Magnesium Deficiency and Cardiometabolic Disease
Abstract
:1. Introduction
2. Magnesium Homeostasis
3. Magnesium Concentration Measurement and Supplementation
4. Magnesium Deficiency in Obesity and Diabetes
4.1. Type 1 Diabetes
4.2. Type 2 Diabetes and Obesity
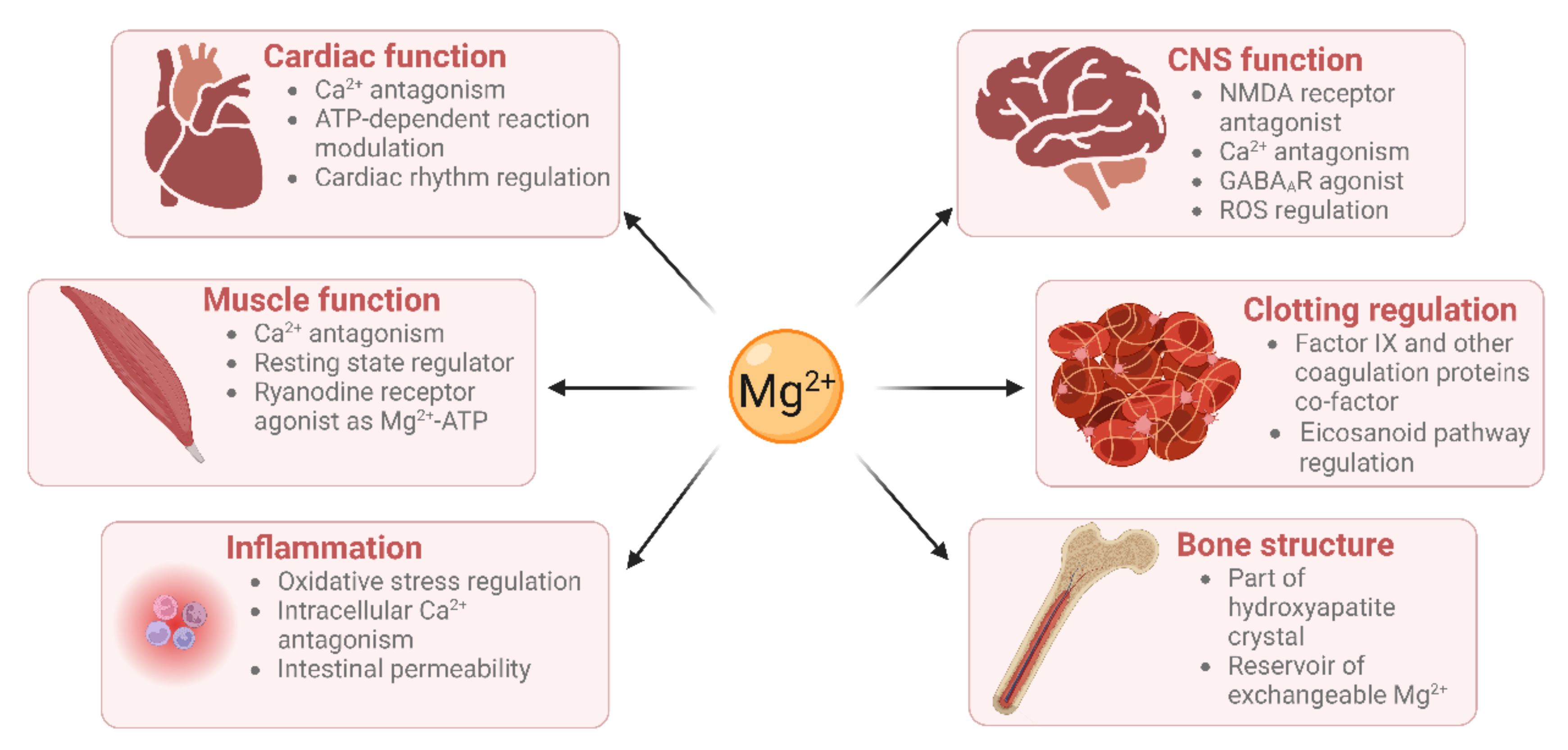
5. Cardiovascular Roles of Magnesium
5.1. Cardiac Muscle Contraction
5.2. Vascular Functioning
5.3. Haemostasis
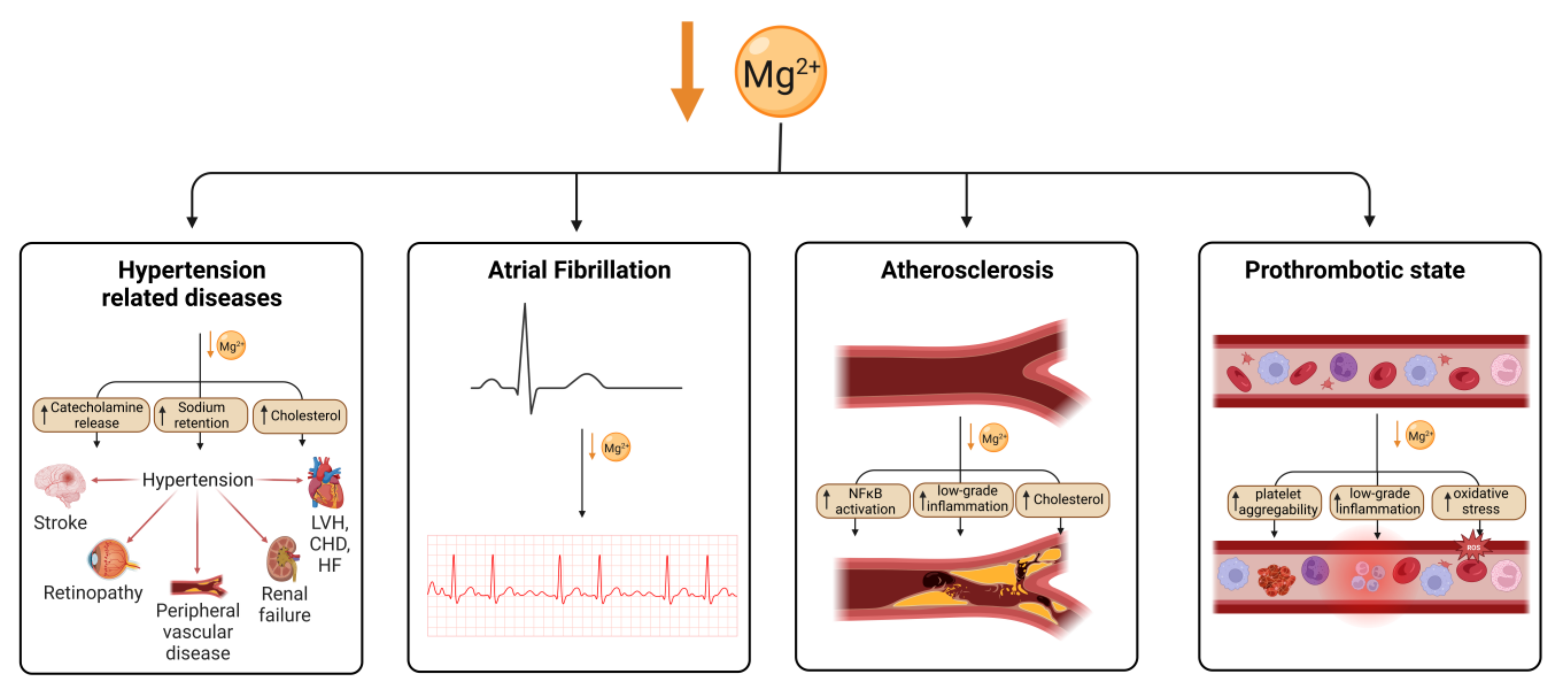
6. Effects of Magnesium Deficiency on the Cardiovascular System
6.1. Hypertension
6.2. Cardiac Functioning
6.3. Vascular Disease
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPI | ankle-brachial pressure index |
| AF | atrial fibrillation |
| ARIC | atherosclerosis risk in communities |
| BP | blood pressure |
| CAD | coronary artery disease |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| ECM | extracellular matrix |
| GABAAR | γ-aminobutyric acid A receptor |
| GLA | γ-carboxyglutamate-rich |
| HbA1c | glycated haemoglobin |
| HF | heart failure |
| IL-n | interleukin-n |
| LVH | left ventricular hypertrophy |
| NFκB | nuclear factor-kappa B |
| NMDA | N-methyl-D-aspartate |
| NHANES | American National Health and Nutrition Examination Survey |
| PAD | peripheral arterial disease |
| PAI-1 | plasminogen activator inhibitor-1 |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| T1DM | type-1 diabetes mellitus |
| T2DM | type-2 diabetes mellitus |
| TNF | tumour necrosis factor |
| TRPM | transient receptor potential channel |
| VCAM | vascular cell adhesion molecule |
| WHO | World Health Organisation |
References
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Rude, R. Magnesium disorders. In Fluids and Electrolytes; Kokko, J., Tannen, R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 421–445. [Google Scholar]
- Rosanoff, A.; West, C.; Elin, R.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Wang, K.C.; Hayward, S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients 2017, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89. [Google Scholar] [PubMed]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef]
- Buelens, F.P.; Leonov, H.; de Groot, B.L.; Grubmüller, H. ATP-magnesium coordination: Protein structure-based force field evaluation and corrections. J. Chem. Theory Comput. 2021, 17, 1922–1930. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Steitz, T.A. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998, 8, 54–63. [Google Scholar] [CrossRef]
- Suh, W.C.; Leirmo, S.; Record, M.T., Jr. Roles of Mg2+ in the mechanism of formation and dissociation of open complexes between Escherichia coli RNA polymerase and the lambda PR promoter: Kinetic evidence for a second open complex requiring Mg2+. Biochemistry 1992, 31, 7815–7825. [Google Scholar] [CrossRef] [PubMed]
- Salehidoost, R.; Taghipour Boroujeni, G.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of oral magnesium supplement on cardiometabolic markers in people with prediabetes: A double blind randomized controlled clinical trial. Sci. Rep. 2022, 12, 18209. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Marfella, C.; Scarpa, A. Cell magnesium transport and homeostasis: Role of intracellular compartments. Miner. Electrolyte Metab. 1993, 19, 282–289. [Google Scholar]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar] [PubMed]
- Romani, A.M.P. Intracellular magnesium homeostasis. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Firoz, M.; Graber, M. Bioavailability of US commercial magnesium preparations. Magnes. Res. 2001, 14, 257–262. [Google Scholar] [PubMed]
