S.; Amirsavadkouhi, A.; Ardehali, S.H.; Khalooeifard, R. Comparison of Modified NUTRIC, NRS-2002, and MUST Scores in Iranian Critically Ill Patients Admitted to Intensive Care Units: A Prospective Cohort Study. J. Parenter. Enter. Nutr. 2021, 45, 1504–1513. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Zhang, Q.; Wu, B. Modified Nutrition Risk in Critically ill is an effective nutrition risk screening tool in severely burned patients, compared with Nutrition Risk Screening 2002. Front. Nutr. 2022, 9, 1007885. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Guenter, P.; Howell, W.H.; Kochevar, M.E.; Roth, J.; Seidner, D.L. Definition of terms, style, and conventions used in A.S.P.E.N. guidelines and standards. Nutr. Clin. Pract. 2005, 20, 281–285. [Google Scholar] [CrossRef]
- Lochs, H.; Allison, S.P.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; van den Berghe, G.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin. Nutr. 2006, 25, 180–186. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, E.Y. Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients 2022, 14, 946. [Google Scholar] [CrossRef]
- Ishibashi, N.; Plank, L.D.; Sando, K.; Hill, G.L. Optimal protein requirements during the first 2 weeks after the onset of critical illness. Crit. Care Med. 1998, 26, 1529–1535. [Google Scholar] [CrossRef]

| Nutritional Screening Group on Day 2 (N = 490) | Nutritional Screening Group on Day 7 (N = 266) | p Value | |
|---|---|---|---|
| Age (years) | 67.9 ± 15.0 | 68.8 ± 14.5 | 0.408 |
| Sex (N, %) | 0.685 | ||
| Male | 317 (64.7%) | 176 (66.2%) | |
| Female | 173 (35.3%) | 90 (33.8%) | |
| Body mass index (kg/m2) | 23.5 ± 5.6 | 23.8 ± 6.6 | 0.509 |
| Weight at ICU admission (kg) | 62.1 ± 15.2 | 62.6 ± 17.4 | 0.673 |
| Days from hospital to ICU (days) | 5.4 ± 11.4 (1, 0–6) | 6.9 ± 13.2 (1, 0–8) | 0.110 |
| Source of admission to ICU (N, %) | 0.346 | ||
| Ward | 189 (38.6%) | 104 (39.1%) | |
| Emergency room | 254 (51.8%) | 128 (48.1%) | |
| ICU | 47 (9.6%) | 34 (12.08) | |
| Comorbidities ≥ 2 (N, %) | 411 (83.88%) | 233 (87.59%) | 0.170 |
| APACHE II score | 28.6 ± 8.9 (29, 22–35) | 17.1 ± 7.8 (16, 12–22) | <0.001 |
| SOFA score | 7.5 ± 3.7 (8, 5–10) | 6.8 ± 3.5 (6.5, 4–9) | 0.012 |
| Vasopressors (N, %) | 335 (68.5%) | 135 (51.1%) | <0.001 |
| Renal dialysis (N, %) | 120 (24.5%) | 56 (21.1%) | 0.286 |
| Antibiotics (N, %) | 408 (83.4%) | 214 (80.5%) | 0.304 |
| Route of administration § | <0.001 | ||
| NPO | 35 (7.2%) | 3 (1.1%) | |
| EN | 91 (18.7%) | 68 (25.6%) | |
| PN | 214 (43.9%) | 54 (20.3%) | |
| EN + PN | 148 (30.3%) | 141 (53.0%) | |
| Calorie ENPN (kcal/day) | 587.5 ± 505.4 | 1074.5 ± 589.8 | <0.001 |
| Hypocaloric §§ | 299 (61.0%) | 99 (37.2%) | <0.001 |
| Protein Supply (g/kg/day) | 0.4 ± 0.5 | 0.8 ± 0.5 | <0.001 |
| Hypoprotein (<1.0 g/kg/day) | 441 (90.0%) | 166 (62.4%) | <0.001 |
| Hypoprotein (<1.3 g/kg/day) | 463 (94.5%) | 220 (82.7%) | <0.001 |
| Diagnosis at ICU admission | 0.734 | ||
| Respiratory system | 123 (25.1%) | 74 (27.8%) | |
| Circulatory system | 104 (21.2%) | 56 (21.1%) | |
| Neoplasm | 76 (15.5%) | 37 (13.9%) | |
| Digestive system | 38 (7.8%) | 15 (5.6%) | |
| Infectious (Including COVID-19) | 33 (6.8%) | 23 (8.8%) | |
| Others | 116 (23.7%) | 61 (22.9%) | |
| Albumin (mg/dL) | 2.9 ± 0.9 (2.8, 2.4–3.2) | 2.7 ± 0.5 (2.7, 2.5–3.0) | 0.013 |
| CRP (mg/L) | 11.6 ± 9.1 (9.0, 4.0–17.6) | 8.5 ± 7.2 (6.3, 3.3–11.2) | <0.001 |
| Lactate (mg/dL) | 3.4 ± 4.2 (2.0, 1.3–3.3) | 2.1 ±2.6 (1.4, 1.1–2.2) | <0.001 |
| WBC (/mm3) | 13.6 ± 12.6 (11.1, 7.8–15.7) | 12.4 ± 8.0 (10.8, 7.5–15.2) | 0.163 |
| mNUTRIC | <0.001 | ||
| Low risk | 103 (21.0%) | 129 (48.5%) | |
| High risk | 387 (79.0%) | 137 (51.5%) | |
| NRS2002 | <0.001 | ||
| Low risk | 4 (0.8%) | 20 (7.5%) | |
| High risk | 486 (99.2%) | 246 (92.5%) | |
| MNA-SF | 0.851 | ||
| Low risk | 93 (19.0%) | 49 (18.4%) | |
| High risk | 397 (81.0%) | 217 (81.6%) | |
| 28-day mortality | 0.515 | ||
| Death (N, %) | 119 (24.3%) | 59 (22.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, S.H.; Kim, Y.; Lee, G.H.; Kim, H.-s.; Lim, S.Y.; Choi, S.A. Optimal Nutritional Support Strategy Based on the Association between Modified NUTRIC Score and 28-Day Mortality in Critically Ill Patients: A Prospective Study. Nutrients 2023, 15, 2465. https://doi.org/10.3390/nu15112465
Park S, Park SH, Kim Y, Lee GH, Kim H-s, Lim SY, Choi SA. Optimal Nutritional Support Strategy Based on the Association between Modified NUTRIC Score and 28-Day Mortality in Critically Ill Patients: A Prospective Study. Nutrients. 2023; 15(11):2465. https://doi.org/10.3390/nu15112465
Chicago/Turabian StylePark, Sunny, So Hyang Park, Yeju Kim, Geon Ho Lee, Hyung-sook Kim, Sung Yoon Lim, and Soo An Choi. 2023. "Optimal Nutritional Support Strategy Based on the Association between Modified NUTRIC Score and 28-Day Mortality in Critically Ill Patients: A Prospective Study" Nutrients 15, no. 11: 2465. https://doi.org/10.3390/nu15112465
APA StylePark, S., Park, S. H., Kim, Y., Lee, G. H., Kim, H.-s., Lim, S. Y., & Choi, S. A. (2023). Optimal Nutritional Support Strategy Based on the Association between Modified NUTRIC Score and 28-Day Mortality in Critically Ill Patients: A Prospective Study. Nutrients, 15(11), 2465. https://doi.org/10.3390/nu15112465







