Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
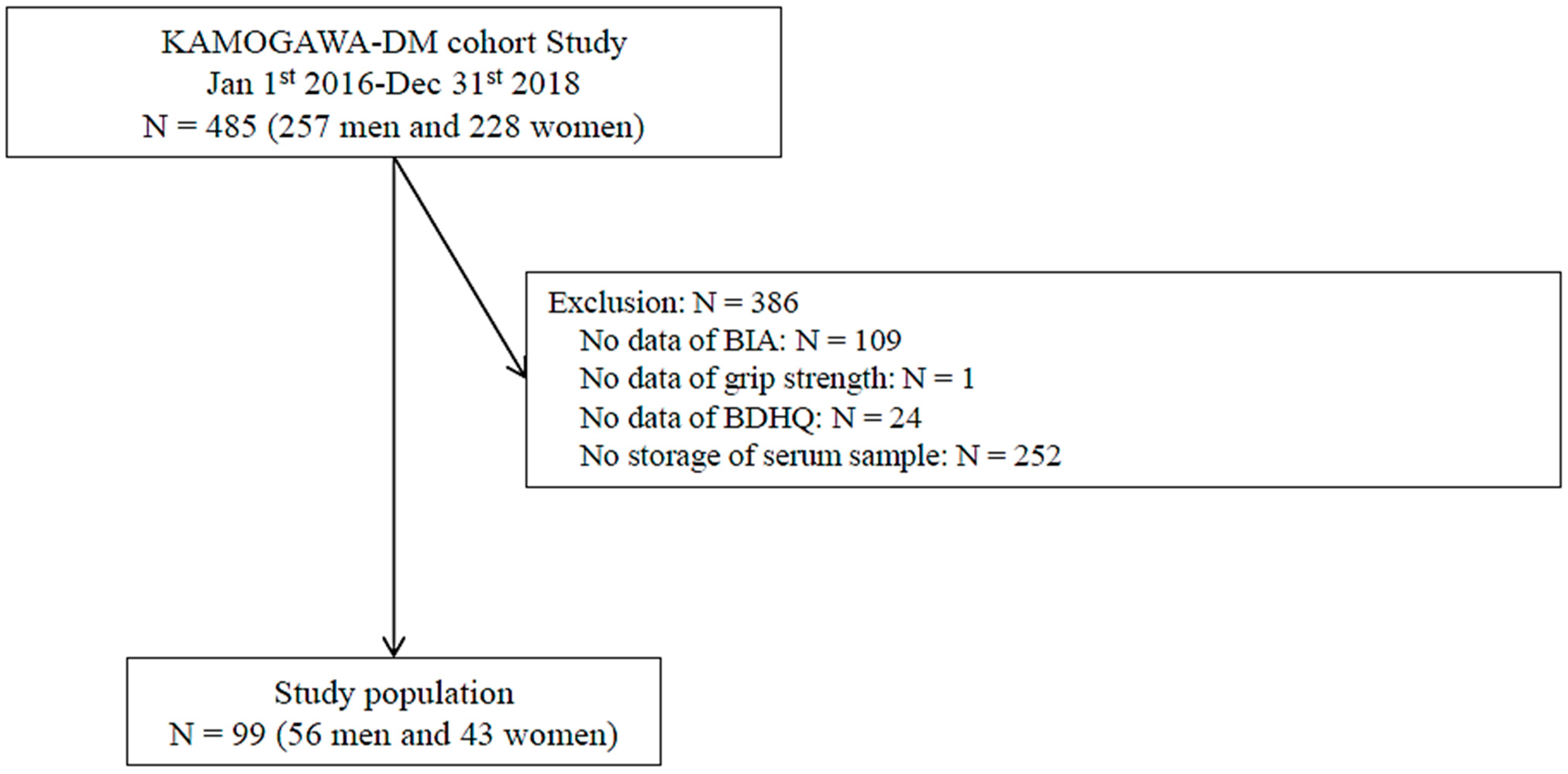
2.1. Study Population
2.2. Data Collection
2.3. Definition of Sarcopenic Risk
2.4. Metabolomic Analyses
2.5. Statistical Analyses
3. Results
3.1. Study Participants
3.2. Sarcopenic Risk and Plasma Metabolites
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; Di Daniele, N.; et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1646. [Google Scholar] [CrossRef] [PubMed]
- Purnamasari, D.; Tetrasiwi, E.; Kartiko, G.; Astrella, C.; Husam, K.; Laksmi, P. Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev. Diabet. Stud. 2022, 18, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della, P. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, K.; Kim, M.; Kim, S.; Cho, Y.; Park, S. Type 2 Diabetes Is Associated with Low Muscle Mass in Older Adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pollakova, D.; Tubili, C.; Folco, U.; Giuseppe, R.; Battino, M.; Giampieri, F. Muscular Involvement in Long Term Type 1 Diabetes: Does It Represent an Underestimated Complication? Nutrition 2023, 112060, in press. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Synergistic Effect of Bodyweight Resistance Exercise and Protein Supplementation on Skeletal Muscle in Sarcopenic or Dynapenic Older Adults. Geriatr. Gerontol. Int. 2019, 19, 429–437. [Google Scholar] [CrossRef]
- Amasene, M.; Besga, A.; Echeverria, I.; Urquiza, M.; Ruiz, J.R.; Rodriguez-Larrad, A.; Aldamiz, M.; Anaut, P.; Irazusta, J.; Labayen, I. Effects of Leucine-Enriched Whey Protein Supplementation on Physical Function in Post-Hospitalized Older Adults Participating in 12-Weeks of Resistance Training Program: A Randomized Controlled Trial. Nutrients 2019, 11, 2337. [Google Scholar] [CrossRef]
- Björkman, M.P.; Suominen, M.H.; Kautiainen, H.; Jyväkorpi, S.K.; Finne-Soveri, H.U.; Strandberg, T.E.; Pitkälä, K.H.; Tilvis, R.S. Effect of Protein Supplementation on Physical Performance in Older People With Sarcopenia-A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2020, 21, 226–231. [Google Scholar] [CrossRef]
- Xu, Z.; Tan, Z.; Zhang, Q.; Gui, Q.; Yang, Y. The Effectiveness of Leucine on Muscle Protein Synthesis, Lean Body Mass and Leg Lean Mass Accretion in Older People: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef]
- Aoyama, S.; Kim, H.-K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of Dietary Protein Intake in Daily Meals Influences Skeletal Muscle Hypertrophy via the Muscle Clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef]