- Marier, J.R. Magnesium content of the food supply in the modern-day world. Magnesium 1986, 5, 1–8. [Google Scholar]
- Crinnion, W.J. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Altern. Med. Rev. 2010, 15, 4–12. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Schuchardt, J.P.; Hahn, A. Intestinal absorption and factors influencing bioavailability of magnesium—An update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Karbach, U.; Rummel, W. Cellular and paracellular magnesium transport across the terminal ileum of the rat and its interaction with the calcium transport. Gastroenterology 1990, 98, 985–992. [Google Scholar] [CrossRef]
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: A mouse model. PLoS Genet. 2013, 9, e1003983. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, L.L.; Jones, M.R.; Buddington, R.K.; Clemens, R.A.; Lee, D.B. Comparison of calcium and magnesium absorption: In vivo and in vitro studies. Am. J. Physiol. Gastrointest. Liver Physiol. 1990, 259, G720–G726. [Google Scholar] [CrossRef]
- Schweigel, M.; Martens, H. Magnesium transport in the gastrointestinal tract. Front. Biosci. 2000, 5, D666–D677. [Google Scholar] [CrossRef]
- Walser, M. Magnesium metabolism. Ergeb. Physiol. 1967, 59, 185–296. [Google Scholar] [CrossRef]
- Alfrey, A.C.; Miller, N.L. Bone magnesium pools in uremia. J. Clin. Investig. 1973, 52, 3019–3027. [Google Scholar] [CrossRef]
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1985, 1, 119–122. [Google Scholar] [CrossRef]
- Cunningham, J.; Rodríguez, M.; Messa, P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin. Kidney J. 2012, 5, i39–i51. [Google Scholar] [CrossRef]
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM 2018, 111, 759–763. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Taal, M.W.; Brenner, B.M.; Rector, F.C. Brenner & Rector’s the Kidney, 9th ed.; Elsevier: Amsterdam, The Netherlands; Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Le Grimellec, C.; Roinel, N.; Morel, F. Simultaneous Mg, Ca, P, K, Na and Cl analysis in rat tubular fluid. II. During acute Mg plasma loading. Pflug. Arch. 1973, 340, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Brunette, M.G.; Vigneault, N.; Carriere, S. Micropuncture study of magnesium transport along the nephron in the young rat. Am. J. Physiol. 1974, 227, 891–896. [Google Scholar] [CrossRef]
- Ryan, M.P.; Devane, J.; Ryan, M.F.; Counihan, T.B. Effects of diuretics on the renal handling of magnesium. Drugs 1984, 28, 167–181. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Ryan, M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991, 28, 19–26. [Google Scholar] [CrossRef]
- Alfrey, A.C.; Miller, N.L.; Trow, R. Effect of age and magnesium depletion on bone magnesium pools in rats. J. Clin. Investig. 1974, 54, 1074–1081. [Google Scholar] [CrossRef]
- Tibbetts, D.M.; Aub, J.C. Magnesium metabolism in health and disease. I. the magnesium and calcium excretion of normal individuals, also the effects of magnesium, chloride, and phosphate ions. J. Clin. Investig. 1937, 16, 491–501. [Google Scholar] [CrossRef]
- Deng, B.; Li, X.; Zhu, P.; Xu, X.; Xu, Q.; Kang, Y. Speciation of magnesium in rat plasma using capillary electrophoresis-inductively coupled plasma-atomic emission spectrometry. Electrophoresis 2008, 29, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Walser, M. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J. Clin. Investig. 1961, 40, 723–730. [Google Scholar]
