Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging
Abstract
1. Introduction
2. Overview of Polyphenolic Compounds with Potential Myoprotective Action
3. Interaction between Phenolic Compounds and Microbiota: Possible Relevance for Sarcopenia
3.1. Ellagitannins and Derivatives
3.2. Hydroxycinnamic Acid Derivatives
3.3. Proanthocyanidins and Flavan-3-ols (Flavanols)
3.4. Flavanones
3.5. Flavones
3.6. Isoflavones
3.7. Flavonols
3.8. Anthocyanins
3.9. Resveratrol
3.10. Lignans
3.11. Curcumin
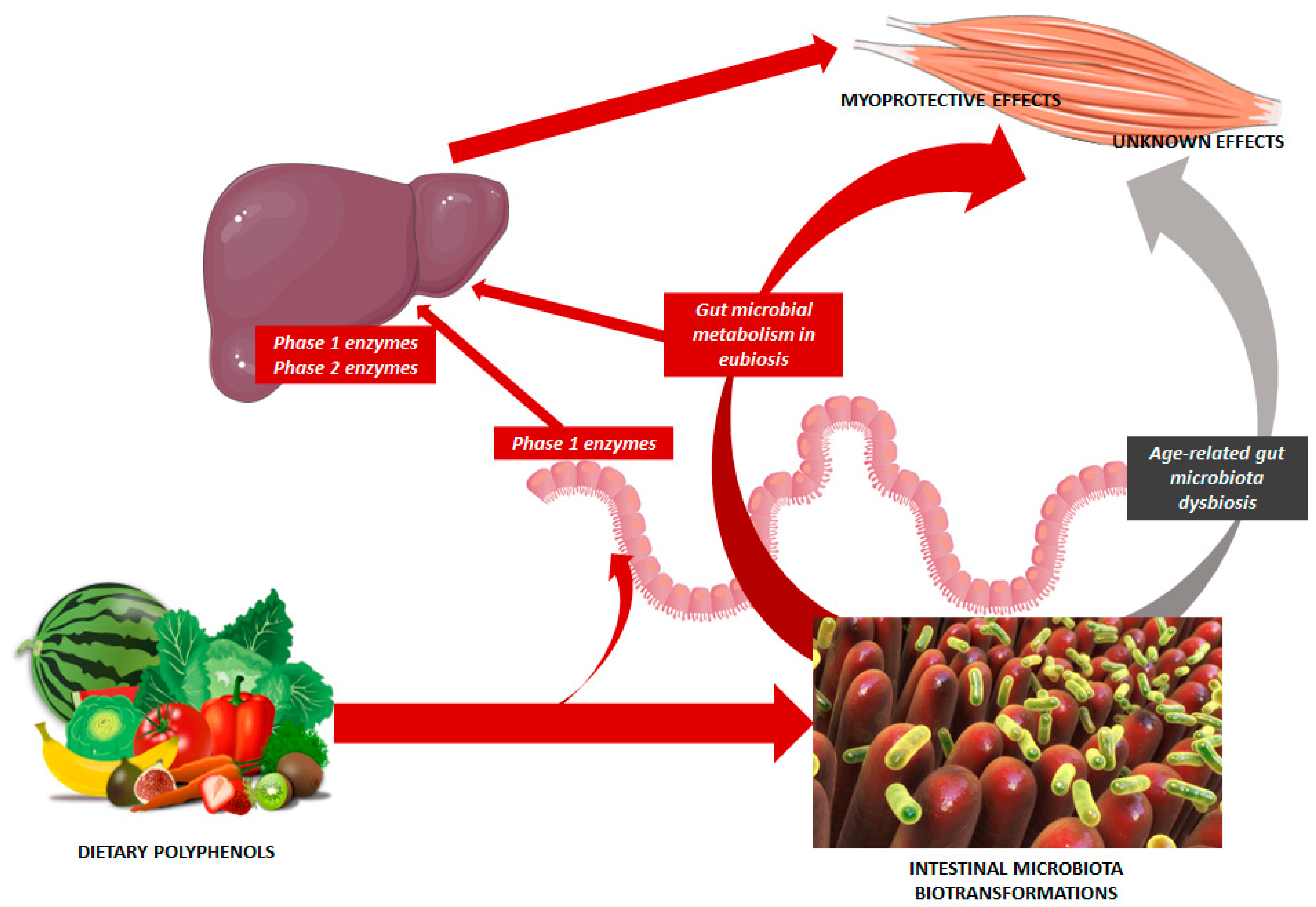
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and me-ta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.; Sun, P.Y.; Davies, K.J.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156–158. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut-muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mule, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; McCormick, B.A. Aging, frailty, and the microbiome-How dysbiosis influences human aging and diseases. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, J.-K.; Hu, Y.-M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res. Rev. 2022, 81, 101739. [Google Scholar] [CrossRef]
- Chen, L.; Arai, H.; Assantachai, P.; Akishita, M.; Chew, S.T.; Dumlao, L.C.; Duque, G.; Woo, J. Roles of nutrition in muscle health of community-dwelling older adults: Evidence-based expert consensus from Asian Working Group for Sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 1653–1672. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to prevent sarcopenia in the aging process: Role of protein intake and exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef]
- Maggi, S.; Tininess, A.; Limongi, F.; Noale, M.; Ecarnot, F. The role of nutrition and the Mediterranean diet on the trajectories of cognitive decline. Exp. Gerontol. 2023, 173, 112110. [Google Scholar] [CrossRef]
- Silva, R.; Pizato, N.; Da Mata, F.; Figueiredo, A.; Ito, M.; Pereira, M.G. Mediterranean Diet and Musculoskeletal-Functional Outcomes in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2018, 22, 655–663. [Google Scholar] [CrossRef]
- Karlsson, M.; Becker, W.; Michaëlsson, K.; Cederholm, T.; Sjögren, P. Associations between dietary patterns at age 71 and the prevalence of sarcopenia 16 years later. Clin. Nutr. 2020, 39, 1077–1084. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Detopoulou, P.; Voulgaridou, G.; Tsoumana, D.; Spanoudaki, M.; Sadikou, F.; Papadopoulou, V.G.; Zidrou, C.; Chatziprodromidou, I.P.; Giaginis, C.; et al. Mediterranean Diet and Sarcopenia Features in Apparently Healthy Adults over 65 Years: A Systematic Review. Nutrients 2023, 15, 1104. [Google Scholar] [CrossRef]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low Adherence to Mediterranean Diet Is Associated with Probable Sarcopenia in Community-Dwelling Older Adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Högger, P. Metabolism of dietary flavonoids in liver microsomes. Curr. Drug Metab. 2013, 14, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.-S.; Kim, K.-M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary poly-phenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef]
- Lai, C.-Q.; Liu, D. Dietary Epicatechin, A Novel Anti-Aging Bioactive Small Molecule. Curr. Med. Chem. 2021, 28, 3–18. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.S.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Tulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnarigenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Křížova, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Liu, J.; Yang, Y.-A.; Zhu, H.-L. A Review: The Anti-inflammatory, Anticancer and Antibacterial Properties of Four Kinds of Licorice Flavonoids Isolated from Licorice. Curr. Med. Chem. 2020, 27, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, F.; Zhang, A.; Wei, W.; Han, Y.; Wang, X. Pharmacokinetic study of schisandrin, schisandrol B, schisantherin A, deoxyschisandrin, and schisandrin B in rat plasma after oral administration of Shengmaisan formula by UPLC-MS. J. Sep. Sci. 2013, 36, 485–491. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Makhamra, S.; Rayan, I.; Barriah, W.; Nasser, A.; Abu Farkh, B.; Rayan, A. Sesamin from Cuscuta palaestina natural plant extracts: Directions for new prospective applications. PLoS ONE 2018, 13, e0195707. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int. J. Mol. Sci. 2020, 21, 5254. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-S.; Kao, J.-H. Sarcopenia and chronic liver diseases. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1229–1244. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A. The intestinal microbiome and its relevance for functionality in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 4–12. [Google Scholar] [CrossRef]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.-P.; Lu, Q.-Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.-H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Iso-lation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Villalba, R.G.; Vaquero, M.R.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef]
- Rodriguez, J.; Pierre, N.; Naslain, D.; Bontemps, F.; Ferreira, D.; Priem, F.; Deldicque, L.; Francaux, M. Urolithin B, a newly identified regulator of skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2017, 8, 583–597. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Singh, A.; D’amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Meroño, T.; Peron, G.; Gargari, G.; González-Domínguez, R.; Miñarro, A.; Vegas-Lozano, E.; Hidalgo-Liberona, N.; Del Bò, C.; Bernardi, S.; Kroon, P.A.; et al. The relevance of uro-lithins-based metabotyping for assessing the effects of a polyphenol-rich dietary intervention on intestinal permeability: A post-hoc analysis of the MaPLE trial. Food Res. Int. 2022, 159, 111632. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Noh, J.-R.; Choe, D.; Lee, N.; Song, Y.; Cho, S.; Kang, E.-J.; Go, M.-J.; Ha, S.K.; Chang, D.-H.; et al. Ageing and rejuvenation models reveal changes in key microbial communities associated with healthy ageing. Microbiome 2021, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Picca, A.; Marzetti, E.; Calvani, R.; Conta, G.; Del Chierico, F.; Capuani, G.; Faccia, M.; Fianchi, F.; Funaro, B.; et al. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021, 41, 1320–1334. [Google Scholar] [CrossRef]