- Shin, H.E.; Won, C.W.; Kim, M. Metabolomic Profiles to Explore Biomarkers of Severe Sarcopenia in Older Men: A Pilot Study. Exp. Gerontol. 2022, 167, 111924. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.; Bossola, M.; Urbani, A.; et al. A Distinct Pattern of Circulating Amino Acids Characterizes Older Persons with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2018, 10, 1691. [Google Scholar] [CrossRef]
- Nakajima, H.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Takahashi, F.; Yoshimura, Y.; Bamba, R.; Okamura, T.; Kitagawa, N.; Majima, S.; et al. Impact of Extracellular-to-intracellular Fluid Volume Ratio on Albuminuria in Patients with Type 2 Diabetes: A Cross-sectional and Longitudinal Cohort Study. J. Diabetes Investig. 2021, 12, 1202–1211. [Google Scholar] [CrossRef]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Sakai, R.; Okamura, T.; Miki, A.; Hamaguchi, M.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Sarcopenia Is Associated with Tongue Pressure in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort Study: Sarcopenia and Tongue Pressure. Geriatr. Gerontol. Int. 2019, 19, 153–158. [Google Scholar] [CrossRef]
- Lemmens, H.J.M.; Brodsky, J.B.; Bernstein, D.P. Estimating Ideal Body Weight—A New Formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Nakajima, H.; Nakanishi, N.; Miyoshi, T.; Okamura, T.; Hashimoto, Y.; Senmaru, T.; Majima, S.; Ushigome, E.; Asano, M.; Yamaguchi, M.; et al. Inulin Reduces Visceral Adipose Tissue Mass and Improves Glucose Tolerance through Altering Gut Metabolites. Nutr. Metab. 2022, 19, 50. [Google Scholar] [CrossRef]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W.; Kraus, W.E. Multimarkers of Metabolic Malnutrition and Inflammation and Their Association with Mortality Risk in Cardiac Catheterisation Patients: A Prospective, Longitudinal, Observational, Cohort Study. Lancet Healthy Longev. 2023, 4, e72–e82. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and Contractile Regulation of Human Skeletal Muscle Protein Synthesis and MTORC1 Signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef]
- Zaromskyte, G.; Prokopidis, K.; Ioannidis, T.; Tipton, K.D.; Witard, O.C. Evaluating the Leucine Trigger Hypothesis to Explain the Post-Prandial Regulation of Muscle Protein Synthesis in Young and Older Adults: A Systematic Review. Front. Nutr. 2021, 8, 685165. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, A. Muscle Amino Acid Metabolism at Rest and during Exercise: Role in Human Physiology and Metabolism. Exerc. Sport Sci. Rev. 1998, 26, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Wade, C.E. Metabolic Consequences of Muscle Disuse Atrophy. J. Nutr. 2005, 135, 1824S–1828S. [Google Scholar] [CrossRef] [PubMed]
