Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Anthropometrics and Clinical Evaluation
2.3. Samples Collection and Cytokines Analysis
2.4. Lipid Extraction from RBC Membranes and Gas Chromatography Analysis of Fatty Acid Methyl Esters (FAME)
2.5. Statistical Analysis
3. Results
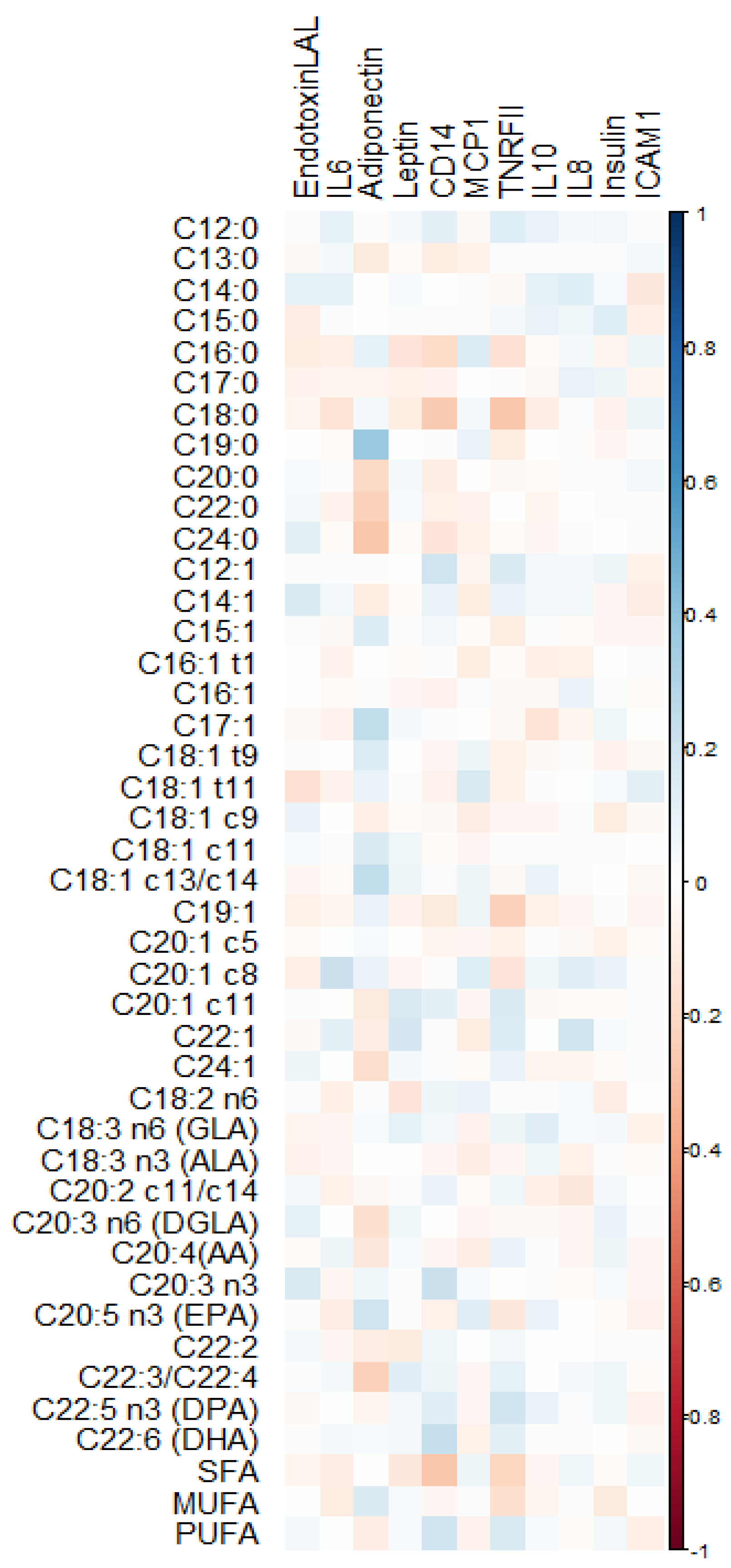
3.1. Distribution of RBC Fatty Acids and Circulating Cytokines
3.2. Quantile Regression between RBC Fatty Acids and Cytokines
3.3. Multiple Quantile Regression between RBC Fatty Acids and Cytokines
3.4. Mediation Analysis
4. Discussion
4.1. Adipokines: Leptin and Adiponectin
4.2. Inflammatory and Anti-Inflammatory Cytokines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene expression profiling of human decidual macrophages: Evidence for immunosuppressive phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Desoye, G. Maternal and fetal metabolism during pregnancy and its relationship with gestational diabetes. Endocrinology 2016, 26, 28–35. [Google Scholar]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. JPEN J. Parenter. Enter. Nutr. 2015, 39 (Suppl. 1), 18S–32S. [Google Scholar] [CrossRef]
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 2), S5–S21. [Google Scholar] [CrossRef]
- Cinelli, G.; Fabrizi, M.; Ravà, L.; Signore, F.; Vernocchi, P.; Semeraro, M.; Vallone, C.; Lanciotti, R.; Ciofi Degli Atti, M.; Manco, M. Association between Maternal and Foetal Erythrocyte Fatty Acid Profiles and Birth Weight. Nutrients 2018, 10, 402. [Google Scholar] [CrossRef]
- Cinelli, G.; Fabrizi, M.; Ravà, L.; Ciofi Degli Atti, M.; Vernocchi, P.; Vallone, C.; Pietrantoni, E.; Lanciotti, R.; Signore, F.; Manco, M. Influence of Maternal Obesity and Gestational Weight Gain on Maternal and Foetal Lipid Profile. Nutrients 2016, 8, 368. [Google Scholar] [CrossRef]
- SIGO: Società Italiana di Ginecologia ed Ostetricia. Available online: www.sigo.it (accessed on 17 January 2016).
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual, 1st ed.; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37, S14–S40. [Google Scholar]
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Gilli, G.; Bona, G.; Fabris, C.; De Curtis, M.; et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Cruz-Hernandez, C. Fast analysis by gas-liquid chromatography. Perspect. Resolut. Complex. Fat. Acid. Compos. J. Chromatogr. A 2007, 1169, 175–178. [Google Scholar] [CrossRef]