- Peters, T., Jr. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Midtvedt, K.; Dolva, L.O.; Norseth, J.; Kjekshus, J. The magnesium loading test: Reference values in healthy subjects. Scand. J. Clin. Lab. Investig. 1994, 54, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.N.; Jepsen, J.M.; Sjøgaard, G.; Hessov, I. A magnesium load test in the diagnosis of magnesium deficiency. Hum. Nutr. Clin. Nutr. 1987, 41, 301–306. [Google Scholar] [PubMed]
- Rosner, M.H.; Ha, N.; Palmer, B.F.; Perazella, M.A. Acquired Disorders of Hypomagnesemia. Mayo Clin. Proc. 2023, 98, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Cheteu Wabo, T.M.; Wu, X.; Sun, C.; Boah, M.; Ngo Nkondjock, V.R.; Kosgey Cheruiyot, J.; Amporfro Adjei, D.; Shah, I. Association of dietary calcium, magnesium, sodium, and potassium intake and hypertension: A study on an 8-year dietary intake data from the National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2022, 16, 74–93. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; McGuire, L.C.; Mokdad, A.H.; Liu, S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity 2007, 15, 1139–1146. [Google Scholar] [CrossRef]
- Kesteloot, H.; Joossens, J.V. Relationship of dietary sodium, potassium, calcium, and magnesium with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 1988, 12, 594–599. [Google Scholar] [CrossRef] [PubMed]
- van Leer, E.M.; Seidell, J.C.; Kromhout, D. Dietary calcium, potassium, magnesium, and blood pressure in the Netherlands. Int. J. Epidemiol. 1995, 24, 1117–1123. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; van Horn, L.; Jacobs, D.R., Jr.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low dietary magnesium and overweight/obesity in a Mediterranean population: A detrimental synergy for the development of hypertension. The SUN project. Nutrients 2020, 13, 125. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between dietary magnesium intake and metabolic syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K. Magnesium. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MA, USA, 2012; pp. 159–175. [Google Scholar]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Rudser, K.D.; Alonso, A.; Harnack, L.; Saenger, A.K.; Lutsey, P.L. Circulating ionized magnesium: Comparisons with circulating total magnesium and the response to magnesium supplementation in a randomized controlled trial. Nutrients 2020, 12, 263. [Google Scholar] [CrossRef]
- Epstein, M.; McGrath, S.; Law, F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N. Engl. J. Med. 2006, 355, 1834–1836. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H.; et al. Effects of magnesium citrate, magnesium oxide, and magnesium sulfate supplementation on arterial stiffness: A randomized, double-blind, placebo-controlled intervention trial. J. Am. Heart Assoc. 2022, 11, e021783. [Google Scholar] [CrossRef]
- Gross, P.; Heduschka, P. Chapter 47—Inherited Disorders of Sodium and Water Handling. In Comprehensive Clinical Nephrology, 4th ed.; Mosby: St. Louis, MO, USA, 2010; pp. 573–583. ISBN 9780323058766. [Google Scholar]
- Classen, H.G. Magnesium orotate—Experimental and clinical evidence. Rom. J. Intern. Med. 2004, 42, 491–501. [Google Scholar]
- De Franceschi, L.; Bachir, D.; Galacteros, F.; Tchernia, G.; Cynober, T.; Neuberg, D.; Beuzard, Y.; Brugnara, C. Oral magnesium pidolate: Effects of long-term administration in patients with sickle cell disease. Br. J. Haematol. 2000, 108, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Schuette, S.A.; Lashner, B.A.; Janghorbani, M. Bioavailability of magnesium diglycinate vs magnesium oxide in patients with ileal resection. J. Parenter. Enter. Nutr. 1994, 18, 430–435. [Google Scholar] [CrossRef]