- Ilaiwy, A.; Quintana, M.T.; Bain, J.R.; Muehlbauer, M.J.; Brown, D.I.; Stansfield, W.E.; Willis, M.S. Cessation of Biomechanical Stretch Model of C2C12 Cells Models Myocyte Atrophy and Anaplerotic Changes in Metabolism Using Non-Targeted Metabolomics Analysis. Int. J. Biochem. Cell Biol. 2016, 79, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place? Int. J. Mol. Sci. 2020, 21, 5635. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, H.; Liu, J.; Chiu, C.-Y.; Su, K.-J.; Tian, Q.; Kakhniashvili, D.; Qiu, C.; Zhao, L.-J.; Luo, Z.; et al. Pathway-Based Metabolomics Study of Sarcopenia-Related Traits in Two US Cohorts. Aging 2022, 14, 2101–2112. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in Health and Disease. Review on Human Studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef]
- Le Plénier, S.; Walrand, S.; Noirt, R.; Cynober, L.; Moinard, C. Effects of Leucine and Citrulline versus Non-Essential Amino Acids on Muscle Protein Synthesis in Fasted Rat: A Common Activation Pathway? Amino Acids 2012, 43, 1171–1178. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Serum Amino Acids in Association with Prevalent and Incident Type 2 Diabetes in A Chinese Population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef]
- Yun, J.H.; Lee, H.-S.; Yu, H.-Y.; Kim, Y.-J.; Jeon, H.J.; Oh, T.; Kim, B.-J.; Choi, H.J.; Kim, J.-M. Metabolomics Profiles Associated with HbA1c Levels in Patients with Type 2 Diabetes. PLoS ONE 2019, 14, e0224274. [Google Scholar] [CrossRef]
- Horton, W.; Barrett, E. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Rea, S.; Della-Morte, D.; Pacifici, F.; Capuani, B.; Pastore, D.; Coppola, A.; Arriga, R.; Andreadi, A.; Donadel, G.; Di Daniele, N.; et al. Insulin and Exendin-4 Reduced Mutated Huntingtin Accumulation in Neuronal Cells. Front. Pharmacol. 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]

| Total | Sarcopenic Risk | p Value | ||
|---|---|---|---|---|
| (−) | (+) | |||
| N | 99 | 72 | 27 | - |
| Age, years | 63.7 ± 11.9 | 61.3 ± 1.3 | 70.2 ± 2.2 | 0.001 |
| Male, % | 56.6 | 55.6 | 59.3 | 0.741 |
| Duration of diabetes, years | 11.3 ± 7.7 | 10.0 ± 0.9 | 14.8 ± 1.4 | 0.005 |
| Height, cm | 162.0 ± 9.3 | 162.3 ± 1.1 | 161.3 ± 1.8 | 0.627 |
| Body weight, kg | 62.8 ± 12.6 | 65.3 ± 1.4 | 56.1 ± 2.3 | 0.001 |
| Body mass index, kg/m2 | 23.9 ± 4.1 | 24.7 ± 0.5 | 21.6 ± 0.7 | 0.001 |