- Alves-Santos, N.H.; Cocate, P.G.; Eshriqui, I.; Benaim, C.; Barros, É.G.; Emmett, P.M.; Kac, G. Dietary patterns and their association with adiponectin and leptin concentrations throughout pregnancy: A prospective cohort. Br. J. Nutr. 2018, 119, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Basu, S.; Presley, L.; Serre, D.; Catalano, P.M.; Hauguel-de Mouzon, S. Patterns of adiponectin expression in term pregnancy: Impact of obesity. J. Clin. Endocrinol. Metab. 2014, 99, 3427–3434. [Google Scholar] [CrossRef]
- Ericsson, A.; Hamark, B.; Jansson, N.; Johansson, B.R.; Powell, T.L.; Jansson, T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R656–R662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.W.; Vatish, M.; Randeva, H.S. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia 2006, 49, 1292–1302. [Google Scholar] [CrossRef]
- Moyce Gruber, B.L.; Cole, L.K.; Xiang, B.; Fonseca, M.A.; Klein, J.; Hatch, G.M.; Doucette, C.A.; Dolinsky, V.W. Adiponectin deficiency induces hepatic steatosis during pregnancy and gestational diabetes in mice. Diabetologia 2022, 65, 733–747. [Google Scholar] [CrossRef]
- Salvator, H.; Grassin-Delyle, S.; Brollo, M.; Couderc, L.J.; Abrial, C.; Victoni, T.; Naline, E.; Devillier, P. Adiponectin Inhibits the Production of TNF-α, IL-6 and Chemokines by Human Lung Macrophages. Front. Pharmacol. 2021, 12, 718929. [Google Scholar] [CrossRef]
- Sauerwald, T.U.; Hachey, D.L.; Jensen, C.L.; Chen, H.; Anderson, R.E.; Heird, W.C. Intermediates in endogenous synthesis of C22:6 omega 3 and C20:4 omega 6 by term and preterm infants. Pediatr. Res. 1997, 41, 183–187. [Google Scholar] [CrossRef]
- Sandhir, R.; Khan, M.; Chahal, A.; Singh, I. Localization of nervonic acid beta-oxidation in human and rodent peroxisomes: Impaired oxidation in Zellweger syndrome and X-linked adrenoleukodystrophy. J. Lipid Res. 1998, 39, 2161–2171. [Google Scholar] [CrossRef]
- Hester, M.S.; Tulina, N.; Brown, A.; Barila, G.; Elovitz, M.A. Intrauterine inflammation reduces postnatal neurogenesis in the hippocampal subgranular zone and leads to accumulation of hilar ectopic granule cells. Brain Res. 2018, 1685, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef] [PubMed]
- Brünnert, D.; Kumar, V.; Kaushik, V.; Ehrhardt, J.; Chahar, K.R.; Sharma, P.K.; Zygmunt, M.; Goyal, P. Thrombin impairs the angiogenic activity of extravillous trophoblast cells via monocyte chemotactic protein-1 (MCP-1): A possible link with preeclampsia. Reprod. Biol. 2021, 21, 100516. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Knöfler, M. Human tumour necrosis factor: Physiological and pathological roles in placenta and endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Haghiac, M.; Yang, X.H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef]
