No Evidence That Circulating GLP-1 or PYY Are Associated with Increased Satiety during Low Energy Diet-Induced Weight Loss: Modelling Biomarkers of Appetite
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participant Recruitment and Eligibility
2.3. LED Intervention
= 0.4 × Basal Metabolic Rate (BMR, Harris–Benedict Equation for women) × Estimated Physical Activity Level (PAL)
= 0.4 × (655 + (9.6 × weight in kg) + (1.8 × height in cm) − (4.7 × age in years)) × 4.184 (conversion from kcal to kJ) × 1.375 (assumed undertaking light activity at work)
2.4. Clinic Visits and Preload Challenge Protocol
2.5. Laboratory Analysis
2.6. Statistical Analysis
3. Results
3.1. Body Weight Change and Body Composition
3.2. Effect of LED Intervention on VAS–Appetite Responses and Blood Biomarkers
3.2.1. VAS
3.2.2. Blood Biomarkers
3.3. Associations between VAS–Appetite Responses and Blood Biomarkers
3.3.1. Incremental Increase in Postprandial VAS–Appetite Responses Were Strongly Inversely Predicted by Fasted Responses
3.3.2. High Circulating GLP-1 was Unexpectedly Positively Associated with Hunger and TOF and Inversely Associated with Fullness and Satisfaction
3.3.3. Glycine as a Potential Biomarker for VAS–Appetite Responses
4. Discussion
4.1. The Utility of Measuring Circulating GLP-1 and PYY during Appetite Assessments
4.2. The Utility of Measuring Circulating GIP during Appetite Assessments
4.3. The Utility of Measuring Circulating AAs during Appetite Assessments
4.4. Considerations When Modelling the Biomarkers of Appetite
4.5. Strengths and Limitations
4.6. Recommendations for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Noronha, J.C.; Thom, G.; Lean, M.E.J. Total dietreplacement within an integrated intensive lifestyle intervention for remission of type 2 diabetes: Lessons from DiRECT. Front. Endocrinol. 2022, 13, 888557. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Davis, L.; Wood, G.C.; Benotti, P.; Cook, A.; Dove, J.; Mowery, J.; Ramasamy, A.; Iyer, N.N.; Smolarz, B.G.; Kumar, N.; et al. Impact of sustained weight loss on cardiometabolic outcomes. Am. J. Cardiol. 2022, 162, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Andersen, S.V.; Astrup, A.; Blundell, J.; Sjodin, A. Is reducing appetite beneficial for body weight management in the context of overweight and obesity? A systematic review and meta-analysis from clinical trials assessing body weight management after exposure to satiety enhancing and/or hunger reducing products. Obes. Rev. 2019, 20, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Mead, B.R.; García-Gavilán, J.F.; Korndal, S.K.; Harrold, J.A.; Camacho-Barcía, L.; Ritz, C.; Christiansen, P.; Salas-Salvadó, J.; Hjorth, M.F.; et al. Is reduction in appetite beneficial for body weight management in the context of overweight and obesity? Yes, according to the SATIN (Satiety Innovation) study. J. Nutr. Sci. 2019, 8, e39. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.H.; Alvarez-Leite, J.I. Appetite control: Hormones or diet strategies? Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 328–335. [Google Scholar] [CrossRef]
- Hintze, L.J.; Mahmoodianfard, S.; Auguste, C.B.; Doucet, É. Weight loss and appetite control in women. Curr. Obes. Rep. 2017, 6, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Byrne, N.M.; Cleghorn, G.J.; Näslund, E.; King, N.A. The effects of weight loss strategies on gastric emptying and appetite control. Obes. Rev. 2011, 12, 935–951. [Google Scholar] [CrossRef]
- MacLean, P.S.; Blundell, J.E.; Mennella, J.A.; Batterham, R.L. Biological control of appetite: A daunting complexity. Obesity 2017, 25, S8–S16. [Google Scholar] [CrossRef]