- Margiotta, E.; Caldiroli, L.; Callegari, M.L.; Miragoli, F.; Zanoni, F.; Armelloni, S.; Rizzo, V.; Messa, P.; Vettoretti, S. Association of Sarcopenia and Gut Microbiota Composition in Older Patients with Advanced Chronic Kidney Disease, Investigation of the Interactions with Uremic Toxins, Inflammation and Oxidative Stress. Toxins 2021, 13, 472. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Li, H.; Dai, Y.; Chen, D.; Wang, M.; Jiang, X.; Huang, Z.; Yu, H.; Huang, J.; et al. Altered Fecal Microbiota Composition in Older Adults with Frailty. Front. Cell. Infect. Microbiol. 2021, 11, 696186. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwa-ni-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef]
- Mortelé, O.; Xavier, B.B.; Lammens, C.; Malhotra-Kumar, S.; Jorens, P.G.; Dirinck, E.; van Nuijs, A.L.; Hermans, N. Obesity influences the microbiotic biotransformation of chlorogenic acid. J. Pharm. Biomed. Anal. 2022, 211, 114550. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food Funct. 2019, 10, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Pan, H.; Lin, H.; Nakanishi, R.; Hirabayashi, T.; Nakayama, E.; Ma, X.; Maeshige, N.; Kondo, H.; Fujino, H. Protective effects of chlorogenic acid on capillary regression caused by disuse muscle atrophy. Biomed. Res. 2021, 42, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Koorbanally, N.A.; Islam, S. Ferulic acid promotes muscle glucose uptake and modulate dysregulated redox balance and metabolic pathways in ferric-induced pancreatic oxidative injury. J. Food Biochem. 2021, 46, e13641. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic Acid Stimulates Glucose Transport in Skeletal Muscle via AMPK Activation: A Contributor to the Beneficial Effects of Coffee on Diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Duan, Y.; Guo, Q.; Zhang, L.; Yang, Y.; Yin, Y.; Han, M.; Gong, S.; Li, J.; et al. Effects of Dietary Chlorogenic Acid Supplementation Derived from Lonicera macranthoides Hand-Mazz on Growth Performance, Free Amino Acid Profile, and Muscle Protein Synthesis in a Finishing Pig Model. Oxid. Med. Cell. Longev. 2022, 2022, 6316611. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Edwards, S.J.; Carter, S.; Nicholson, T.; Allen, S.L.; Morgan, P.T.; Jones, S.W.; Rendeiro, C.; Breen, L. (−)-Epicatechin and its colonic metabolite hippuric acid protect against dexamethasone-induced atrophy in skeletal muscle cells. J. Nutr. Biochem. 2022, 110, 109150. [Google Scholar] [CrossRef]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Freitas, C.M.T.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Deji, Y.; Gao, S.; Wu, C.; Song, Q.; Shi, Z.; Xiang, X.; Zang, J.; Su, J. Bifidobacterium as a Potential Biomarker of Sarcopenia in Elderly Women. Nutrients 2023, 15, 1266. [Google Scholar] [CrossRef]
- Alessandri, G.; Fontana, F.; Tarracchini, C.; Rizzo, S.M.; Bianchi, M.G.; Taurino, G.; Chiu, M.; Lugli, G.A.; Mancabelli, L.; Ar-gentini, C.; et al. Identification of a prototype human gut Bifidobacterium longum subsp. longum strain based on comparative and functional genomic approaches. Front. Microbiol. 2023, 14, 1130592. [Google Scholar] [CrossRef]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Júnior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2019, 12, 65. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. BioFactors 2016, 42, 5–12. [Google Scholar] [CrossRef]
- Zbinden-Foncea, H.; Castro-Sepulveda, M.; Fuentes, J.; Speisky, H. Effect of epicatechin on skeletal muscle. Curr. Med. Chem. 2022, 29, 1110–1123. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.R.; et al. (−)-Epicatechin induces mitochondrial bio-genesis and markers of muscle regeneration in adults with Becker muscular dystrophy. Muscle Nerve 2021, 63, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.-H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.-H. Catechins activate muscle stem cells by Myf5 induction and stimulate muscle regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef]
- Mafi, F.; Biglari, S.; Afousi, A.G.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.J. The Green Tea Pol-yphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Edens, N.K.; Pereira, S.L. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp. Gerontol. 2014, 50, 82–94. [Google Scholar] [CrossRef]
- Pence, B.D.; Gibbons, T.E.; Bhattacharya, T.K.; Mach, H.; Ossyra, J.M.; Petr, G.; Martin, S.A.; Wang, L.; Rubakhin, S.S.; Sweedler, J.W.; et al. Effects of exercise and dietary epigal-locatechin gallate and β-alanine on skeletal muscle in aged mice. Appl. Physiol. Nutr. Metab. 2016, 41, 181–190. [Google Scholar] [CrossRef]
- Mirza, K.A.; Pereira, S.L.; Edens, M.K.; Tisdale, M.J. Attenuation of muscle wasting in murine C2C 12 myotubes by epigallo-catechin-3-gallate. J. Cachexia Sarcopenia Muscle 2014, 5, 339–345. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Chioua, M.; Garrido, I.; Cueva, C.; Samadi, A.; Marco-Contelles, J.; Moreno-Arribas, M.V.; Bartolomé, B.; Monagas, M. Synthesis, Analytical Features, and Biological Relevance of 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone, a Microbial Metabolite Derived from the Catabolism of Dietary Flavan-3-ols. J. Agric. Food Chem. 2011, 59, 7083–7091. [Google Scholar] [CrossRef]
- Takagaki, A.; Yoshioka, Y.; Yamashita, Y.; Nagano, T.; Ikeda, M.; Hara-Terawaki, A.; Seto, R.; Ashida, H. Effects of Microbial Metabolites of (−)-Epigallocatechin Gallate on Glucose Uptake in L6 Skeletal Muscle Cell and Glucose Tolerance in ICR Mice. Biol. Pharm. Bull. 2019, 42, 212–221. [Google Scholar] [CrossRef]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3′,4′-Dihydroxyphenyl-γ-valerolactone), a major microbial metabolite of proanthocyanidin, attenuates THP-1 monocyte-endothelial adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef]
- Marin, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Anti-microbial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Takagaki, A.; Kato, Y.; Nanjo, F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (−)-epigallocatechin. Arch. Microbiol. 2014, 196, 681–695. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Maffei, V.J.; Kim, S.; Blanchard, E.; Luo, M.; Jazwinski, S.M.; Taylor, C.M.; A Welsh, D. Biological Aging and the Human Gut Microbiota. J. Gerontol. Ser. A 2017, 72, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Maslennikov, R.; Ivashkin, V.; Alieva, A.; Poluektova, E.; Kudryavtseva, A.; Krasnov, G.; Zharkova, M.; Zharikov, Y. Gut dysbiosis and body composition in cirrhosis. World J. Hepatol. 2022, 14, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; MROS Research Group; Johnson, A.; Demmer, R.T.; Fino, N.; Orwoll, E.S.; Ensrud, K.E.; Hoffman, A.R.; Cauley, J.A.; Shmagel, A.; et al. The Association between Objectively Measured Physical Activity and the Gut Microbiome among Older Community Dwelling Men. J. Nutr. Health Aging 2019, 23, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Imperatrice, M.; Cuijpers, I.; Troost, F.J.; Sthijns, M.M.J.P.E. Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients 2022, 14, 2955. [Google Scholar] [CrossRef]