- Pietrantoni, E.; Del Chierico, F.; Rigon, G.; Vernocchi, P.; Salvatori, G.; Manco, M.; Signore, F.; Putignani, L. Docosahexaenoic acid supplementation during pregnancy: A potential tool to prevent membrane rupture and preterm labor. Int. J. Mol. Sci. 2014, 15, 8024–8036. [Google Scholar] [CrossRef]


| Mothers (n = 250) | |
| Age (years) | 32.6 (29.0–37.0) |
| Maternal smoking | 37 (10.8%) |
| Gestational diabetes | 9 (3.6%) |
| Body mass index and nutritional status | |
| Pre–pregnancy BMI (kg/m2) | 23.0 (19.7–24.8) |
| Underweight | 20 (8%) |
| Normal weight | 169 (67.6%) |
| Overweight | 40 (16%) |
| Obese | 21 (8.4%) |
| Education | |
| Primary school | 5 (2%) |
| Secondary school | 37 (14.8%) |
| High school | 117 (46.8%) |
| Bachelor’s degree | 91 (36.4%) |
| Gestational weight gain | |
| GWG (kg) | 13.37 (10.0–17.00) |
| Inadequate | 67 (26.8%) |
| Adequate | 91 (36.4%) |
| Excessive | 92 (36.8%) |
| Delivery | |
| Vaginal | 181 (72.4%) |
| Cesarean | 69 (27.6) |
| Gestational age | 39.1 (38–40) |
| Newborn birth weight (g) | 3383 (3060–3670) |
| Newborn birth length (cm) | 50.6 (49–52) |
| Cytokines | |
|---|---|
| Adiponectin (mcg/mL) | 4.92 (2.39–9) |
| Leptin (ng/mL) | 25.05 (12.76–43.54) |
| Endotoxin (EU/mL) | 2.35 (1.85–3.13) |
| Soluble cluster of differentiation 14 (sCD14) (mcg/mL) | 1.84 (1.14–3.06) |
| Tumor necrosis factor receptor II (TNFR-II) (pg/mL) | 797.6 (531.0–1010.0) |
| Interleukin-6 (IL-6) (pg/mL) | 24.09 (7.69–64.61) |
| Interleukin-8 (IL-8) (pg/mL) | 6.94 (3.39–12.66) |
| Interleukin-10 (IL-10) (pg/mL) | 19.59 (10.26–35.38) |
| Insulin (μUI/mL) | 16.2 (8.3–22) |
| Intercellular Adhesion Molecule 1 (ICAM-1) (pg/mL) | 354.55 (226.82–662.15) |
| Monocyte Chemoattractant Protein 1 (MCP-1) (ng/mL) | 26.19(16.23–36.96) |
| FA (%) | Coefficient | p-Value |
|---|---|---|
| Adiponectin (mcg/mL) | ||
| C12:0 | 0.82 | 0.020 |
| C20:0 | −5.16 | 0.028 |
| C24:0 | 1.20 | <0.001 |
| C18:1 c13/c14 | 1.50 | 0.015 |
| C20:1 c11 | −4.89 | 0.037 |
| C24:1 | −0.52 | 0.037 |
| C22:3/C22:4 | −1.07 | 0.002 |
| Leptin (ng/mL) | ||
| C22:1 | −6.90 | 0.040 |
| sCD14 (mcg/mL) | ||
| C12:0 | 0.25 | 0.009 |
| C16:0 | −0.12 | 0.010 |
| C18:0 | −0.15 | 0.001 |
| C20:0 | −1.20 | 0.023 |
| C24:0 | −0.16 | 0.008 |
| C12:1 | 0.43 | <0.001 |
| C20:1 c11 | 1.50 | 0.020 |
| C18:2 n6 | 0.07 | 0.040 |
| C22:6 (DHA) | 0.23 | <0.001 |
| SFA | −0.06 | 0.001 |
| TNRF-II (pg/mL) | ||
| C16:0 | −31.50 | 0.010 |
| C18:0 | −55.80 | <0.001 |
| C12:1 | 81.70 | 0.003 |
| C19:1 | −517.8 | 0.002 |
| C20:1 c11 | −385.60 | 0.040 |
| C22:3/C22:4 | −70.08 | 0.040 |
| C22:5 n3 (DPA) | 74.82 | 0.040 |
| SFAs | −14.77 | 0.010 |
| MUFAs | −13.36 | <0.001 |
| PUFAs | 12.81 | 0.006 |