- Melby, C.L.; Paris, H.L.; Foright, R.M.; Peth, J. Attenuating the biologic drive for weight regain following weight loss: Must what goes down always go back up? Nutrients 2017, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Liu, Y.; Lu, L.W.; Barnett, D.; Sequeira, I.R.; Poppitt, S.D. Does a higher protein diet promote satiety and weight loss independent of carbohydrate content? An 8-week low-energy diet (LED) intervention. Nutrients 2022, 14, 538. [Google Scholar] [CrossRef] [PubMed]
- Tochikubo, O.; Nakamura, H.; Jinzu, H.; Nagao, K.; Yoshida, H.; Kageyama, N.; Miyano, H. Weight loss is associated with plasma free amino acid alterations in subjects with metabolic syndrome. Nutr. Diabetes 2016, 6, e197. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. The metabolomic signatures of weight change. Metabolites 2019, 9, 67. [Google Scholar] [CrossRef]
- Dockray, G. Gut endocrine secretions and their relevance to satiety. Curr. Opin. Pharmacol. 2004, 4, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Madsbad, S.; Bojsen-Møller, K.N.; Svane, M.S.; Jørgensen, N.B.; Dirksen, C.; Martinussen, C. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018, 14, 708–714. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Aylwin, S.J.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut hormones as mediators of appetite and weight loss after oux-en-Y Gastric Bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Pedersen, S.D.; Gregersen, N.T.; Vestergaard, L.; Nielsen, M.S.; Ritz, C.; Madsbad, S.; Worm, D.; Hansen, D.L.; Clausen, T.R.; et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: A randomized controlled clinical trial. Int. J. Obes. 2016, 40, 281–290. [Google Scholar] [CrossRef]
- Lim, J.J.; Poppitt, S.D. How satiating are the ‘satiety’ peptides: A problem of pharmacology versus physiology in the development of novel foods for regulation of food intake. Nutrients 2019, 11, 1517. [Google Scholar] [CrossRef]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.J.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J. Clin. Endocrinol. Metab. 2020, 105, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; Blaak, E.E.; Saris, W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef]
- Mars, M.; Stafleu, A.; de Graaf, C. Use of satiety peptides in assessing the satiating capacity of foods. Physiol. Behav. 2012, 105, 483–488. [Google Scholar] [CrossRef]
- Santo, M.A.; Riccioppo, D.; Pajecki, D.; Kawamoto, F.; de Cleva, R.; Antonangelo, L.; Marçal, L.; Cecconello, I. Weight regain after gastric bypass: Influence of gut hormones. Obes. Surg. 2016, 26, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef]
- Cooper, J.A. Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutr. Res. Rev. 2014, 27, 186–197. [Google Scholar] [CrossRef]
- Watkins, J.D.; Koumanov, F.; Gonzalez, J.T. Protein- and calcium-mediated GLP-1 secretion: S narrative review. Adv. Nutr. 2021, 12, 2540–2552. [Google Scholar] [CrossRef]
- Korompokis, K.; Ostman, E.; Dougkas, A. The impact of liquid preloads varying in macronutrient content on postprandial kinetics of amino acids relative to appetite in healthy adults. Appetite 2016, 107, 511–520. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin. Nutr. 2009, 28, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, P.; Berry, S.E.; Finlayson, G.; O’Driscoll, R.; Hadjigeorgiou, G.; Drew, D.A.; Khatib, H.A.; Nguyen, L.H.; Linenberg, I.; Chan, A.T.; et al. Postprandial glycaemic dips predict appetite and energy intake in healthy individuals. Nat. Metab. 2021, 3, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Sequeira, I.R.; Yip, W.C.Y.; Lu, L.W.; Barnett, D.; Cameron-Smith, D.; Poppitt, S.D. Postprandial glycine as a biomarker of satiety: A dose-rising randomised control trial of whey protein in overweight women. Appetite 2022, 169, 105871. [Google Scholar] [CrossRef]