- Noguera, F.J.M.; Alcaraz, P.E.; Vivas, J.C.; Chung, L.H.; Cascales, E.M.; Pagán, C.M. 8 weeks of 2S-Hesperidin supplementation improves muscle mass and reduces fat in amateur competitive cyclists: Randomized controlled trial. Food Funct. 2021, 12, 3872–3882. [Google Scholar] [CrossRef]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef]
- Pellegrini, M.; Bulzomi, P.; Galluzzo, P.; Lecis, M.; Leone, S.; Pallottini, V.; Marino, M. Naringenin modulates skeletal muscle differentiation via estrogen receptor α and β signal pathway regulation. Genes Nutr. 2014, 9, 425. [Google Scholar] [CrossRef]
- Ke, J.-Y.; Cole, R.M.; Hamad, E.M.; Hsiao, Y.-H.; Cotten, B.M.; Powell, K.A.; Belury, M.A. Citrus flavonoid, naringenin, increases locomotor activity and reduces diacylglycerol accumulation in skeletal muscle of obese ovariectomized mice. Mol. Nutr. Food Res. 2016, 60, 313–324. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Riedl, K.M.; Cooperstone, J.L.; Högel, J.; Bosy-Westphal, A.; Schwartz, S.J.; Carle, R.; Schweiggert, R.M. Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol. Nutr. Food Res. 2016, 60, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Borges, G.; Van Der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Plagmann, L.S.; Yang, L.; Zielke, R.; Gombart, A.F.; Maier, C.S.; Sikora, A.E.; Blakemore, P.R.; Stevens, J.F. Re-ductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the Intestinal Bacterium Eubacterium ramulus. Mol. Nutr. Food Res. 2019, 63, e1800923. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Rabot, S.; Espín, J.C.; Bruneau, A.; Philippe, C.; González-Sarrías, A.; Heyerick, A.; Tomás-Barberán, F.A.; De Keukeleire, D.; Verstraete, W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr. 2008, 138, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Saatokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant Apigenin Relieves Age-Related Muscle Atrophy by Inhibiting Oxidative Stress and Hyperactive Mitophagy and Apoptosis in Skeletal Muscle of Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2081–2088. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Choi, Y.M.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin enhances skeletal muscle hypertrophy and myoblast differentiation by regulating Prmt7. Oncotarget 2017, 8, 78300–78311. [Google Scholar] [CrossRef]
- Arango, D.; Diosa-Toro, M.; Rojas-Hernandez, L.S.; Cooperstone, J.; Schwartz, S.; Mo, X.; Jiang, J.; Schmittgen, T.D.; Doseff, A.I. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 2015, 59, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, D.; Wu, W. Inhibitory Effects and Mechanisms of Luteolin on Proliferation and Migration of Vascular Smooth Muscle Cells. Nutrients 2013, 5, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, S.; Kwon, E. Luteolin Protects Against Obese Sarcopenia in Mice with High-Fat Diet-Induced Obesity by Ameliorating Inflammation and Protein Degradation in Muscles. Mol. Nutr. Food Res. 2023, 67, e2200729. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez- López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of Exercise Performance by 48 Hours, and 15-Day Sup-plementation with Mangiferin and Luteolin in Men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The bioavailability of apigenin-7-glucoside is influenced by human in-testinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic Degradation of Flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Elderman, M.E.; El Aidy, S.; Burgerhof, J.G.M.; Visser, H.; Vaughan, E.E.; Faas, M.M.; de Vos, P. Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long-Chain Inulin Intake. Mol. Nutr. Food Res. 2021, 65, e2000390. [Google Scholar] [CrossRef]
- Hata, S.; Okamura, T.; Kobayashi, A.; Bamba, R.; Miyoshi, T.; Nakajima, H.; Kitagawa, N.; Hashimoto, Y.; Majima, S.; Senmaru, T.; et al. Gut Microbiota Changes by an SGLT2 Inhibitor, Luseogliflozin, Alters Metabolites Compared with Those in a Low Carbohydrate Diet in db/db Mice. Nutrients 2022, 14, 3531. [Google Scholar] [CrossRef]
- Chamarande, J.; Cunat, L.; Pavlov, N.; Alauzet, C.; Cailliez-Grimal, C. Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics. Nutrients 2022, 14, 4176. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut, 2023; online ahead of print. [Google Scholar]
- Rodriguez-Castaño, G.P.; Dorris, M.R.; Liu, X.; Bolling, B.; Acosta-Gonzalez, A.; Rey, F.E. Bacteroides thetaiotaomicron Starch Utilization Promotes Quercetin Degradation and Butyrate Production by Eubacterium ramulus. Front. Microbiol. 2019, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, X.; Fu, W.; Xiang, F.; He, X.; Yang, B.; Wang, X.; Ma, W.-L. Gut Microbiota Dysbiosis Correlates with Abnormal Immune Response in Moderate COVID-19 Patients with Fever. J. Inflamm. Res. 2021, 14, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I.; Barceló, A. Gut Microbiota Bacterial Species Associated with Mediterranean Diet-Related Food Groups in a Northern Spanish Population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhao, Y.; Zhu, S.; Liu, L.; Cheng, K.; Ye, B.; Han, Y.; Fan, J.; Xia, M. Flavonifractor plautii Protects Against Elevated Arterial Stiffness. Circ. Res. 2023, 132, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, T.; Yang, Y.; Chaudhary, P.P.; Teh, J.P.Y.; Cheon, B.K.; Moses, D.; Schuster, S.C.; Schlundt, J.; Li, J.; et al. Integrative multiomics analysis reveals host-microbe-metabolite interplays associated with the aging process in Singaporeans. Gut Microbes 2022, 14, 2070392. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Kitamura, K.; Erlangga, J.S.; Tsukamoto, S.; Sakamoto, Y.; Mabashi-Asazuma, H.; Iida, K. Daidzein promotes the expression of oxidative phosphorylation- and fatty acid oxidation-related genes via an estrogen-related receptor α pathway to decrease lipid accumulation in muscle cells. J. Nutr. Biochem. 2020, 77, 108315. [Google Scholar] [CrossRef]