- Uysal, N.; Kizildag, S.; Yuce, Z.; Guvendi, G.; Kandis, S.; Koc, B.; Karakilic, A.; Camsari, U.M.; Ates, M. Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best? Biol. Trace Elem. Res. 2019, 187, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Sarma, D.; Saikia, U.K. Hypomagnesemia in type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16, 1000–1003. [Google Scholar] [CrossRef]
- Kocyigit, E.; Akturk, M.; Koksal, E. Relationships between serum and dietary magnesium, calcium, and metabolic parameters in women with type 2 diabetes mellitus. Clin. Nutr. ESPEN 2023, 54, 304–310. [Google Scholar] [CrossRef]
- Rodrigues, A.K.; Melo, A.E.; Domingueti, C.P. Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Stefanowicz, F.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Total plasma magnesium, zinc, copper and selenium concentrations in type-I and type-II diabetes. Biometals 2019, 32, 123–138. [Google Scholar] [CrossRef]
- van Dijk, P.R.; Waanders, F.; Qiu, J.; de Boer, H.H.R.; van Goor, H.; Bilo, H.J.G. Hypomagnesemia in persons with type 1 diabetes: Associations with clinical parameters and oxidative stress. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820980240. [Google Scholar] [CrossRef]
- Oost, L.J.; van Heck, J.I.P.; Tack, C.J.; de Baaij, J.H.F. The association between hypomagnesemia and poor glycaemic control in type 1 diabetes is limited to insulin resistant individuals. Sci. Rep. 2022, 12, 6433. [Google Scholar] [CrossRef] [PubMed]
- Markovits, N.; Loebstein, R.; Halkin, H.; Bialik, M.; Landes-Westerman, J.; Lomnicky, J.; Kurnik, D. The association of proton pump inhibitors and hypomagnesemia in the community setting. J. Clin. Pharmacol. 2014, 54, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Kietsiriroje, N.; Pearson, S.; Campbell, M.; Ariëns, R.A.S.; Ajjan, R.A. Double diabetes: A distinct high-risk group? Diabetes Obes. Metab. 2019, 21, 2609–2618. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef]
- Iafusco, D.; Franceschi, R.; Maguolo, A.; Guercio Nuzio, S.; Crinò, A.; Delvecchio, M.; Iughetti, L.; Maffeis, C.; Calcaterra, V.; Manco, M. From metabolic syndrome to type 2 diabetes in youth. Children 2023, 10, 516. [Google Scholar] [CrossRef]
- Balkhiyarova, Z.; Luciano, R.; Kaakinen, M.; Ulrich, A.; Shmeliov, A.; Bianchi, M.; Chioma, L.; Dallapiccola, B.; Prokopenko, I.; Manco, M. Relationship between glucose homeostasis and obesity in early life-a study of Italian children and adolescents. Hum. Mol. Genet. 2022, 31, 816–826. [Google Scholar] [CrossRef]
- Carroll, S.; Dudfield, M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sport. Med. 2004, 34, 371–418. [Google Scholar] [CrossRef]
- Gorodeski Baskin, R.; Alfakara, D. Root Cause for Metabolic Syndrome and Type 2 Diabetes: Can Lifestyle and Nutrition Be the Answer for Remission. Endocrinol. Metab. Clin. N. Am. 2023, 52, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Joshi, S.; Shaw, J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res. Clin. Pract. 2010, 87, 261–266. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Wu, J.; Xun, P.; Tang, Q.; Cai, W.; He, K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Xun, P.; Liu, K.; Loria, C.; Yokota, K.; Jacobs, D.R., Jr.; He, K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010, 33, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.T.; Dai, Q.; Yang, G.; Cai, H.; Li, H.; Zheng, W.; Shu, X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Nevárez-Sida, A. Cost-effectiveness analysis of using oral magnesium supplementation in the treatment of prediabetes. Prim. Care Diabetes 2022, 16, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hierons, S.J.; Catchpole, A.; Abbas, K.; Wong, W.; Giles, M.S.; Miller, G.V.; Ajjan, R.A.; Stewart, A.J. Total plasma magnesium, zinc, copper and selenium concentrations in obese patients before and after bariatric surgery. Biometals 2023, 36, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hsu, Y.H.; Niu, T.; Manson, J.E.; Buring, J.E.; Liu, S. Common genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (TRPM6 and TRPM7), magnesium intake, and risk of type 2 diabetes in women. BMC Med. Genet. 2009, 10, 4. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Jing, Z.; Wang, Y.; Cheng, Y.; Wang, W.; Sun, W. Role of magnesium in type 2 diabetes mellitus. Biol. Trace Elem. Res. 2020, 196, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Ryul Shim, S.; Jamali, N.; Hassanzadeh-Rostami, Z.; Soltani, S.; Sasani, N.; Mohsenpour, M.A.; Firoozi, D.; Basirat, R.; Hosseini, R.; et al. Comparison of nutritional supplements for glycemic control in type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Res. Clin. Pract. 2022, 191, 110037. [Google Scholar] [CrossRef]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef]
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 457–473. [Google Scholar] [CrossRef]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef]
- Zdanowicz, M.M.; Barletta, M.A. Protective role of magnesium in catecholamine-induced arrhythmia and toxicity in vitro. Magnes. Res. 1991, 4, 153–162. [Google Scholar]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef]
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Haenni, A.; Johansson, K.; Lind, L.; Lithell, H. Magnesium infusion improves endothelium-dependent vasodilation in the human forearm. Am. J. Hypertens. 2002, 15, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Landau, R.; Scott, J.A.; Smiley, R.M. Magnesium-induced vasodilation in the dorsal hand vein. BJOG 2004, 111, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Dietrich, H.H.; Horiuchi, T.; Hongo, K.; Dacey, R.G., Jr. Mechanisms of magnesium-induced vasodilation in cerebral penetrating arterioles. Neurosci. Res. 2016, 107, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Oshima, T.; Matsuura, H.; Ishida, T.; Kambe, M.; Kajiyama, G. Extracellular Mg2+ inhibits capacitative Ca2+ entry in vascular smooth muscle cells. Circulation 1997, 95, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.M.; Pashley, D.H.; Anderson, R.W. Response of pulpal blood flow to intra-arterial infusion of endothelin. J. Endod. 1992, 18, 228–231. [Google Scholar] [CrossRef]
- Lansman, J.B.; Hess, P.; Tsien, R.W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986, 88, 321–347. [Google Scholar] [CrossRef]
- Soltani, N.; Keshavarz, M.; Sohanaki, H.; Zahedi Asl, S.; Dehpour, A.R. Relaxatory effect of magnesium on mesenteric vascular beds differs from normal and streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2005, 508, 177–181. [Google Scholar] [CrossRef]
- Shimosawa, T.; Takano, K.; Ando, K.; Fujita, T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension 2004, 44, 897–902. [Google Scholar] [CrossRef]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Satake, K.; Lee, J.D.; Shimizu, H.; Uzui, H.; Mitsuke, Y.; Yue, H.; Ueda, T. Effects of magnesium on prostacyclin synthesis and intracellular free calcium concentration in vascular cells. Magnes. Res. 2004, 17, 20–27. [Google Scholar] [PubMed]