- Giezenaar, C.; Luscombe-Marsh, N.D.; Hutchison, A.T.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr. Diabetes 2018, 8, 40. [Google Scholar] [CrossRef]
- Gregersen, N.; Møller, B.; Raben, A.; Kristensen, S.; Holm, L.; Flint, A.; Astrup, A. Determinants of appetite ratings: The role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr. Res. 2011, 55, 7028. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Larsen, T.M.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with prediabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef]
- Bhogal, M.S.; Langford, R. Gender differences in weight loss; evidence from a NHS weight management service. Public Health 2014, 128, 811–813. [Google Scholar] [CrossRef]
- Crane, M.M.; Jeffery, R.W.; Sherwood, N.E. Exploring gender differences in a randomized trial of weight loss maintenance. Am. J. Men’s Health 2017, 11, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wiessing, K.R.; Xin, L.; McGill, A.T.; Budgett, S.C.; Strik, C.M.; Poppitt, S.D. Sensitivity of ad libitum meals to detect changes in hunger. Restricted-item or multi-item testmeals in the design of preload appetite studies. Appetite 2012, 58, 1076–1082. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, U.K.; Pundir, S.; Chiang, V.S.; Milan, A.M.; Barnett, M.P.G.; Smith, G.C.; Markworth, J.F.; Knowles, S.O.; Cameron-Smith, D. Comparable postprandial amino acid and gastrointestinal hormone responses to beef steak cooked using different methods: A randomised crossover trial. Nutrients 2020, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Drapeau, V.; Astrup, A.; Tremblay, A. Relationship between diet-induced changes in body fat and appetite sensations in women. Appetite 2009, 52, 809–812. [Google Scholar] [CrossRef]
- Hansen, T.T.; Hjorth, M.F.; Sandby, K.; Andersen, S.V.; Astrup, A.; Ritz, C.; Bulló, M.; Camacho-Barcía, M.L.; García-Gavilán, J.F.; Salas-Salvadó, J.; et al. Predictors of successful weight loss with relative maintenance of fat-free mass in individuals with overweight and obesity on an 8-week low-energy diet. Br. J. Nutr. 2019, 122, 468–479. [Google Scholar] [CrossRef]
- Turicchi, J.; O’Driscoll, R.; Finlayson, G.; Duarte, C.; Hopkins, M.; Martins, N.; Michalowska, J.; Larsen, T.M.; van Baak, M.A.; Astrup, A.; et al. Associations between the proportion of fat-free mass loss during weight loss, changes in appetite, and subsequent weight change: Results from a randomized 2-stage dietary intervention trial. Am. J. Clin. Nutr. 2020, 111, 536–544. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS Statistics 27 Step by Step: A Simple Guide and Reference, 17th ed.; Routledge: Abingdon, UK, 2021. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Hopkins, M.; Beaulieu, K.; Oustric, P.; Blundell, J.E. Issues in measuring and interpreting human appetite (satiety/satiation) and its contribution to obesity. Curr. Obes. Rep. 2019, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Pareja, J.C.; Chaim, E.A.; Geloneze, B.; Barreto, M.R.; Magro, D.O. GLP-1 and GLP-2 levels are correlated with satiety regulation after Roux-en-Y gastric bsypass: Results of an exploratory prospective study. Obes. Surg. 2017, 27, 703–708. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghatei, M.A.; et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Van den Broek, M.; de Heide, L.J.M.; Emous, M.; Wijma, R.B.; Veeger, N.; Wolthuis, A.; Laskewitz, A.J.; Heiner-Fokkema, M.R.; Muller Kobold, A.C.; Wolffenbuttel, B.H.R.; et al. Satiety and gastrointestinal hormones during a Mixed Meal Tolerance Test after gastric bypass surgery: Association with plasma amino acid concentrations. Surg. Obes. Relat. Dis. 2018, 14, 1106–1117. [Google Scholar] [CrossRef]