| Plasma glucose, mg/dL | 143.2 ± 49.8 | 144.2 ± 5.9 | 140.6 ± 9.6 | 0.748 |
| HbA1c, % | 7.3 ± 1.3 | 7.4 ± 0.2 | 7.3 ± 0.3 | 0.717 |
| Cr, mg/dL | 0.9 ± 0.5 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.151 |
| Skeletal muscle mass, kg | 18.7 ± 4.2 | 19.4 ± 0.5 | 17.1 ± 0.8 | 0.017 |
| SMI, kg/m2 | 7.0 ± 0.9 | 7.2 ± 0.1 | 6.5 ± 0.2 | <0.001 |
| GS, kg | 28.5 ± 9.6 | 30.9 ± 1.0 | 22.1 ± 1.7 | <0.001 |
| Use of SGLT2 inhibitor, % | 18.2 | 20.8 | 11.1 | 0.264 |
| Use of insulin, % | 19.2 | 18.1 | 22.2 | 0.639 |
| Exercise habit (−/+) | 50/49 (50.1/49.5) | 34/38 (47.2/52.8) | 16/11 (59.3/40.7) | 0.286 |
| Total | Sarcopenic Risk | p Value | |
|---|---|---|---|
| (−) | (+) | ||
| N | 72 | 27 | |
| Alanine, nmol/L | 0.418 ± 0.011 | 0.380 ± 0.018 | 0.077 |
| Valine, nmol/L | 0.272 ± 0.007 | 0.254 ± 0.011 | 0.164 |
| Leucine, nmol/L | 0.151 ± 0.004 | 0.135 ± 0.007 | 0.043 |
| Isoleucine, nmol/L | 0.077 ± 0.002 | 0.073 ± 0.004 | 0.437 |
| Proline, nmol/L | 0.184 ± 0.008 | 0.183 ± 0.014 | 0.937 |
| Glycine, nmol/L | 0.240 ± 0.007 | 0.262 ± 0.012 | 0.103 |
| Serine, nmol/L | 0.126 ± 0.003 | 0.115 ± 0.005 | 0.06 |
| Threonine, nmol/L | 0.117 ± 0.004 | 0.119 ± 0.006 | 0.773 |
| Malic acid, nmol/L | 0.011 ± 0.003 | 0.007 ± 0.004 | 0.417 |
| Aspartic acid, nmol/L | 0.016 ± 0.001 | 0.012 ± 0.002 | 0.051 |
| Methionine, nmol/L | 0.018 ± 0.001 | 0.018 ± 0.001 | 0.764 |
| Glutamic acid, nmol/L | 0.062 ± 0.005 | 0.041 ± 0.008 | 0.031 |
| Phenylalanine, nmol/L | 0.082 ± 0.002 | 0.074 ± 0.004 | 0.082 |
| Citric acid, nmol/L | 0.021 ± 0.001 | 0.020 ± 0.002 | 0.785 |
| Lysine, nmol/L | 0.120 ± 0.004 | 0.117 ± 0.006 | 0.708 |
| Tyrosine, nmol/L | 0.053 ± 0.002 | 0.048 ± 0.003 | 0.171 |
| Cystine, nmol/L | 0.011 ± 0.001 | 0.015 ± 0.002 | 0.086 |
| Total | SMI, kg/m2 | GS, kg | Age, years | HbA1c, % | ||||
|---|---|---|---|---|---|---|---|---|
| γ | p | γ | p | γ | p | γ | p | |
| Alanine, nmol/L | 0.093 | 0.359 | 0.07 | 0.491 | −0.184 | 0.069 | 0.045 | 0.658 |
| Valine, nmol/L | 0.19 | 0.059 | 0.183 | 0.07 | −0.227 | 0.024 | 0.197 | 0.051 |
| Leucine, nmol/L | 0.338 | 0.001 | 0.302 | 0.002 | −0.267 | 0.008 | 0.163 | 0.108 |
| Isoleucine, nmol/L | 0.286 | 0.004 | 0.208 | 0.039 | −0.099 | 0.331 | 0.08 | 0.43 |
| Proline, nmol/L | 0.149 | 0.141 | 0.016 | 0.878 | −0.031 | 0.763 | −0.095 | 0.348 |
| Glycine, nmol/L | −0.063 | 0.537 | −0.193 | 0.056 | 0.112 | 0.272 | −0.091 | 0.368 |
| Serine, nmol/L | 0.064 | 0.53 | 0.062 | 0.543 | −0.24 | 0.017 | 0.17 | 0.093 |