- Longo, M.; Jain, V.; Vedernikov, Y.P.; Facchinetti, F.; Saade, G.R.; Garfield, R.E. Endothelium dependence and gestational regulation of inhibition of vascular tone by magnesium sulfate in rat aorta. Am. J. Obstet. Gynecol. 2001, 184, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.C.; Ponce, X.; González-González, M.E.; Larrea, F.; Halhali, A. Effects of magnesium sulphate on placental expression of endothelin 1 and its receptors in preeclampsia. Clin. Biochem. 2007, 40, 976–980. [Google Scholar] [CrossRef]
- Locatelli, L.; Fedele, G.; Castiglioni, S.; Maier, J.A. Magnesium deficiency induces lipid accumulation in vascular endothelial cells via oxidative stress—The potential contribution of EDF-1 and PPARγ. Int. J. Mol. Sci. 2021, 22, 1050. [Google Scholar] [CrossRef]
- Maier, J.A.; Malpuech-Brugère, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 2004, 1689, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, S.; Baldoli, E.; Leidi, M.; Maier, J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim. Biophys. Acta 2010, 1802, 952–958. [Google Scholar] [CrossRef]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Chandrasekran, S.; Quon, M.J. Role of lipotoxicity in endothelial dysfunction. Heart Fail. Clin. 2012, 8, 589–607. [Google Scholar] [CrossRef]
- Cai, Z.; Gong, Z.; Li, Z.; Li, L.; Kong, W. Vascular extracellular matrix remodeling and hypertension. Antioxid. Redox Signal. 2021, 34, 765–783. [Google Scholar] [CrossRef]
- Weigel, P.H.; DeAngelis, P.L. Hyaluronan synthases: A decade-plus of novel glycosyltransferases. J. Biol. Chem. 2007, 282, 36777–36781. [Google Scholar] [CrossRef]
- Malinin, N.L.; Pluskota, E.; Byzova, T.V. Integrin signaling in vascular function. Curr. Opin. Hematol. 2012, 19, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.M.; Zhang, L. Structure-function of the putative I-domain within the integrin beta 2 subunit. J. Biol. Chem. 2001, 276, 19340–19349. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, A.; Li, J.; Chien, S.; Engler, A.J. Cation type specific cell remodeling regulates attachment strength. PLoS ONE 2014, 9, e102424. [Google Scholar] [CrossRef]
- Trache, A.; Trzeciakowski, J.P.; Meininger, G.A. Mg2+ modulates integrin-extracellular matrix interaction in vascular smooth muscle cells studied by atomic force microscopy. J. Mol. Recognit. 2010, 23, 316–321. [Google Scholar]
- Mould, A.P.; Garratt, A.N.; Puzon-McLaughlin, W.; Takada, Y.; Humphries, M.J. Regulation of integrin function: Evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha5 beta1. Biochem. J. 1998, 331, 821–828. [Google Scholar] [CrossRef]
- Lawson, J.H.; Mann, K.G. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J. Biol. Chem. 1991, 266, 11317–11327. [Google Scholar] [CrossRef] [PubMed]
- Sidonio, R.F., Jr.; Malec, L. Hemophilia B (factor IX deficiency). Hematol. Oncol. Clin. N. Am. 2021, 35, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, F.; Yoshida, M.; Yamashita, T.; Morita, T. Magnesium(II) is a crucial constituent of the blood coagulation cascade. Potentiation of coagulant activities of factor IX by Mg2+ ions. J. Biol. Chem. 1996, 271, 8541–8544. [Google Scholar] [CrossRef]
- Gajsiewicz, J.M.; Nuzzio, K.M.; Rienstra, C.M.; Morrissey, J.H. Tissue factor residues that modulate magnesium-dependent rate enhancements of the tissue factor/factor VIIa complex. Biochemistry 2015, 54, 4665–4671. [Google Scholar] [CrossRef]
- van den Besselaar, A.M. Magnesium and manganese ions accelerate tissue factor-induced coagulation independently of factor IX. Blood Coagul. Fibrinolysis 2002, 13, 19–23. [Google Scholar] [CrossRef]