- Coutinho, S.R.; With, E.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. The impact of rate of weight loss on body composition and compensatory mechanisms during weight reduction: A randomized control trial. Clin. Nutr. 2018, 37, 1154–1162. [Google Scholar] [CrossRef]
- Diepvens, K.; Soenen, S.; Steijns, J.; Arnold, M.; Westerterp-Plantenga, M. Long-term effects of consumption of a novel fat emulsion in relation to body-weight management. Int. J. Obes. 2007, 31, 942–949. [Google Scholar] [CrossRef]
- Halliday, T.M.; Polsky, S.; Schoen, J.A.; Legget, K.T.; Tregellas, J.R.; Williamson, K.M.; Cornier, M.-A. Comparison of surgical versus diet-induced weight loss on appetite regulation and metabolic health outcomes. Physiol. Rep. 2019, 7, e14048. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.; Stengel, A. Control of food intake by gastrointestinal peptides: Mechanisms of action and possible modulation in the treatment of obesity. J. Neurogastroenterol. Motil. 2017, 23, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Papamargaritis, D.; le Roux, C.W. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery? Nutrients 2021, 13, 762. [Google Scholar] [CrossRef]
- Adam, T.C.; Lejeune, M.P.; Westerterp-Plantenga, M.S. Nutrient-stimulated glucagon-like peptide 1 release after body-weight loss and weight maintenance in human subjects. Br. J. Nutr. 2006, 95, 160–167. [Google Scholar] [CrossRef]
- Nymo, S.; Coutinho, S.R.; Eknes, P.H.; Vestbostad, I.; Rehfeld, J.F.; Truby, H.; Kulseng, B.; Martins, C. Investigation of the long-term sustainability of changes in appetite after weight loss. Int. J. Obes. 2018, 42, 1489–1499. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Holst, J.J. Glucagon and other proglucagon-derived peptides in the pathogenesis of obesity. Front. Nutr. 2022, 9, 964406. [Google Scholar] [CrossRef]
- Iepsen, E.W.; Lundgren, J.; Holst, J.J.; Madsbad, S.; Torekov, S.S. Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3-36. Eur. J. Endocrinol. 2016, 174, 775–784. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review). Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019, 30, 987–996.e6. [Google Scholar] [CrossRef] [PubMed]
- De Block, C.E.M.; Dirinck, E.; Verhaegen, A.; Van Gaal, L.F. Efficacy and safety of high-dose glucagon-like peptide-1, glucagon-like peptide-1/glucose-dependent insulinotropic peptide, and glucagon-like peptide-1/glucagon receptor agonists in type 2 diabetes. Diabetes Obes. Metab. 2022, 24, 788–805. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Jepsen, S.L.; Xu, S.F.S.; Engelstoft, M.S.; Egerod, K.L.; Schwartz, T.W.; Ørskov, C.; Rosenkilde, M.M.; Holst, J.J. Amino acids differ in their capacity to stimulate GLP-1 release from the perfused rat small intestine and stimulate secretion by different sensing mechanisms. Am. J. Physiol. -Endocrinol. Metab. 2021, 320, E874–E885. [Google Scholar] [CrossRef]
- Heeley, N.; Blouet, C. Central amino acid sensing in the control of feeding behavior. Front. Endocrinol. 2016, 7, 148. [Google Scholar] [CrossRef]
- Blundell, J.E.; Halford, J.C. Serotonin and appetite regulation. CNS Drugs 1998, 9, 473–495. [Google Scholar] [CrossRef]
- Gietzen, D.W.; Aja, S.M. The brain’s response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol. Neurobiol. 2012, 46, 332–348. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Ishizaka, Y.; Mitushima, T.; Tani, M.; Toda, A.; Toda, E.; Okada, M.; Miyano, H.; et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin. Obes. 2012, 2, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, L.; Xiao, Q.; Wang, Y.; Meng, X.; Xu, R.; Wang, S.; Na, R. Obesity and diabetes related plasma amino acid alterations. Clin. Biochem. 2013, 46, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Barcia, L.; García-Gavilán, J.; Papandreou, C.; Hansen, T.T.; Harrold, J.A.; Finlayson, G.; Blundell, J.E.; Sjödin, A.; Halford, J.C.G.; Bulló, M. Circulating metabolites associated with postprandial satiety in overweight/obese participants: The SATIN study. Nutrients 2021, 13, 549. [Google Scholar] [CrossRef]