- Ogawa, M.; Kitano, T.; Kawata, N.; Sugihira, T.; Kitakaze, T.; Harada, N.; Yamaji, R. Daidzein down-regulates ubiqui-tin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J. Nutr. Biochem. 2017, 49, 63–70. [Google Scholar] [CrossRef]
- Zhang, H.; Chi, M.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Daidzein alleviates cisplatin-induced muscle atrophy by regulating Glut4/AMPK/FoxO pathway. Phytother. Res. 2021, 35, 4363–4376. [Google Scholar] [CrossRef]
- Yoshino, M.; Naka, A.; Sakamoto, Y.; Shibasaki, A.; Toh, M.; Tsukamoto, S.; Kondo, K.; Iida, K. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J. Nutr. Biochem. 2015, 26, 1193–1199. [Google Scholar] [CrossRef]
- Aoyama, S.; Jia, H.; Nakazawa, K.; Yamamura, J.; Saito, K.; Kato, H. Dietary Genistein Prevents Denervation-Induced Muscle Atrophy in Male Rodents via Effects on Estrogen Receptor-α. J. Nutr. 2016, 146, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Ma, J.; Chen, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Li, X.; Zhu, L.; Shen, L. miR-222 Is Involved in the Amelioration Effect of Genistein on Dexamethasone-Induced Skeletal Muscle Atrophy. Nutrients 2022, 14, 1861. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Shen, L.; Liu, L.; Guo, Z.; Wang, S.; Chen, L.; Zheng, T.; Fan, Y.; Tan, Y.; Jiang, D.; et al. miR-222 is involved in the regulation of genistein on skeletal muscle fiber type. J. Nutr. Biochem. 2020, 80, 108320. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liao, T.; Chen, J.; Ma, J.; Wang, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Zeng, C.; et al. Genistein Promotes Skeletal Muscle Regeneration by Regulating miR-221/222. Int. J. Mol. Sci. 2022, 23, 13482. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Yamashita, Y.; Zhang, T.; Nakagawa, K.; Ashida, H. Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells. Mol. Cell. Endocrinol. 2014, 393, 99–108. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kubota, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch. Biochem. Biophys. 2019, 664, 157–166. [Google Scholar] [CrossRef]
- Hirasaka, K.; Saito, S.; Yamaguchi, S.; Miyazaki, R.; Wang, Y.; Haruna, M.; Taniyama, S.; Higashitani, A.; Terao, J.; Nikawa, T.; et al. Dietary Supplementation with Isoflavones Prevents Muscle Wasting in Tumor-Bearing Mice. J. Nutr. Sci. Vitaminol. 2016, 62, 178–184. [Google Scholar] [CrossRef]
- Tabata, S.; Aizawa, M.; Kinoshita, M.; Ito, Y.; Kawamura, Y.; Takebe, M.; Pan, W.; Sakuma, K. The influence of isoflavone for denervation-induced muscle atrophy. Eur. J. Nutr. 2019, 58, 291–300. [Google Scholar] [CrossRef]
- Aubertinleheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese–sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Costa, J.G.; Giolo, J.S.; Rossato, L.T.; Nahas, P.C.; Mariano, I.M.; Batista, J.P.; Puga, G.M.; de Oliveira, E.P. Isoflavone supplementation plus combined aerobic and resistance exercise do not change phase angle values in postmenopausal women: A randomized placebo-controlled clinical trial. Exp. Gerontol. 2019, 117, 31–37. [Google Scholar] [CrossRef]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of whey and soy protein sup-plementation on inflammatory cytokines in older adults: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [PubMed]
- Seeley, A.D.; Jacobs, K.A.; Signorile, J.F. Acute Soy Supplementation Improves 20-km Time Trial Performance, Power, and Speed. Med. Sci. Sport. Exerc. 2019, 52, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.A.; Peirotén, A.; Landete, J.M. Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2017, 39, 198–205. [Google Scholar] [CrossRef]
- Benvenuti, C.; Setnikar, I. Effect of Lactobacillus sporogenes on oral isoflavones bioavailability: Single dose pharmacokinetic study in menopausal women. Arzneimittelforschung 2011, 61, 605–609. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Aiso, C.; Tabata, Y.; Gotoh, Y.; Kosaka, H.; Suzuki, T. Lactococcus cremoris subsp. cremoris FC-fermented milk activates protein synthesis and increases skeletal muscle mass in middle-aged mice. Biochem. Biophys. Res. Commun. 2022, 612, 176–180. [Google Scholar] [CrossRef]
- Ford, A.L.; Nagulesapillai, V.; Piano, A.; Auger, J.; Girard, S.-A.; Christman, M.; Tompkins, T.A.; Dahl, W.J. Microbiota Stability and Gastrointestinal Tolerance in Response to a High-Protein Diet with and without a Prebiotic, Probiotic, and Synbiotic: A Randomized, Double-Blind, Placebo-Controlled Trial in Older Women. J. Acad. Nutr. Diet. 2020, 120, 500–516.e10. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.H.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut mi-crobiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef]
- Almeida, H.M.; Sardeli, A.V.; Conway, J.; Duggal, N.A.; Cavaglieri, C.R. Comparison between frail and non-frail older adults’ gut microbiota: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 82, 101773. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Pla-cebo-Controlled Trial. Nutrients 2022, 14, 4566. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Gargari, G.; Meroño, T.; Miñarro, A.; Vegas Lozano, E.; Castellano Escuder, P.; González-Domínguez, R.; Hidal-go-Liberona, N.; Del Bò, C.; Bernardi, S.; et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: The MaPLE trial. Clin. Nutr. 2021, 40, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Gan, R.-Y.; Li, H.-B.; Wu, D.-T.; Atanasov, A.G.; Gul, K.; Zhang, J.-R.; Yang, Q.-Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, M.-S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.-H.; Kim, Y. Rutin Increases Muscle Mitochondrial Biogenesis with AMPK Activation in High-Fat Diet-Induced Obese Rats. Nutrients 2015, 7, 8152–8169. [Google Scholar] [CrossRef]
- Hah, Y.S.; Lee, W.K.; Lee, S.J.; Lee, S.Y.; Seo, J.H.; Kim, E.J.; Choe, Y.I.; Kim, S.G.; Yoo, J.I. Rutin Prevents Dexame-thasone-Induced Muscle Loss in C2C12 Myotube and Mouse Model by Controlling FOXO3-Dependent Signaling. Antioxidants 2023, 12, 639. [Google Scholar] [CrossRef]
- Liu, S.; Adewole, D.; Yu, L.; Sid, V.; Wang, V.; Karmin, O.; Yang, C. Rutin attenuates inflammatory responses induced by lipopoly-saccharide in an in vitro mouse muscle cell (C2C12) model. Poult. Sci. 2019, 98, 2756–2764. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.S.; Lu, C.C.; Chiu, Y.J.; Chen, H.C.; Chung, M.I.; Wu, Y.T.; Chen, F.A. Effect of Quercetin on Dexame-thasone-Induced C2C12 Skeletal Muscle Cell Injury. Molecules 2020, 25, 3267. [Google Scholar] [CrossRef]