- Falls, L.A.; Furie, B.C.; Jacobs, M.; Furie, B.; Rigby, A.C. The omega-loop region of the human prothrombin gamma-carboxyglutamic acid domain penetrates anionic phospholipid membranes. J. Biol. Chem. 2001, 276, 23895–23902. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hsiao, G.; Shen, M.Y.; Fong, T.H.; Chen, Y.W.; Lin, C.H.; Chou, D.S. Mechanisms involved in the antiplatelet activity of magnesium in human platelets. Br. J. Haematol. 2002, 119, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Li, X.; Wu, J.; Liang, Z.; Deng, F.; Xie, W.; Zhu, M.; Zhu, J.; Zhu, W.; Geng, S.; et al. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation 2015, 38, 1639–1648. [Google Scholar] [CrossRef]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and cardiovascular disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- Rosanoff, A.; Costello, R.B.; Johnson, G.H. Effectively prescribing oral magnesium therapy for hypertension: A categorized systematic review of 49 clinical trials. Nutrients 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef]
- Enaruna, N.O.; Ande, A.; Okpere, E.E. Clinical significance of low serum magnesium in pregnant women attending the University of Benin Teaching Hospital. Niger. J. Clin. Pract. 2013, 16, 448–453. [Google Scholar] [CrossRef]
- Jafrin, W.; Mia, A.R.; Chakraborty, P.K.; Hoque, M.R.; Paul, U.K.; Shaha, K.R.; Akhter, S.; Roy, A.S. An evaluation of serum magnesium status in pre-eclampsia compared to the normal pregnancy. Mymensingh Med. J. 2014, 23, 649–653. [Google Scholar]
- Kanagal, D.V.; Rajesh, A.; Rao, K.; Devi, U.H.; Shetty, H.; Kumari, S.; Shetty, P.K. Levels of Serum Calcium and Magnesium in Pre-eclamptic and Normal Pregnancy: A Study from Coastal India. J. Clin. Diagn. Res. 2014, 8, OC01–OC04. [Google Scholar] [CrossRef] [PubMed]
- The Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet 2002, 359, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S.; Michaëlsson, K. Serum magnesium levels and risk of coronary artery disease: Mendelian randomisation study. BMC Med. 2018, 16, 68. [Google Scholar] [CrossRef]
- He, B.; Xia, L.; Zhao, J.; Yin, L.; Zhang, M.; Quan, Z.; Ou, Y.; Huang, W. Causal Effect of Serum Magnesium on Osteoporosis and Cardiometabolic Diseases. Front. Nutr. 2021, 8, 738000. [Google Scholar] [CrossRef]
- Migdady, I.; Russman, A.; Buletko, A.B. Atrial fibrillation and ischemic stroke: A clinical review. Semin. Neurol. 2021, 41, 348–364. [Google Scholar] [CrossRef]
- Khan, A.M.; Lubitz, S.A.; Sullivan, L.M.; Sun, J.X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; Benjamin, E.J.; Wang, T.J. Low serum magnesium and the development of atrial fibrillation in the community: The Framingham heart study. Circulation 2013, 127, 33–38. [Google Scholar] [CrossRef]
- Topol, E.J.; Lerman, B.B. Hypomagnesemic torsades de pointes. Am. J. Cardiol. 1983, 52, 1367–1368. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Xie, J.; Chow, M.S.; Kluger, J. Prophylactic magnesium to decrease the arrhythmogenic potential of class III antiarrhythmic agents in a rabbit model. Pharmacotherapy 1999, 19, 635–640. [Google Scholar] [CrossRef]
- Shimaoka, T.; Wang, Y.; Morishima, M.; Miyamoto, S.; Ono, K. Magnesium deficiency causes transcriptional downregulation of Kir2.1 and Kv4.2 channels in cardiomyocytes resulting in QT interval prolongation. Circ. J. 2020, 84, 1244–1253. [Google Scholar] [CrossRef]
- ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collabora-tive Group. Lancet 1995, 345, 669–685.