- Karnani, M.M.; Venner, A.; Jensen, L.T.; Fugger, L.; Burdakov, D. Direct and indirect control of orexin/hypocretin neurons by glycine receptors. J. Physiol. 2011, 589, 639–651. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Yan-Do, R.; MacDonald, P.E. Impaired “glycine”-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef]
- Dalton, M.; Hollingworth, S.; Blundell, J.; Finlayson, G. Weak satiety responsiveness is a reliable trait associated with hedonic risk factors for overeating among women. Nutrients 2015, 7, 7421–7436. [Google Scholar] [CrossRef] [PubMed]
- Barkeling, B.; King, N.A.; Näslund, E.; Blundell, J.E. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int. J. Obes. 2007, 31, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Nymo, S.; Truby, H.; Rehfeld, J.F.; Hunter, G.R.; Gower, B.A. Association between ketosis and changes in appetite markers with weight loss following a very low-energy diet. Obesity 2020, 28, 2331–2338. [Google Scholar] [CrossRef] [PubMed]

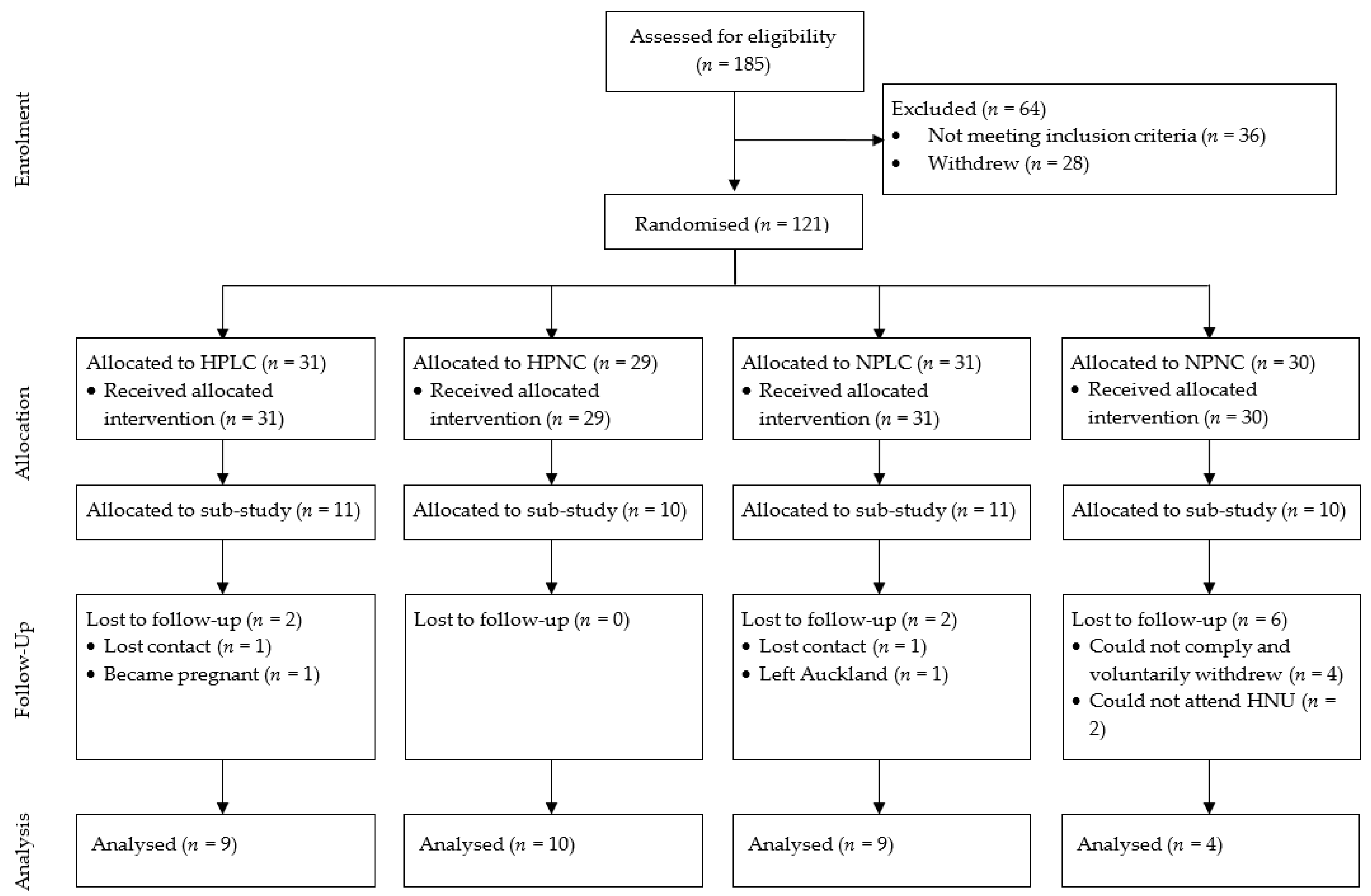
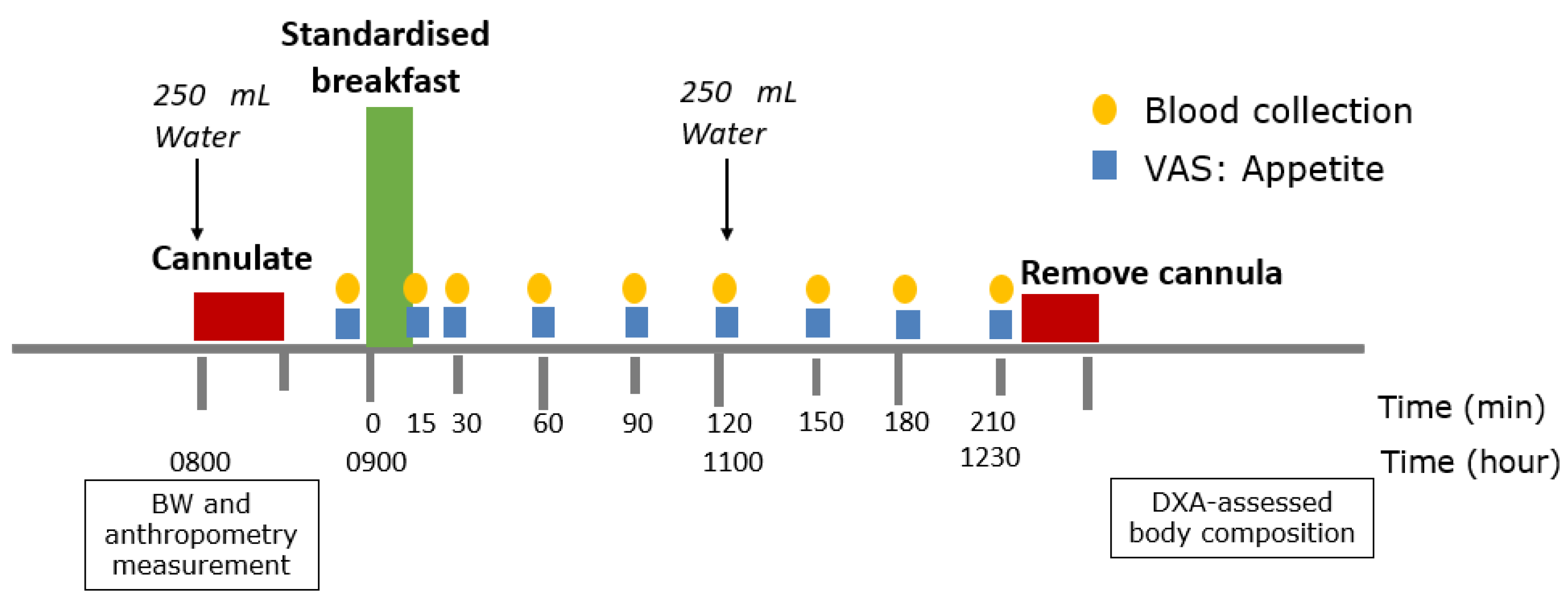
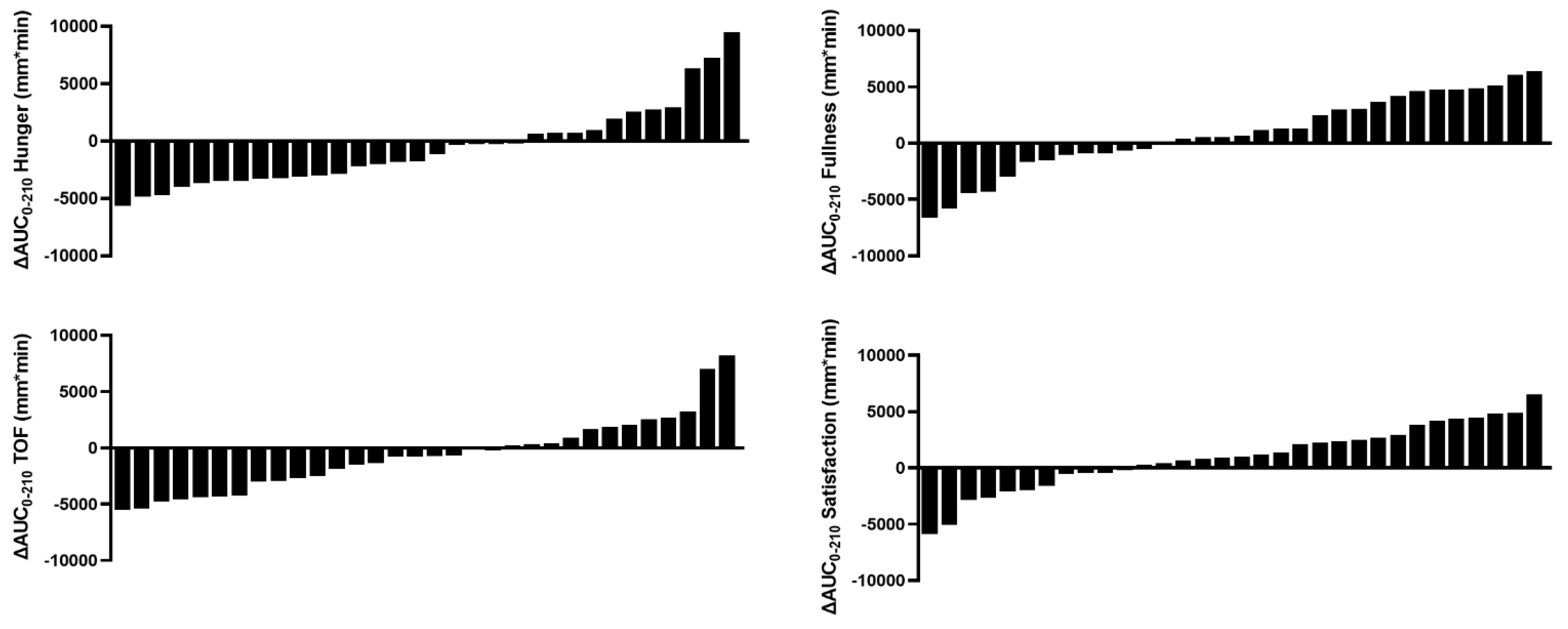
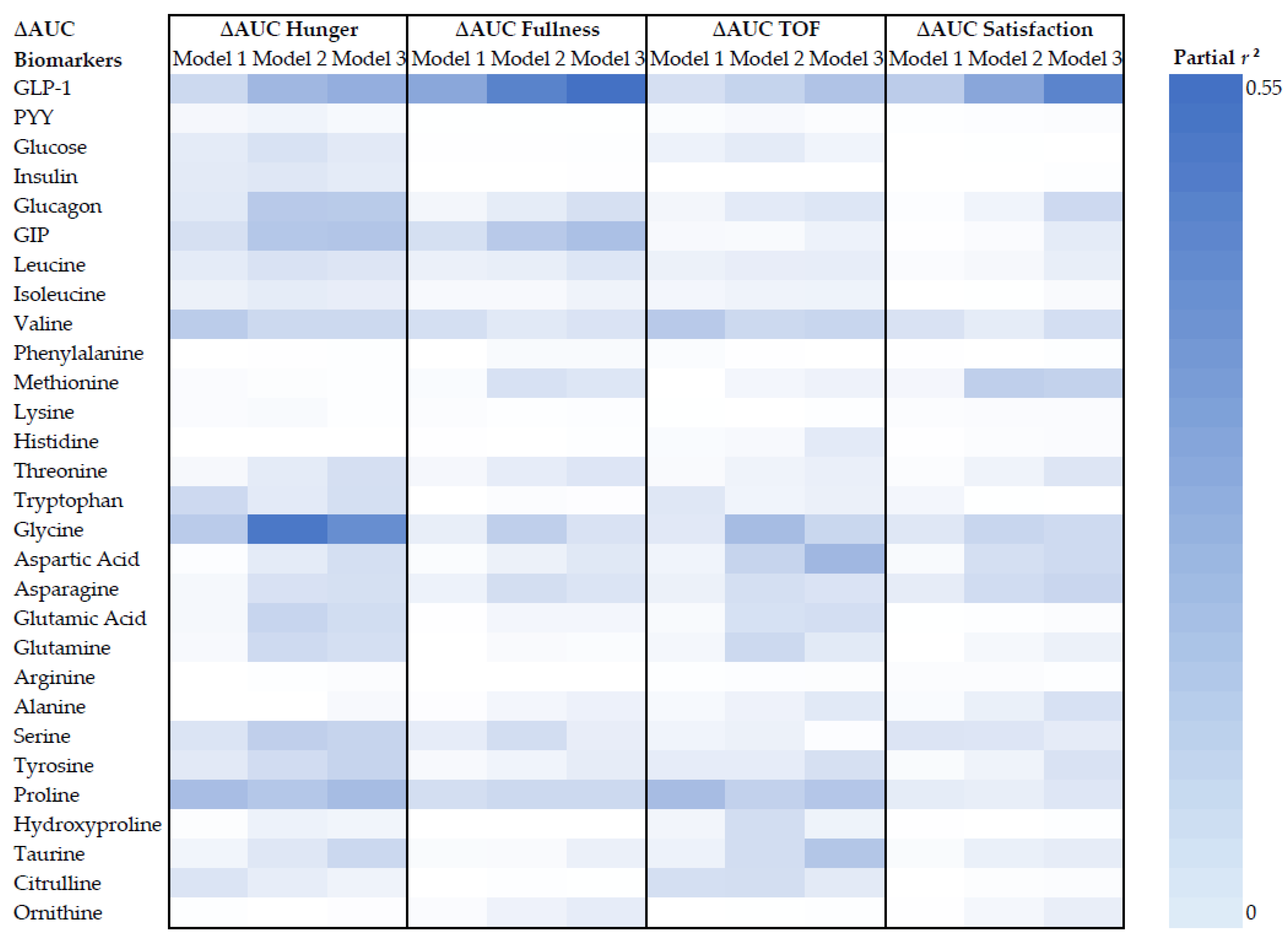
| Characteristics | Week 0 | Week 8 | ∆Week 8 | p-Value |
|---|---|---|---|---|
| Weight (kg) | 90.9 ± 8.7 | 82.6 ± 8.9 | −8.4 ± 0.5 | <0.001 |
| BMI (kg/m2) | 34.4 ± 3.2 | 31.2 ± 3.4 | −3.2 ± 0.2 | <0.001 |
| FM (kg) | 41.6 ± 6.3 | 35.0 ± 6.5 | −6.6 ± 0.4 | <0.001 |
| Percentage FM (%) | 45.9 ± 3.9 | 42.2 ± 4.9 | −3.7 ± 0.3 | <0.001 |
| FFM (kg) | 48.7 ± 4.8 | 47.3 ± 4.8 | −1.3 ± 0.2 | <0.001 |
| VAS–Appetite Responses | Week 0 | Week 8 | ∆Week 8 | p-Value |
|---|---|---|---|---|
| Fasted hunger (mm) | 41 ± 19 | 51 ± 27 | 10 ± 6 | 0.100 |
| AUC0-210 hunger (mm×min) | 6213 ± 3207 | 5647 ± 3215 | −566 ± 633 | 0.378 |
| iAUC0-210 hunger (mm×min) | −2403 ± 3949 | −5160 ± 5644 | −2757 ± 1084 | 0.016 |
| Fasted fullness (mm) | 33 ± 17 | 31 ± 20 | −2 ± 3 | 0.548 |
| AUC0-210 fullness (mm×min) | 12,970 ± 3525 | 13,824 ± 3900 | 854 ± 608 | 0.170 |
| iAUC0-210 fullness (mm×min) | 6146 ± 4181 | 7449 ± 4905 | 1303 ± 663 | 0.058 |
| Fasted TOF (mm) | 52 ± 16 | 60 ± 15 | 8 ± 4 | 0.029 |
| AUC0-210 TOF (mm×min) | 7279 ± 2945 | 6621 ± 3549 | −657 ± 584 | 0.269 |
| iAUC0-210 TOF (mm×min) | −3616 ± 3839 | −5939 ± 4280 | −2323 ± 605 | 0.001 |
| Fasted satisfaction (mm) | 32 ± 15 | 32 ± 18 | 0 ± 3 | 0.952 |
| AUC0-210 satisfaction (mm×min) | 12,338 ± 3268 | 13,309 ± 3487 | 970 ± 513 | 0.068 |
| iAUC0-210 satisfaction (mm×min) | 5658 ± 3619 | 6751 ± 4323 | 1093 ± 623 | 0.089 |
| Biomarkers | Week 0 | Week 8 | ∆Week 8 | p-Value |
|---|---|---|---|---|
| Glucose and glucoregulatory peptides | ||||
| Fasted Glucose (mM) | 5.7 ± 0.95 | 5.2 ± 0.5 | −0.5 ± 0.2 | 0.006 |
| AUC0-210 Glucose (mM×min) | 1160 ± 237 | 1124 ± 118 | −36 ± 37 | 0.330 |
| iAUC0-210 Glucose (mM×min) | −30 ± 81 | 30 ± 102 | 61 ± 20 | 0.004 |
| Fasted Insulin (pg/mL) | 981.2 ± 1006.4 | 985.7 ± 1137.5 | 4.5 ± 64.5 | 0.945 |
| AUC0-210 Insulin (pg/mL×min) | 387,611 ± 242,789 | 387,372 ± 221,294 | −239 ± 19,712 | 0.990 |
| iAUC0-210Insulin (pg/mL×min) | 181,711 ± 125,434 | 180,575 ± 107,820 | −1136 ± 124,721 | 0.939 |
| Fasted Glucagon (pg/mL) | 53.9 ± 32.0 | 43.9 ± 23.4 | −9.9 ± 5.2 | 0.066 |
| AUC0-210 Glucagon (pg/mL×min) | 15,877 ± 7385 | 13,550 ± 5336 | −2327 ± 1161 | 0.054 |