- Chen, X.; Liang, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity. J. Food Biochem. 2021, 45, e13968. [Google Scholar] [CrossRef]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sport. Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Chen, X.; Liang, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Quercetin regulates skeletal muscle fiber type switching via adiponectin signaling. Food Funct. 2021, 12, 2693–2702. [Google Scholar] [CrossRef]
- Hour, T.-C.; Vo, T.C.T.; Chuu, C.-P.; Chang, H.-W.; Su, Y.-F.; Chen, C.-H.; Chen, Y.-K. The Promotion of Migration and Myogenic Differentiation in Skeletal Muscle Cells by Quercetin and Underlying Mechanisms. Nutrients 2022, 14, 4106. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, S.; Akazawa, S.; Takahashi, T.; Izumo, T.; Rogi, T.; Nakai, M. Quercetin attenuates adipogenesis and fibrosis in human skeletal muscle. Biochem. Biophys. Res. Commun. 2022, 615, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Holobar, A. Quercetin ingestion modifies human motor unit firing patterns and muscle contractile properties. Exp. Brain Res. 2021, 239, 1567–1579. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sabatini, S.; Sgrò, P.; Di Luigi, L.; Sacchetti, M. Quercetin Supplementation Improves Neuromuscular Function Recovery from Muscle Damage. Nutrients 2020, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Gelabert-Rebato, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Lopez-Rios, L.; Wiebe, J.C.; Martin-Rodriguez, S.; Arteaga-Ortiz, R.; Dorado, C.; et al. Supplementation with a Mango Leaf Extract (Zynamite®) in Combination with Quercetin Attenuates Muscle Damage and Pain and Accelerates Recovery after Strenuous Damaging Exercise. Nutrients 2020, 12, 614. [Google Scholar] [CrossRef]
- Otsuka, Y.; Miyamoto, N.; Nagai, A.; Izumo, T.; Nakai, M.; Fukuda, M.; Arimitsu, T.; Yamada, Y.; Hashimoto, T. Effects of Quercetin Glycoside Supplementation Combined With Low-Intensity Resistance Training on Muscle Quantity and Stiffness: A Randomized, Controlled Trial. Front. Nutr. 2022, 9, 912217. [Google Scholar] [CrossRef]
- Ulla, A.; Ozaki, K.; Rahman, M.; Nakao, R.; Uchida, T.; Maru, I.; Mawatari, K.; Fukawa, T.; Kanayama, H.-O.; Sakakibara, I.; et al. Morin improves dexamethasone-induced muscle atrophy by modulating atrophy-related genes and oxidative stress in female mice. Biosci. Biotechnol. Biochem. 2022, 86, 1448–1458. [Google Scholar] [CrossRef]
- Yoshimura, T.; Saitoh, K.; Sun, L.; Wang, Y.; Taniyama, S.; Yamaguchi, K.; Uchida, T.; Ohkubo, T.; Higashitani, A.; Nikawa, T.; et al. Morin suppresses cachexia-induced muscle wasting by binding to ribosomal protein S10 in carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 506, 773–779. [Google Scholar] [CrossRef]
- Issac, P.K.; Karan, R.; Guru, A.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Raj, J.A. Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol. Biol. Rep. 2021, 48, 5857–5872. [Google Scholar] [CrossRef]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Bucci, V.; Dutta, P.; Ward, D.; McCormick, B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J. Med Microbiol. 2018, 67, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Zeamer, A.; Ward, D.V.; Dutta, P.; Bucci, V.; A McCormick, B. The Nursing Home Older Adult Gut Microbiome Composition Shows Time-dependent Dysbiosis and Is Influenced by Medication Exposures, Age, Environment, and Frailty. J. Gerontol. Ser. A 2021, 76, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Wu, J.-C.; Huang, Q.; Shahidi, F.; Wang, Y.-J.; Ho, C.-T.; Pan, M.-H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Luan, Z.; Sun, G.; Huang, Y.; Yang, Y.; Yang, R.; Li, C.; Wang, T.; Tan, D.; Qi, S.; Jun, C.; et al. Metagenomics Study Reveals Changes in Gut Microbiota in Centenarians: A Cohort Study of Hainan Centenarians. Front. Microbiol. 2020, 11, 1474. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar] [CrossRef]
- Renson, A.; Harris, K.M.; Dowd, J.B.; Gaydosh, L.; McQueen, M.B.; Krauter, K.S.; Shannahan, M.; E Aiello, A. Early Signs of Gut Microbiome Aging: Biomarkers of Inflammation, Metabolism, and Macromolecular Damage in Young Adulthood. J. Gerontol. Ser. A 2020, 75, 1258–1266. [Google Scholar] [CrossRef]
- Murata, M.; Nonaka, H.; Komatsu, S.; Goto, M.; Morozumi, M.; Yamada, S.; Lin, I.-C.; Yamashita, S.; Tachibana, H. Delphinidin Prevents Muscle Atrophy and Upregulates miR-23a Expression. J. Agric. Food Chem. 2017, 65, 45–50. [Google Scholar] [CrossRef]
- Murata, M.; Kosaka, R.; Kurihara, K.; Yamashita, S.; Tachibana, H. Delphinidin prevents disuse muscle atrophy and reduces stress-related gene expression. Biosci. Biotechnol. Biochem. 2016, 80, 1636–1640. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Y.; Li, H.; Chen, X.; Zhang, C.; Wang, H.; Deng, Z. The antioxidant activity and active sites of delphinidin and petunidin measured by DFT, in vitro chemical-based and cell-based assays. J. Food Biochem. 2019, 43, e12968. [Google Scholar] [CrossRef]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Copetti, C.L.K.; Diefenthaeler, F.; Hansen, F.; Vieira, F.G.K.; Di Pietro, P.F. Fruit-Derived Anthocyanins: Effects on Cy-cling-Induced Responses and Cycling Performance. Antioxidants 2022, 11, 387. [Google Scholar] [CrossRef]
- Pekas, E.J.; Shin, J.; Headid, R.J.; Son, W.M.; Layec, G.; Yadav, S.K.; Scott, S.D.; Park, S.Y. Combined anthocyanins and bro-melain supplement improves endothelial function and skeletal muscle oxygenation status in adults: A double-blind place-bo-controlled randomised crossover clinical trial. Br. J. Nutr. 2021, 125, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Bonfanti, C.; Antonini, S.; Angelini, G.; Mura, G.; Zanaglio, F.; Taglietti, V.; Romanello, V.; Sandri, M.; Tonelli, C.; et al. Nutritional intervention with cyanidin hinders the progression of muscular dystrophy. Cell Death Dis. 2020, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–26, online first. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, X.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Zheng, P.; Chen, H.; Yan, H.; Huang, Z. Effects of protocatechuic acid on antioxidant capacity, mitochondrial biogenesis and skeletal muscle fiber transformation. J. Nutr. Biochem. 2023, 116, 109327. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Cesare, M.M.; Fredianelli, L.; De Leo, M.; Conti, V.; Braca, A.; Di Stefano, R. Effect of Tomato Peel Extract Grown under Drought Stress Condition in a Sarcopenia Model. Molecules 2022, 27, 2563. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-B.; Lee, H.-S.; Hong, J.S.; Kim, D.H.; Moon, J.M.; Park, Y. Effects of tannase-converted green tea extract on skeletal muscle development. BMC Complement. Med. Ther. 2020, 20, 47. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honda, S.; Ogura, M.; Kato, M.; Tanigawa, R.; Fujino, H.; Kawamoto, S. Lemon Myrtle (Backhousia citriodora) Extract and Its Active Compound, Casuarinin, Activate Skeletal Muscle Satellite Cells In Vitro and In Vivo. Nutrients 2022, 14, 1078. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Angelino, D.; Vivas, E.I.; Kerby, R.L.; García-Viguera, C.; Del Rio, D.; Rey, F.E.; Mena, P. Differential Catabolism of an Anthocyanin-Rich Elderberry Extract by Three Gut Microbiota Bacterial Species. J. Agric. Food Chem. 2020, 68, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Fabjanowicz, M.; Płotka-Wasylka, J.; Namieśnik, J. Detection, identification and determination of resveratrol in wine. Problems and challenges. TrAC Trends Anal. Chem. 2018, 103, 21–33. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial actions of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef]
- Anwar, M.; Pradhan, R.; Dey, S.; Kumar, R. The Role of Sirtuins in Sarcopenia and Frailty. Aging Dis. 2023, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Haramizu, S.; Asano, S.; Butler, D.C.; Stanton, D.A.; Hajira, A.; Mohamed, J.S.; Alway, S.E. Dietary resveratrol confers apop-totic resistance to oxidative stress in myoblasts. J. Nutr. Biochem. 2017, 50, 103–115. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef]
- Tuntevski, K.; Hajira, A.; Nichols, A.; Alway, S.E.; Mohamed, J.S. Muscle-specific sirtuin1 gain-of-function ameliorates skeletal muscle atrophy in a pre-clinical mouse model of cerebral ischemic stroke. FASEB Bioadv. 2020, 2, 387–397. [Google Scholar] [CrossRef]
- Liao, Z.-Y.; Chen, J.-L.; Xiao, M.-H.; Sun, Y.; Zhao, Y.-X.; Pu, D.; Lv, A.-K.; Wang, M.-L.; Zhou, J.; Zhu, S.-Y.; et al. The effect of exercise, resveratrol or their combination on Sarcopenia in aged rats via regulation of AMPK/Sirt1 pathway. Exp. Gerontol. 2017, 98, 177–183. [Google Scholar] [CrossRef]
- Joseph, A.M.; Malamo, A.G.; Silvestre, J.; Wawrzyniak, N.; Carey-Love, S.; Nguyen, L.M.D.; Dutta, D.; Xu, J.; Leeuwenburgh, C.; Adhihetty, P.J. Short-term caloric restriction, resveratrol, or combined treatment regimens in late-life alter mitochondrial protein expression profiles in a fiber-type specific manner in aged animals. Exp. Gerontol. 2013, 48, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Toniolo, L.; Crea, E.; Giacomello, E. A short-term treatment with resveratrol improves the inflammatory conditions of Middle-aged mice skeletal muscles. Int. J. Food Sci. Nutr. 2022, 73, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, A.S.; Nordstrøm Kjær, T.; Juul Ornstrup, M.; Bønløkke Pedersen, S. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: A randomized, placebo-controlled clinical trial on the effects of resveratrol after four months’ treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-P.; Bai, C.-H.; Alizargar, J.; Peng, C.-Y. Combination of exercise training and resveratrol attenuates obese sarcopenia in skeletal muscle atrophy. Chin. J. Physiol. 2020, 63, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, Z.; Jia, J.; Chen, J.-L.; Xiao, Q. The effects of resveratrol feeding and exercise training on the skeletal muscle function and transcriptome of aged rats. PeerJ 2019, 7, e7199. [Google Scholar] [CrossRef]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of Resveratrol on the Recovery of Muscle Mass Following Disuse in the Plantaris Muscle of Aged Rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef]
- Jackson, J.R.; Ryan, M.J.; Alway, S.E. Long-Term Supplementation With Resveratrol Alleviates Oxidative Stress but Does Not Attenuate Sarcopenia in Aged Mice. J. Gerontol. Ser. A 2011, 66, 751–764. [Google Scholar] [CrossRef]
- Ballak, S.B.; Jaspers, R.T.; Deldicque, L.; Chalil, S.; Peters, E.L.; de Haan, A.; Degens, H. Blunted hypertrophic response in old mouse muscle is associated with a lower satellite cell density and is not alleviated by resveratrol. Exp. Gerontol. 2015, 62, 23–31. [Google Scholar] [CrossRef]
- Negro, M.; Perna, S.; Spadaccini, D.; Castelli, L.; Calanni, L.; Barbero, M.; Cescon, C.; Rondanelli, M.; D’Antona, G. Effects of 12 weeks of essential amino acids (EEA)-based multi-ingredient nutritional supplementation on muscle mass, muscle strength, muscle power and fatigue in healthy elderly subjects: A randomized controlled double-blind study. J. Nutr. Health Aging 2019, 23, 414–424. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Echeverri, J.H.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and in humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; E Kulling, S. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gong, H.; Lyu, X.; Liu, Y.; Li, S.; Tan, S.; Dong, L.; Zhang, X. Characteristics of the fecal microbiome and metabolome in older patients with heart failure and sarcopenia. Front. Cell. Infect. Microbiol. 2023, 13, 1127041. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Aguilar-Aguilar, E.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Lunularin Producers versus Non-producers: Novel Human Metabotypes Associated with the Metabolism of Resveratrol by the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 10521–10531. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of stilbenoids by human fecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Yeon, M.; Choi, H.; Jun, H.-S. Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients 2020, 12, 1255. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Lee, H.; Hong, M.; Park, S.-Y.; Bae, H. Magnolol Attenuates Cisplatin-Induced Muscle Wasting by M2c Macrophage Activation. Front. Immunol. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, Y.; Komura, T.; Kashima, N.; Tamura, M.; Kage-Nakadai, E.; Saeki, S.; Terao, K.; Nishikawa, Y. Influence of oral supplementation with sesamin on longevity of Caenorhabditis elegans and the host defense. Eur. J. Nutr. 2014, 53, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Le, T.D.; Nakahara, Y.; Ueda, M.; Komura, K.; Hirai, J.; Sato, Y.; Takemoto, D.; Tomimori, N.; Ono, Y.; Nakai, M.; et al. Sesamin suppresses aging phenotypes in adult muscular and nervous systems and intestines in a Drosophila se-nescence-accelerated model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1826–1839. [Google Scholar] [PubMed]