- Woods, K.L.; Fletcher, S.; Roffe, C.; Haider, Y. Intravenous magnesium sulphate in suspected acute myocardial infarction: Results of the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2). Lancet 1992, 339, 1553–1558. [Google Scholar] [CrossRef]
- Woods, K.L.; Fletcher, S. Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: The second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2). Lancet 1994, 343, 816–819. [Google Scholar] [CrossRef]
- Sakaguchi, Y. The emerging role of magnesium in CKD. Clin. Exp. Nephrol. 2022, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Shanahan, C.M.; de Baaij, J.H.F. Magnesium counteracts vascular calcification: Passive interference or active modulation? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B., Sr. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Virmani, R. Correlation between carotid intimal/medial thickness and atherosclerosis: A point of view from pathology. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ehrenpreis, E.D.; Jarrouj, G.; Meader, R.; Wagner, C.; Ellis, M. A comprehensive review of hypomagnesemia. Dis. Mon. 2022, 68, 101285. [Google Scholar] [CrossRef]
- Reffelmann, T.; Dörr, M.; Ittermann, T.; Schwahn, C.; Völzke, H.; Ruppert, J.; Robinson, D.; Felix, S.B. Low serum magnesium concentrations predict increase in left ventricular mass over 5 years independently of common cardiovascular risk factors. Atherosclerosis 2010, 213, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Shugaa Addin, N.; Schlett, C.L.; Bamberg, F.; Thorand, B.; Linseisen, J.; Seissler, J.; Peters, A.; Rospleszcz, S. Subclinical Cardiovascular Disease Markers in Relation to Serum and Dietary Magnesium in Individuals from the General Population: The KORA-MRI Study. Nutrients 2022, 14, 4954. [Google Scholar] [CrossRef] [PubMed]
- Nordanstig, J.; Behrendt, C.A.; Bradbury, A.W.; de Borst, G.J.; Fowkes, F.; Golledge, J.; Gottsater, A.; Hinchliffe, R.J.; Nikol, S.; Norgren, L. Peripheral arterial disease (PAD)—A challenging manifestation of atherosclerosis. Prev. Med. 2023, 171, 107489. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhuang, X.; Huo, M.; Feng, P.; Zhang, S.; Zhong, X.; Zhou, H.; Guo, Y.; Hu, X.; Du, Z.; et al. Serum magnesium and the prevalence of peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2019, 282, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jin, X.; Liu, J.; Sun, T.; Xie, M.; Bao, W.; Yu, X.; Yang, X.; Zhang, Y.; Zhang, H.; et al. Association of plasma magnesium with prediabetes and type 2 diabetes mellitus in adults. Sci. Rep. 2017, 7, 12763. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Plat, J.; Bakker, S.J.; Mensink, R.P. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci. Rep. 2017, 7, 106. [Google Scholar] [CrossRef]
- Farshidi, H.; Sobhani, A.R.; Eslami, M.; Azarkish, F.; Eftekhar, E.; Keshavarz, M.; Soltani, N. Magnesium sulfate administration in moderate coronary artery disease patients improves atherosclerotic risk factors: A double-blind clinical trial study. J. Cardiovasc. Pharmacol. 2020, 76, 321–328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium Deficiency and Cardiometabolic Disease. Nutrients 2023, 15, 2355. https://doi.org/10.3390/nu15102355
Fritzen R, Davies A, Veenhuizen M, Campbell M, Pitt SJ, Ajjan RA, Stewart AJ. Magnesium Deficiency and Cardiometabolic Disease. Nutrients. 2023; 15(10):2355. https://doi.org/10.3390/nu15102355
Chicago/Turabian StyleFritzen, Remi, Amy Davies, Miriam Veenhuizen, Matthew Campbell, Samantha J. Pitt, Ramzi A. Ajjan, and Alan J. Stewart. 2023. "Magnesium Deficiency and Cardiometabolic Disease" Nutrients 15, no. 10: 2355. https://doi.org/10.3390/nu15102355