| iAUC0-210 Glucagon (pg/mL×min) | 4562 ± 4192 | 4330 ± 3134 | −232 ± 864 | 0.790 |
| Fasted GIP (pg/mL) | 58.2 ± 52.8 | 49.9 ± 31.2 | −8.3 ± 9.2 | 0.375 |
| AUC0-210 GIP (pg/mL×min) | 50,651 ± 16,790 | 65,093 ± 19,705 | 14,442 ± 2688 | <0.001 |
| iAUC0-210 GIP (pg/mL×min) | 38,426 ± 16,778 | 54,617 ± 17,795 | 16,191 ± 3155 | <0.001 |
| Gastrointestinal peptides | ||||
| Fasted GLP-1 (pg/mL) | 181.0 ± 89.9 | 164.1 ± 98.3 | −17.0 ± 17.2 | 0.332 |
| AUC0-210 GLP-1 (pg/mL×min) | 54,909 ± 21,691 | 56,045 ± 20,956 | 1136 ± 3734 | 0.763 |
| iAUC0-210 GLP-1 (pg/mL×min) | 16,818 ± 13,534 | 20,222 ± 13,727 | 3405 ± 3459 | 0.333 |
| Fasted PYY (pg/mL) | 44.9 ± 40.8 | 45.7 ± 38.9 | 0.9 ± 3.6 | 0.807 |
| AUC0-210 PYY (pg/mL×min) | 12,750 ± 8158 | 14,031 ± 7697 | 1281 ± 699 | 0.076 |
| iAUC0-210 PYY (pg/mL×min) | 3374 ± 3071 | 4428 ± 4349 | 1054 ± 842 | 0.220 |
| Branched-chain amino acids | ||||
| Fasted Leucine (µM) | 121.6 ± 27.8 | 124.4 ± 29.5 | 2.7 ± 5.8 | 0.638 |
| AUC0-210 Leucine (µM×min) | 38,018 ± 6546 | 37,007 ± 6665 | −1011 ± 1188 | 0.401 |
| iAUC0-210 Leucine (µM×min) | 12,472 ± 4106 | 10,889 ± 3834 | −1584 ± 927 | 0.098 |
| Fasted Isoleucine (µM) | 64.3 ± 18.4 | 68.3 ± 19.4 | 4.0 ± 3.3 | 0.228 |
| AUC0-210 Isoleucine (µM×min) | 21,996 ± 4115 | 22,095 ± 4449 | 99 ± 696 | 0.888 |
| iAUC0-210 Isoleucine (µM×min) | 8498 ± 2904 | 7754 ± 2503 | −745 ± 591 | 0.217 |
| Fasted Valine (µM) | 239.7 ± 62.9 | 242.3 ± 55.3 | 2.6 ± 11.1 | 0.815 |
| AUC0-210 Valine (µM×min) | 63,751 ± 12,903 | 62,849 ± 10,001 | −902 ± 1891 | 0.637 |
| iAUC0-210 Valine (µM×min) | 13,422 ± 4928 | 11,970 ± 4494 | −1452 ± 1147 | 0.215 |
| Other essential amino acids | ||||
| Fasted Phenylalanine (µM) | 56.4 ± 10.9 | 54.0 ± 6.7 | −2.4 ± 2.1 | 0.268 |
| AUC0-210 Phenylalanine (µM×min) | 151,82 ± 1759 | 14,407 ± 986 | −775 ± 244 | 0.003 |
| iAUC0-210 Phenylalanine (µM×min) | 3340 ± 1556 | 3062 ± 1220 | −278 ± 351 | 0.435 |
| Fasted Methionine (µM) | 27.6 ± 9.1 | 24.2 ± 5.9 | −3.3 ± 1.9 | 0.083 |
| AUC0-210 Methionine (µM×min) | 6572 ± 1939 | 5903 ± 1414 | −669 ± 378 | 0.086 |
| iAUC0-210 Methionine (µM×min) | 786 ± 846 | 814 ± 726 | 28 ± 177 | 0.876 |
| Fasted Lysine (µM) | 83.5 ± 14.2 | 82.1 ± 15.2 | −1.4 ± 3.4 | 0.687 |
| AUC0-210 Lysine (µM×min) | 22,271 ± 3451 | 21,541 ± 3926 | −731 ± 840 | 0.391 |
| iAUC0-210 Lysine (µM×min) | 786 ± 846 | 814 ± 726 | 28 ± 177 | 0.876 |
| Fasted Histidine (µM) | 52.3 ± 12.3 | 58.6 ± 14.0 | −6.2 ± 3.2 | 0.058 |
| AUC0-210 Histidine (µM×min) | 11,643 ± 2729 | 12,449 ± 2524 | 806 ± 586 | 0.179 |
| iAUC0-210 Histidine (µM×min) | 656 ± 2165 | 153 ± 1712 | −503 ± 539 | 0.359 |
| Fasted Threonine (µM) | 118.0 ± 23.3 | 106.9 ± 27.6 | −11.1 ± 4.6 | 0.022 |
| AUC0-210 Threonine (µM×min) | 27,652 ± 6083 | 24,870 ± 6143 | −2782 ± 1125 | 0.019 |
| iAUC0-210 Threonine (µM×min) | 2881 ± 3185 | 2420 ± 2683 | −461 ± 775 | 0.556 |
| Fasted Tryptophan (µM) | 42.8 ± 10.4 | 37.8 ± 7.1 | −5.0 ± 2.0 | 0.020 |
| AUC0-210 Tryptophan (µM×min) | 9861 ± 1696 | 8899 ± 1074 | −962 ± 273 | 0.001 |
| iAUC0-210 Tryptophan (µM×min) | 884 ± 1344 | 970 ± 1238 | 86 ± 352 | 0.809 |
| Non-essential amino acids | ||||
| Fasted Glycine (µM) | 237.1 ± 68.2 | 249.2 ± 72.8 | 12.1 ± 8.0 | 0.139 |
| AUC0-210 Glycine (µM×min) | 49,349 ± 13,475 | 51,714 ± 14,718 | 2365 ± 1819 | 0.203 |
| iAUC0-210 Glycine (µM×min) | −434 ± 5734 | −612 ± 5522 | −178 ± 1339 | 0.895 |
| Fasted Aspartic acid (µM) | 6.0 ± 4.2 | 4.7 ± 3.1 | −1.3 ± 1.0 | 0.210 |
| AUC0-210 Aspartic acid (µM×min) | 1218 ± 368 | 1029 ± 447 | −189 ± 99 | 0.065 |
| iAUC0-210 Aspartic acid (µM×min) | 107 ± 474 | 39 ± 662 | −68 ± 175 | 0.698 |
| Fasted Asparagine (µM) | 48.1 ± 8.2 | 47.5 ± 6.4 | −0.6 ± 1.8 | 0.727 |