- Takada, S.; Kinugawa, S.; Matsushima, S.; Takemoto, D.; Furihata, T.; Mizushima, W.; Fukushima, A.; Yokota, T.; Ono, Y.; Shibata, H.; et al. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochon-drial function in mice with high-fat diet-induced diabetes. Exp. Physiol. 2015, 100, 1319–1330. [Google Scholar] [CrossRef]
- Kou, G.; Li, P.; Shi, Y.; Traore, S.S.; Shi, X.; Amoah, A.N.; Cui, Z.; Lyu, Q. Sesamin Activates Skeletal Muscle FNDC5 Expression and Increases Irisin Secretion via the SIRT1 Signaling Pathway. J. Agric. Food Chem. 2022, 70, 7704–7715. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An in-terplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Nurmi, T.; Mursu, J.; Peñalvo, J.L.; Poulsen, H.E.; Voutilainen, S. Dietary intake and urinary excretion of lignans in Finnish men. Br. J. Nutr. 2010, 103, 677–685. [Google Scholar] [CrossRef]
- Eeckhaut, E.; Struijs, K.; Possemiers, S.; Vincken, J.-P.; De Keukeleire, D.; Verstraete, W. Metabolism of the Lignan Macromolecule into Enterolignans in the Gastrointestinal Lumen as Determined in the Simulator of the Human Intestinal Microbial Ecosystem. J. Agric. Food Chem. 2008, 56, 4806–4812. [Google Scholar] [CrossRef]
- Hålldin, E.; Eriksen, A.K.; Brunius, C.; da Silva, A.B.; Bronze, M.; Hanhineva, K.; Aura, A.; Landberg, R. Factors Explaining Interpersonal Variation in Plasma Enterolactone Concentrations in Humans. Mol. Nutr. Food Res. 2019, 63, e1801159. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Eeckhaut, E.; Depypere, H.; Verstraete, W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: Producer phenotyping and relation with intestinal community. FEMS Microbiol. Ecol. 2007, 61, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Factories 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Doré, J.; Blaut, M. Bioavailability of lignans in human subjects. Nutr. Res. Rev. 2006, 19, 187–196. [Google Scholar] [CrossRef]
- Wang, L.Q.; Meselhy, M.R.; Li, Y.; Qin, G.W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef]
- Sugimura, Y.; Kanda, A.; Sawada, K.; Wai, K.M.; Tanabu, A.; Ozato, N.; Midorikawa, T.; Hisada, T.; Nakaji, S.; Ihara, K. As-sociation between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int. J. Environ. Res. Public Health 2022, 19, 7464. [Google Scholar] [CrossRef] [PubMed]
- Fluitman, K.S.; Davids, M.; Olofsson, L.E.; Wijdeveld, M.; Tremaroli, V.; Keijser, B.J.; Visser, M.; Bäckhed, F.; Nieuwdorp, M.; Ijzerman, R.G. Gut microbial characteristics in poor appetite and undernutrition: A cohort of older adults and microbiota transfer in germ-free mice. J. Cachexia Sarcopenia Muscle 2022, 13, 2188–2201. [Google Scholar] [CrossRef] [PubMed]
- Nanavati, K.; Rutherfurd-Markwick, K.; Lee, S.J.; Bishop, N.C.; Ali, A. Effect of curcumin supplementation on exercise-induced muscle damage: A narrative review. Eur. J. Nutr. 2022, 61, 3835–3855. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Córdova Martínez, A.; Caballero García, A.; Fernandez-Lazaro, C.I. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: A systematic review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef]
- Campbell, M.S.; Carlini, N.A.; Fleenor, B.S. Influence of curcumin on performance and post-exercise recovery. Crit. Rev. Food Sci. Nutr. 2021, 61, 1152–1162. [Google Scholar] [CrossRef]
- Basham, S.A.; Waldman, H.S.; Krings, B.M.; Lamberth, J.; Smith, J.W.; McAllister, M.J. Effect of Curcumin Supplementation on Exercise-Induced Oxidative Stress, Inflammation, Muscle Damage, and Muscle Soreness. J. Diet. Suppl. 2020, 17, 401–414. [Google Scholar] [CrossRef]
- Mallard, A.R.; Briskey, D.; Richards, B.A.; Rao, A. Curcumin Improves Delayed Onset Muscle Soreness and Postexercise Lactate Accumulation. J. Diet. Suppl. 2021, 18, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Takada, S.; Kinugawa, S.; Tsutsui, H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp. Physiol. 2015, 100, 1052–1063. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.; Chen, H.; Li, X.; Ye, C.; Zhang, F.; Zhang, Z.; Yao, Q.; Guo, Y. Curcumin targeting NF-κB/ubiquitin-proteasome-system axis ameliorates muscle atrophy in triple-negative breast cancer cachexia mice. Mediat. Inflamm. 2022, 2022, 2567150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Xhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PCG-1α/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Deane, C.S.; Din, U.S.U.; Sian, T.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults. Nutrients 2022, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Chun, Y.-S.; Kim, J.-K.; Lee, J.-O.; Ku, S.-K.; Shim, S.-M. Curcumin Attenuates Sarcopenia in Chronic Forced Exercise Executed Aged Mice by Regulating Muscle Degradation and Protein Synthesis with Antioxidant and Anti-inflammatory Effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Yang, I.-H.; Lin, Y.-W.; Lin, J.-N.; Wu, C.-C.; Chiang, C.-Y.; Lai, K.-H.; Lin, F.-H. Curcumin-Loaded Hydrophobic Surface-Modified Hydroxyapatite as an Antioxidant for Sarcopenia Prevention. Antioxidants 2021, 10, 616. [Google Scholar] [CrossRef]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of prolonged dietaru curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Han, J. Curcuminoid Demethylation as an Alternative Metabolism by Human Intestinal Microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hao, S.; Yang, C.; Yuan, L.; Zhou, X.; Zhao, H.; Yao, J. Alterations of intestinal microbiota in liver cirrhosis with muscle wasting. Nutrition 2021, 83, 111081. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to look into the puzzle of pol-yphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.A.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabo-types? Food Funct. 2021, 12, 10325–10355. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Ticinesi, A.; Mancabelli, L.; Carnevali, L.; Nouvenne, A.; Meschi, T.; Del Rio, D.; Ventura, M.; Sgoifo, A.; Angelino, D. Interac-tion Between Diet and Microbiota in the Pathophysiology of Alzheimer’s Disease: Focus on Polyphenols and Dietary Fibers. J. Alzheimers Dis. 2022, 86, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Ticinesi, A.; Gerritsen, J.; Nouvenne, A.; Lugli, G.A.; Mancabelli, L.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: A metagenomic study. Sci. Rep. 2016, 6, 25945. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