| Threonine, nmol/L | 0.141 | 0.165 | 0.132 | 0.194 | −0.037 | 0.713 | −0.045 | 0.657 |
| Malic acid, nmol/L | 0.019 | 0.867 | 0.038 | 0.738 | −0.106 | 0.351 | −0.091 | 0.421 |
| Aspartic acid, nmol/L | −0.006 | 0.954 | 0.049 | 0.627 | −0.189 | 0.061 | 0.185 | 0.067 |
| Methionine, nmol/L | 0.195 | 0.053 | 0.168 | 0.096 | 0.067 | 0.512 | −0.027 | 0.791 |
| Glutamic acid, nmol/L | 0.164 | 0.105 | 0.262 | 0.009 | −0.391 | <0.001 | 0.263 | 0.008 |
| Phenylalanine, nmol/L | 0.072 | 0.481 | 0.094 | 0.358 | −0.177 | 0.079 | 0.145 | 0.153 |
| Citric acid, nmol/L | 0.145 | 0.199 | 0.072 | 0.528 | 0.188 | 0.095 | −0.1437 | 0.226 |
| Lysine, nmol/L | 0.121 | 0.234 | 0.157 | 0.121 | −0.12 | 0.236 | 0.11 | 0.281 |
| Tyrosine, nmol/L | 0.045 | 0.659 | 0.085 | 0.401 | 0.0004 | 0.997 | 0.061 | 0.547 |
| Cystine, nmol/L | 0.047 | 0.674 | −0.129 | 0.245 | 0.317 | 0.004 | −0.215 | 0.051 |
| Variables | Model 1 Unadjusted | Model 2 Adjusted | Model 3 Adjusted | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Leucine (low) (ref: high) | 1.56 (0.53–4.58) | 0.416 | - | - | 1.09 (0.34–3.51) | 0.888 |
| Glutamic acid (low) (ref: high) | 5.34 (1.53–18.7) | 0.019 | 4.27 (1.07–17.11) | 0.041 | - | - |
| Age, years | 1.10 (1.04–1.17) | <0.001 | 1.1 (1.03–1.17) | 0.005 | 1.1 (1.04–1.17) | 0.002 |
| HbA1c, % | 0.94 (0.66–1.32) | 0.711 | 1.13 (0.72–1.79) | 0.599 | 1.1 (1.04–1.17) | 0.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, H.; Okada, H.; Kobayashi, A.; Takahashi, F.; Okamura, T.; Hashimoto, Y.; Nakanishi, N.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; et al. Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes. Nutrients 2023, 15, 2400. https://doi.org/10.3390/nu15102400
Nakajima H, Okada H, Kobayashi A, Takahashi F, Okamura T, Hashimoto Y, Nakanishi N, Senmaru T, Ushigome E, Hamaguchi M, et al. Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes. Nutrients. 2023; 15(10):2400. https://doi.org/10.3390/nu15102400
Chicago/Turabian StyleNakajima, Hanako, Hiroshi Okada, Ayaka Kobayashi, Fuyuko Takahashi, Takuro Okamura, Yoshitaka Hashimoto, Naoko Nakanishi, Takafumi Senmaru, Emi Ushigome, Masahide Hamaguchi, and et al. 2023. "Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes" Nutrients 15, no. 10: 2400. https://doi.org/10.3390/nu15102400
APA StyleNakajima, H., Okada, H., Kobayashi, A., Takahashi, F., Okamura, T., Hashimoto, Y., Nakanishi, N., Senmaru, T., Ushigome, E., Hamaguchi, M., & Fukui, M. (2023). Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes. Nutrients, 15(10), 2400. https://doi.org/10.3390/nu15102400