| ICAM-1 (pg/mL) | ||
| C18:0 | −20.14 | 0.002 |
| C14:1 | −52.69 | <0.001 |
| SFAs | 8.09 | 0.010 |
| IL-8 (pg/mL) | ||
| C14:0 | 2.49 | 0.002 |
| C15:0 | 3.02 | 0.040 |
| C22:0 | −2.16 | 0.010 |
| C12:1 | 1.11 | 0.020 |
| C24:1 | −0.5 | 0.015 |
| FAs (%) | Coefficient | p-Value |
|---|---|---|
| Adiponectin (mcg/mL) | ||
| C18:1 c13/c14 | 1.20 | 0.020 |
| C22:3/C22:4 | −1.44 | 0.008 |
| C22:6 (DHA) | 0.62 | 0.060 |
| Endotoxin (EU/mL) | ||
| C20:1 c5 | −0.90 | 0.030 |
| C22:1 | −0.40 | 0.050 |
| Leptin (ng/mL) | ||
| Maternal body weight at week 38 | 0.90 | <0.001 |
| sCD14 (mcg/mL) | ||
| C22:6 (DHA) | 0.20 | 0.060 |
| TNRF-II (pg/mL) | ||
| C24:1 | 47.90 | 0.070 |
| GDM | 523.1 | 0.006 |
| ICAM-1 (pg/mL) | ||
| C14:0 | −86.80 | 0.045 |
| C14:1 | −63.30 | 0.060 |
| GDM | 688 | 0.060 |
| Smoking habit | 133 | 0.090 |
| IL-6 (pg/mL) | ||
| C24:1 | 3.70 | 0.10 |
| IL-8 (pg/mL) | ||
| C19:1 | −5.80 | 0.090 |
| C18:2 n6 | 0.50 | 0.060 |
| Maternal age | 0.10 | 0.040 |
| IL-10 (pg/mL) | ||
| C24:1 | −2.50 | 0.060 |
| MCP-1 (ng/mL) | ||
| C15:1 | 0.80 | 0.040 |
| C18:1 t11 | −9.50 | 0.070 |
| Maternal age | 0.40 | 0.090 |
| Association of patterns of FAs with cytokines | ||
| sCD14 (mcg/mL) | ||
| SFAs | −0.07 | 0.003 |
| ICAM-1 (pg/mL) | ||
| SFAs | 7.77 | 0.080 |
| TNRF-II (pg/mL) | ||
| SFAs | −12.1 | 0.050 |
| MUFAs | −17 | 0.003 |
| Cytokine (y) | Fatty acid (x) | Mediator (m) | y~x Pval | m~x Pval | Mediation Pval |
|---|---|---|---|---|---|
| IL-8 | C14:0 | Insulin | 0.018 | 0.034 | 0.408 |
| TNFR-II | C22:5 | Insulin | 0.040 | 0.009 | 0.548 |
| sCD14 | C16:0 | Pre-gravid BMI | 0.012 | 0.015 | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policastro, V.; Righelli, D.; Ravà, L.; Vernocchi, P.; Bianchi, M.; Vallone, C.; Signore, F.; Manco, M. Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy. Nutrients 2023, 15, 2432. https://doi.org/10.3390/nu15112432
Policastro V, Righelli D, Ravà L, Vernocchi P, Bianchi M, Vallone C, Signore F, Manco M. Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy. Nutrients. 2023; 15(11):2432. https://doi.org/10.3390/nu15112432
Chicago/Turabian StylePolicastro, Valeria, Dario Righelli, Lucilla Ravà, Pamela Vernocchi, Marzia Bianchi, Cristina Vallone, Fabrizio Signore, and Melania Manco. 2023. "Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy" Nutrients 15, no. 11: 2432. https://doi.org/10.3390/nu15112432
APA StylePolicastro, V., Righelli, D., Ravà, L., Vernocchi, P., Bianchi, M., Vallone, C., Signore, F., & Manco, M. (2023). Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy. Nutrients, 15(11), 2432. https://doi.org/10.3390/nu15112432