| AUC0-210 Asparagine (µM×min) | 12,610 ± 2299 | 12,159 ± 2018 | −451 ± 397 | 0.266 |
| iAUC0-210 Asparagine (µM×min) | 2506 ± 2064 | 2189 ± 1569 | −317 ± 430 | 0.467 |
| Fasted Glutamic acid (µM) | 45.3 ± 20.8 | 35.2 ± 15.7 | −10.1 ± 3.9 | 0.014 |
| AUC0-210 Glutamic acid(µM×min) | 9408 ± 4765 | 7400 ± 2627 | −2008 ± 684 | 0.006 |
| iAUC0-210 Glutamic acid (µM×min) | −111 ± 3370 | 4 ± 2947 | 115 ± 852 | 0.893 |
| Fasted Glutamine (µM) | 551.5 ± 92.0 | 540.5 ± 77.9 | −11.0 ± 14.7 | 0.460 |
| AUC0-210 Glutamine (µM×min) | 122,671 ± 18,948 | 118,172 ± 16,194 | −4499 ± 2660 | 0.101 |
| iAUC0-210 Glutamine (µM×min) | 6864 ± 11,086 | 4668 ± 11,034 | −2196 ± 2469 | 0.381 |
| Fasted Arginine (µM) | 72.5 ± 13.2 | 79.0 ± 19.0 | 6.4 ± 3.6 | 0.085 |
| AUC0-210 Arginine (µM×min) | 20,122 ± 3943 | 20,601 ± 4232 | 479 ± 901 | 0.598 |
| iAUC0-210 Arginine (µM×min) | 4894 ± 4121 | 4022 ± 2840 | −872 ± 900 | 0.340 |
| Fasted Alanine (µM) | 422.2 ± 95.8 | 347.6 ± 74.9 | −74.5 ± 17.4 | <0.001 |
| AUC0-210 Alanine (µM×min) | 94561 ± 20,182 | 79,243 ± 14,554 | −15,318± 4134 | <0.001 |
| iAUC0-210 Alanine (µM×min) | 5909 ± 10,503 | 6244 ± 12,105 | 335 ± 2589 | 0.898 |
| Fasted Serine (µM) | 107.3 ± 29.4 | 122.0 ± 23.8 | 14.7 ± 5.0 | 0.006 |
| AUC0-210 Serine (µM×min) | 25,181 ± 6155 | 27,432 ± 5421 | 2251 ± 973 | 0.028 |
| iAUC0-210 Serine (µM×min) | 2647 ± 3699 | 1812 ± 846 | −835 ± 1053 | 0.006 |
| Fasted Tyrosine (µM) | 67.7 ± 13.8 | 58.5 ± 10.4 | −9.2 ± 2.3 | <0.001 |
| AUC0-210 Tyrosine (µM×min) | 17,822 ± 2604 | 16,303 ± 2234 | −1518 ± 423 | 0.001 |
| iAUC0-210 Tyrosine (µM×min) | 3613 ± 2154 | 4026 ± 1579 | 413 ± 479 | 0.396 |
| Fasted Proline (µM) | 230.7 ± 81.5 | 182.0 ± 64.7 | −48.7 ± 8.6 | <0.001 |
| AUC0-210 Proline (µM×min) | 60,677 ± 16,967 | 53,784 ± 14,143 | −6892 ± 1864 | <0.001 |
| iAUC0-210 Proline (µM×min) | 12,220 ± 7582 | 15,557 ± 4997 | 3337 ± 1578 | 0.043 |
| Non-proteogenic amino acids | ||||
| Fasted Hydroxyproline (µM) | 13.7 ± 6.7 | 11.5 ± 3.3 | −2.2 ± 1.4 | 0.115 |
| AUC0-210 Hydroxyproline (µM×min) | 2714 ± 1203 | 2341 ± 595 | −374 ± 238 | 0.127 |
| iAUC0-210 Hydroxyproline (µM×min) | −161 ± 367 | −74 ± 225 | 87± 83 | 0.302 |
| Fasted Taurine (µM) | 95.7 ± 39.4 | 100.5 ± 38.2 | 4.9 ± 10.1 | 0.632 |
| AUC0-210 Taurine (µM×min) | 16,472 ± 3967 | 17,009 ± 3313 | 536 ± 699 | 0.449 |
| iAUC0-210 Taurine (µM×min) | −4054 ± 7619 | −4103 ± 8700 | −49 ± 2216 | 0.983 |
| Fasted Citrulline (µM) | 28.9 ± 6.0 | 26.7 ± 6.9 | −2.2 ± 1.2 | 0.071 |
| AUC0-210 Citrulline (µM×min) | 6021 ± 1196 | 5631 ± 1118 | −390 ± 167 | 0.026 |
| iAUC0-210 Citrulline (µM×min) | −48 ± 865 | 14 ± 738 | 63 ± 194 | 0.749 |
| Fasted Ornithine (µM) | 36.0 ± 16.1 | 32.9 ± 15.6 | −3.1 ± 2.5 | 0.217 |
| AUC0-210 Ornithine (µM×min) | 11,389 ± 5152 | 10,047 ± 3190 | −1342 ± 681 | 0.058 |
| iAUC0-210 Ornithine (µM×min) | 3831 ± 2815 | 3142 ± 352 | −689 ± 513 | 0.189 |
| ∆AUC Biomarker | ∆AUC Hunger | ∆AUC Fullness | ∆AUC TOF | ∆AUC Satisfaction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimates | p-Value | ηp2 | Model R2 | Estimates | p-Value | ηp2 | Model R2 | Estimates | p-Value | ηp2 | Model R2 | Estimates | p-Value | ηp2 | Model R2 | |
| Model 1 | ||||||||||||||||
| GLP-1 a | 0.074 ± 0.034 | 0.037 | 0.15 | 0.44 | −0.104 ± 0.032 | 0.004 | 0.28 | 0.39 | 0.082 ± 0.036 | 0.029 | 0.17 | 0.28 | −0.078 ± 0.030 | 0.017 | 0.19 | 0.31 |
| PYY | 0.145 ± 0.151 | 0.346 | 0.03 | 0.34 | −0.050 ± 0.164 | 0.763 | 0.00 | 0.15 | 0.101 ± 0.156 | 0.523 | 0.02 | 0.15 | −0.060 ± 0.137 | 0.666 | 0.01 | 0.16 |
| GIP | 0.071 ± 0.036 | 0.059 | 0.12 | 0.42 | −0.075 ± 0.038 | 0.057 | 0.12 | 0.27 | 0.033 ± 0.039 | 0.396 | 0.03 | 0.17 | −0.013 ± 0.034 | 0.698 | 0.01 | 0.17 |