| Polyphenol Class | Polyphenol Subclass | Compound | Main Dietary Sources | Action in Experimental Models |
|---|---|---|---|---|
| Phenolic Acid | Hydroxybenzoic Acid | Gallic Acid | Berries, plums, grapes, mango, tea, wine | Increased mitochondrial function and biogenesis |
| Ellagic Acid | Berries, grapes, pomegranates, walnuts | Induction of antioxidant enzymes, protection against mitochondrial dysfunction | ||
| Urolithin A | Berries, grapes, pom-egranates, walnuts | Increased muscle angiogenesis, energetic capacity and contractile function | ||
| Urolithin B | Berries, grapes, pom-egranates, walnuts | Increased protein synthesis, myotube differentiation and muscular fiber hypertrophy | ||
| Hydroxycinnamic Acid | Ferulic Acid | Rice, wheat, oats, beans, coffee, artichoke, nuts | Regulation of muscle fiber differentiation and stimulation of myogenic transcriptional factors | |
| Chlorogenic Acid | Apples, artichoke, coffee, grapes, pears, kiwi, plums, potatoes | Improvement of mitochondrial function and energy metabolism | ||
| Caffeic Acid | Coffee, olives, carrots, potatoes, fruits | Stimulation of myocellular differentiation and hypertrophy | ||
| Flavonoids | Flavanols | Epicatechin | Berries, grapes, wine, cocoa, plums, tea | Induction of mitochondrial biogenesis and myogenic differentiation; decreased follistatin and myostatin |
| Epigallocatechin | Berries, grapes, wine, cocoa, plums, tea | Upregulation of myogenic transcriptional factors, antioxidant | ||
| Epigallocatechin Gallate | Berries, grapes, wine, cocoa, plums, tea | Reduction of protein degradation, reduction of proapoptotic signaling, inhibition of NF-κB | ||
| Flavanones | Hesperidin | Citrus fruits | Increased mitochondrial function, reduced oxidative stress | |
| Naringenin | Citrus fruits | Increased glucose uptake, regulation of skeletal muscle cell differentiation | ||
| Flavones | Apigenin | Herbs, tea, wine, citrus fruits, spinach, broccoli, peas | Inhibition of mitophagy and autophagy, enhanced myogenic differentiation, downregulation of TNFα | |
| Luteolin | Herbs, tea, wine, citrus fruits, spinach, broccoli, peas | Downregulation of pro-inflammatory cytokines, antioxidant | ||
| Isoflavones | Genistein | Soybeans, fava beans, lupin, kudzu, psoralea, coffee | Inhibition of apoptosis, increased myocellular differentiation, antioxidant | |
| Daidzein | Soybeans, tofu, kudzu | Inhibition of protein degradation, promotion of myocellular differentiation | ||
| Glabridin | Licorice | Inhibition of protein degradation | ||
| Flavonols | Quercetin | Capers, herbs, coriander, radish, fennel, onion, radicchio, berries | Reduction of myostatin, antioxidation, increased mitochondrial biogenesis, reduction of protein degradation | |
| Morin | Osage orange, guava | Antioxidation, reduction of protein degradation | ||
| Anthocyanins | Delphinidin | Berries, pomegranates, grapes | Antioxidation, reduced atrogin-1 expression and protein degradation | |
| Cyanidin | Grapes, berries, cherry, apple, plum | Reduced inflammation and fibrosis | ||
| Polyphenol | Stilbene | Resveratrol | Grapes, berries, peanuts | Reduction of atrogin-1, reduction of oxidative stress, improvement of mitochondrial function, inc5reased protein synthesis, regulation of mTOR signaling, induction of myotube hypertrophy |
| Lignan | Schisandrin A | Shengmainsan (Chinese traditional herb) | Suppression of protein degradation and stimulation of protein synthesis | |
| Magnolol | Magnolia bark | Stimulation of IGF-1 mediated protein synthesis | ||
| Sesamin | Sesame | Reduced oxidative stress, increased mitochondrial function | ||
| Other | Curcumin | Curcumin | Turmeric, ginger, food additives | Inhibition of atrogin-1, reduction of oxidative stress, promotion of myofibrillar differentiation, reduction of proteasome expression and protein degradation |
| Phenolic Subclass | Bacterial Taxa Involved in Gut Microbiota Biotransformation Pathways | Metabotypes Identified |
|---|---|---|
| Ellagitannins | Akkermansia muciniphila | Yes (UroA, UroB, Uro0) |
| Gordonibacter spp. | ||
| Eggerthellaceae | ||
| Lactobacillus spp. | ||
| Leuconostoc spp. | ||
| Pediococcus spp. | ||
| Chlorogenic acid and derivatives | Bifidobacterium spp. | No |
| Flavanols/Proanthocyanidins | Clostridium coccoides | No |
| Bifidobacterium infantis | ||
| Eggerthella lenta | ||
| Adlercreutzia equolifaciens | ||
| Flavanones | Bifidobacterium spp. | Yes (hesperidin producers or not) |
| Eubacterium limosum | ||
| Eubacterium ramulus | ||
| Flavones | Enterococcus avium | No |
| Parabacteroides distasonis | ||
| Eubacterium ramulus | ||
| Flavonifractor plautii | ||
| Isoflavones | Lactococcus spp. | Yes (equol producers/non producers) |
| Enterococcus spp. | ||
| Bifidobacterium spp. | ||
| Clostridium spp. | ||
| Eggerthella spp. | ||
| Adlercreutzia spp. | ||
| Butyricimonas spp. | ||
| Eubacterium ramulus | ||
| Flavonols | Lachnoclostridium spp. | No |
| Eubacterium ramulus | ||
| Eubacterium oxidoreducens | ||
| Flavonifractor plautii | ||
| Butyrivibrio spp. | ||
| Anthocyanins | Bacteroides spp. | No |
| Clostridium spp. | ||
| Eubacterium spp. | ||
| Resveratrol | Adlercreutzia equilifaciens | Yes (lunularin producers/non producers) |
| Slackia equolifaciens | ||
| Lignans | Bacteroides spp. | Yes (low, middle or high metabolizers) |
| Clostridium spp. | ||
| Eubacterium limosum | ||
| Blautia producta | ||
| Eggerthella lenta | ||
| Curcumin | Escherichia coli | No |
| Blautia spp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Meschi, T. Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging. Nutrients 2023, 15, 2367. https://doi.org/10.3390/nu15102367
Ticinesi A, Nouvenne A, Cerundolo N, Parise A, Meschi T. Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging. Nutrients. 2023; 15(10):2367. https://doi.org/10.3390/nu15102367
Chicago/Turabian StyleTicinesi, Andrea, Antonio Nouvenne, Nicoletta Cerundolo, Alberto Parise, and Tiziana Meschi. 2023. "Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging" Nutrients 15, no. 10: 2367. https://doi.org/10.3390/nu15102367
APA StyleTicinesi, A., Nouvenne, A., Cerundolo, N., Parise, A., & Meschi, T. (2023). Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging. Nutrients, 15(10), 2367. https://doi.org/10.3390/nu15102367