| Valine | 0.164 ± 0.063 | 0.015 | 0.20 | 0.45 | −0.142 ± 0.071 | 0.055 | 0.13 | 0.25 | 0.175 ± 0.064 | 0.011 | 0.21 | 0.33 | −0.113 ± 0.060 | 0.069 | 0.11 | 0.23 |
| Glycine a | −0.172 ± 0.066 | 0.014 | 0.20 | 0.51 | 0.109 ± 0.078 | 0.172 | 0.07 | 0.25 | −0.113 ± 0.069 | 0.114 | 0.09 | 0.33 | 0.107 ± 0.064 | 0.107 | 0.09 | 0.27 |
| Proline | −0.169 ± 0.054 | 0.004 | 0.26 | 0.51 | 0.123 ± 0.059 | 0.047 | 0.13 | 0.38 | −0.178 ± 0.057 | 0.004 | 0.26 | 0.37 | 0.083 ± 0.054 | 0.136 | 0.08 | 0.25 |
| Model 2 | ||||||||||||||||
| GLP-1 a | 0.096 ± 0.031 | 0.005 | 0.28 | 0.67 | −0.132 ± 0.031 | <0.001 | 0.43 | 0.61 | 0.100 ± 0.034 | 0.007 | 0.27 | 0.55 | −0.111 ± 0.028 | 0.001 | 0.39 | 0.57 |
| PYY | 0.150 ± 0.139 | 0.291 | 0.04 | 0.53 | −0.043 ± 0.158 | 0.790 | 0.00 | 0.32 | 0.113 ± 0.140 | 0.428 | 0.03 | 0.41 | −0.063 ± 0.129 | 0.633 | 0.01 | 0.36 |
| GIP | 0.092 ± 0.034 | 0.013 | 0.22 | 0.63 | −0.101 ± 0.039 | 0.016 | 0.21 | 0.45 | 0.030 ± 0.040 | 0.455 | 0.02 | 0.41 | −0.023 ± 0.037 | 0.530 | 0.02 | 0.31 |
| Valine | 0.125 ± 0.059 | 0.046 | 0.15 | 0.59 | −0.111 ± 0.071 | 0.134 | 0.09 | 0.35 | 0.129 ± 0.062 | 0.047 | 0.15 | 0.49 | −0.086 ± 0.060 | 0.160 | 0.08 | 0.35 |
| Glycine a | −0.284 ± 0.055 | <0.001 | 0.53 | 0.77 | 0.193 ± 0.082 | 0.027 | 0.19 | 0.43 | −0.197 ± 0.067 | 0.007 | 0.26 | 0.57 | 0.150 ± 0.070 | 0.042 | 0.16 | 0.43 |
| Proline | −0.155 ± 0.059 | 0.014 | 0.22 | 0.62 | 0.142 ± 0.068 | 0.047 | 0.15 | 0.46 | −0.147 ± 0.062 | 0.026 | 0.18 | 0.50 | 0.083 ± 0.061 | 0.186 | 0.07 | 0.37 |
| Model 3 | ||||||||||||||||
| GLP-1 a | 0.098 ± 0.029 | 0.003 | 0.32 | 0.67 | −0.138 ± 0.028 | <0.001 | 0.50 | 0.64 | 0.108 ± 0.031 | 0.002 | 0.33 | 0.56 | −0.115 ± 0.023 | <0.001 | 0.51 | 0.69 |
| PYY | 0.118 ± 0.141 | 0.409 | 0.03 | 0.51 | −0.041 ± 0.162 | 0.802 | 0.00 | 0.28 | 0.081 ± 0.146 | 0.586 | 0.01 | 0.34 | −0.069 ±0.127 | 0.593 | 0.01 | 0.38 |
| GIP | 0.093 ± 0.034 | 0.012 | 0.23 | 0.62 | −0.108 ± 0.038 | 0.009 | 0.25 | 0.45 | 0.046 ± 0.039 | 0.250 | 0.05 | 0.37 | −0.049 ± 0.033 | 0.152 | 0.08 | 0.41 |
| Valine | 0.128 ± 0.061 | 0.046 | 0.15 | 0.57 | −0.129 ± 0.073 | 0.089 | 0.11 | 0.34 | 0.143 ± 0.065 | 0.037 | 0.16 | 0.46 | −0.109 ± 0.057 | 0.066 | 0.07 | 0.43 |
| Glycine a | −0.241 ± 0.055 | <0.001 | 0.45 | 0.74 | 0.142 ± 0.081 | 0.092 | 0.11 | 0.36 | −0.144 ± 0.067 | 0.043 | 0.16 | 0.50 | 0.126 ± 0.063 | 0.055 | 0.15 | 0.47 |
| Proline | −0.155 ± 0.052 | 0.006 | 0.26 | 0.63 | 0.126 ± 0.060 | 0.045 | 0.15 | 0.48 | −0.152 ± 0.057 | 0.013 | 0.22 | 0.49 | 0.084 ± 0.051 | 0.110 | 0.10 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.J.; Liu, Y.; Lu, L.W.; Sequeira, I.R.; Poppitt, S.D. No Evidence That Circulating GLP-1 or PYY Are Associated with Increased Satiety during Low Energy Diet-Induced Weight Loss: Modelling Biomarkers of Appetite. Nutrients 2023, 15, 2399. https://doi.org/10.3390/nu15102399
Lim JJ, Liu Y, Lu LW, Sequeira IR, Poppitt SD. No Evidence That Circulating GLP-1 or PYY Are Associated with Increased Satiety during Low Energy Diet-Induced Weight Loss: Modelling Biomarkers of Appetite. Nutrients. 2023; 15(10):2399. https://doi.org/10.3390/nu15102399
Chicago/Turabian StyleLim, Jia Jiet, Yutong Liu, Louise W. Lu, Ivana R. Sequeira, and Sally D. Poppitt. 2023. "No Evidence That Circulating GLP-1 or PYY Are Associated with Increased Satiety during Low Energy Diet-Induced Weight Loss: Modelling Biomarkers of Appetite" Nutrients 15, no. 10: 2399. https://doi.org/10.3390/nu15102399
APA StyleLim, J. J., Liu, Y., Lu, L. W., Sequeira, I. R., & Poppitt, S. D. (2023). No Evidence That Circulating GLP-1 or PYY Are Associated with Increased Satiety during Low Energy Diet-Induced Weight Loss: Modelling Biomarkers of Appetite. Nutrients, 15(10), 2399. https://doi.org/10.3390/nu15102399








