An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications
Abstract
1. Introduction
2. Epidemiology
3. Etiology
4. Intestinal Rehabilitation Programs
5. Intestinal Adaptation
6. Prognostic Criteria
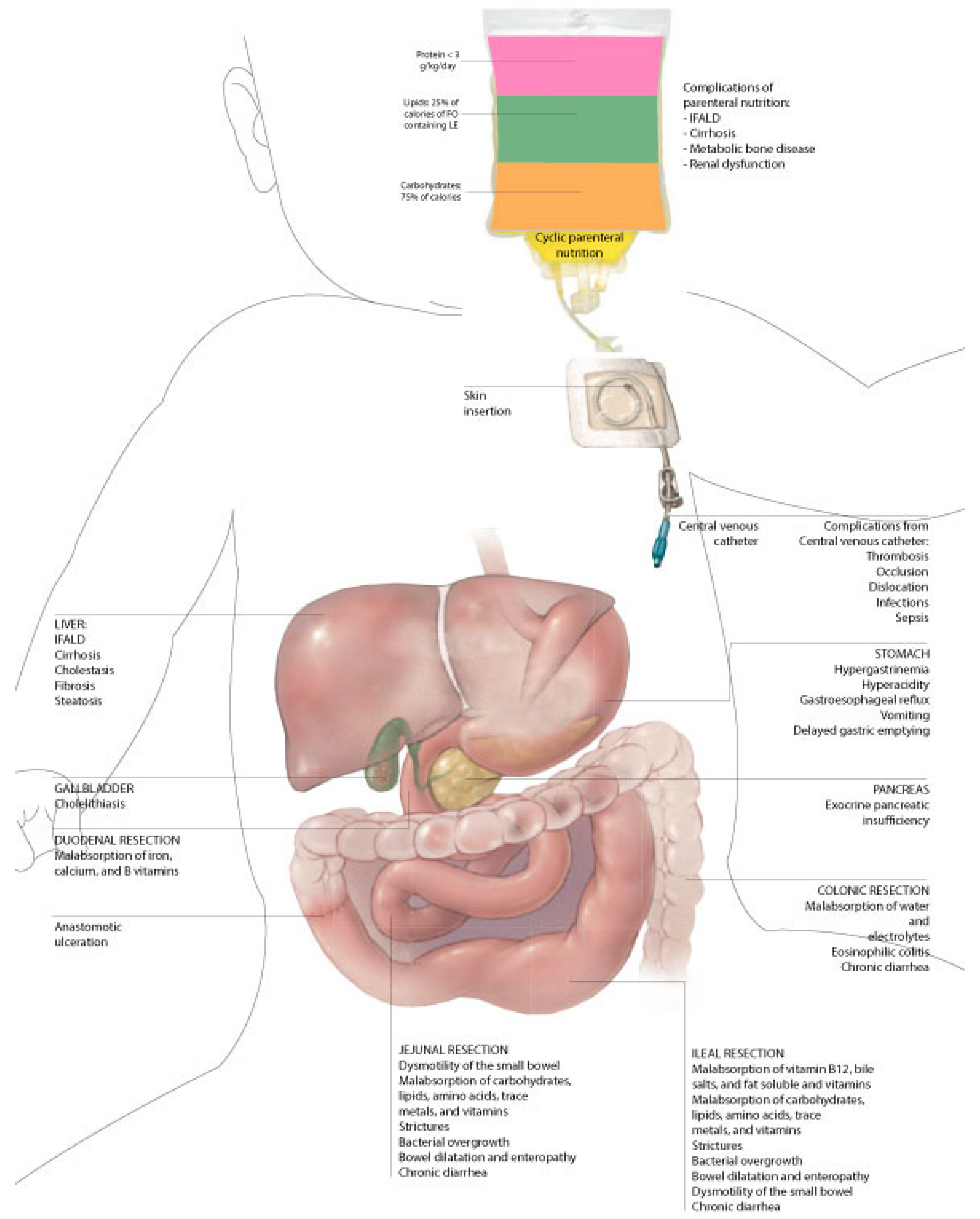
7. Complications
7.1. Catheter-Related Complications
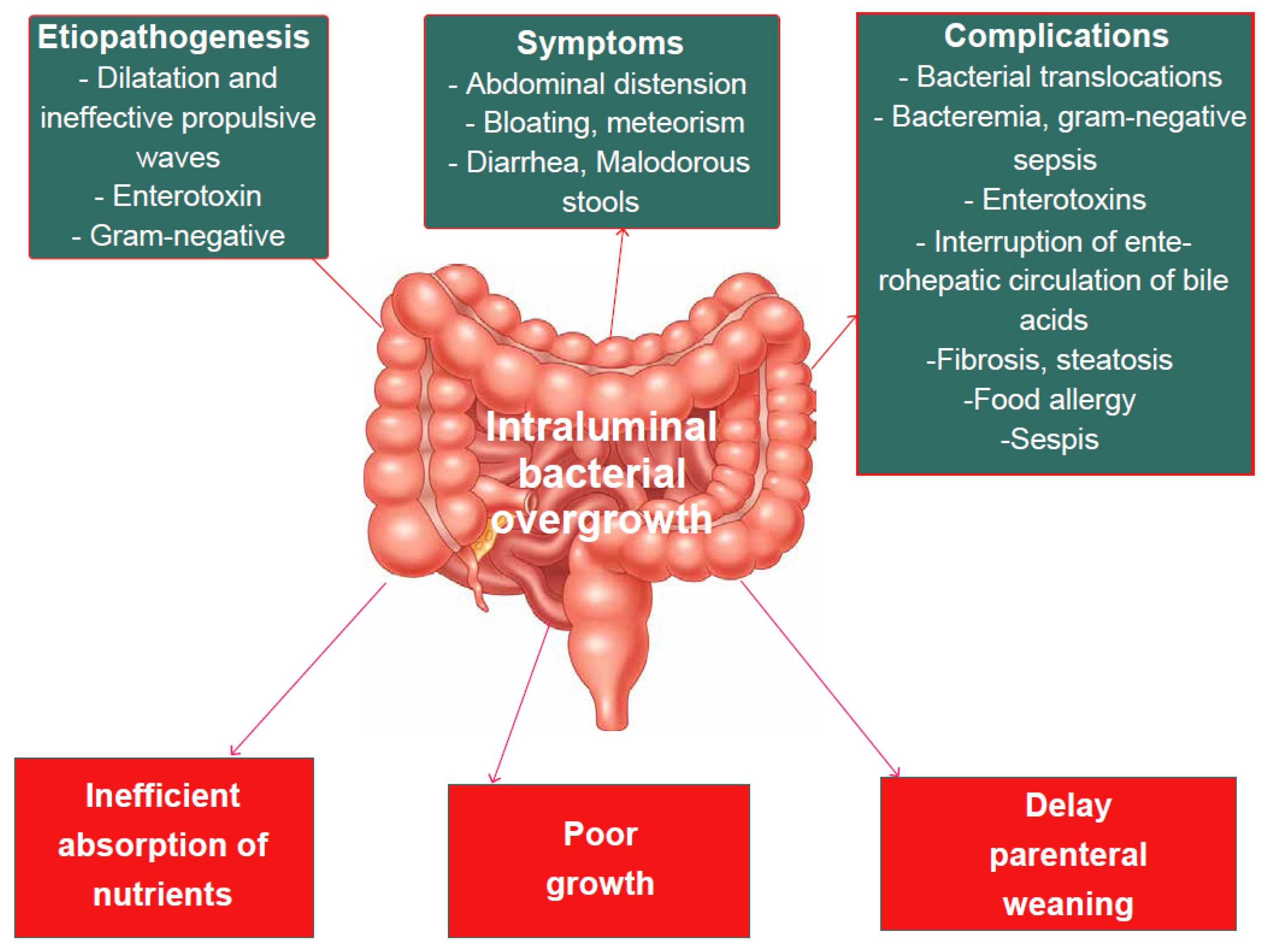
7.2. Small Intestine Bacterial Overgrowth (SIBO)
7.3. D-Lactic Acidosis
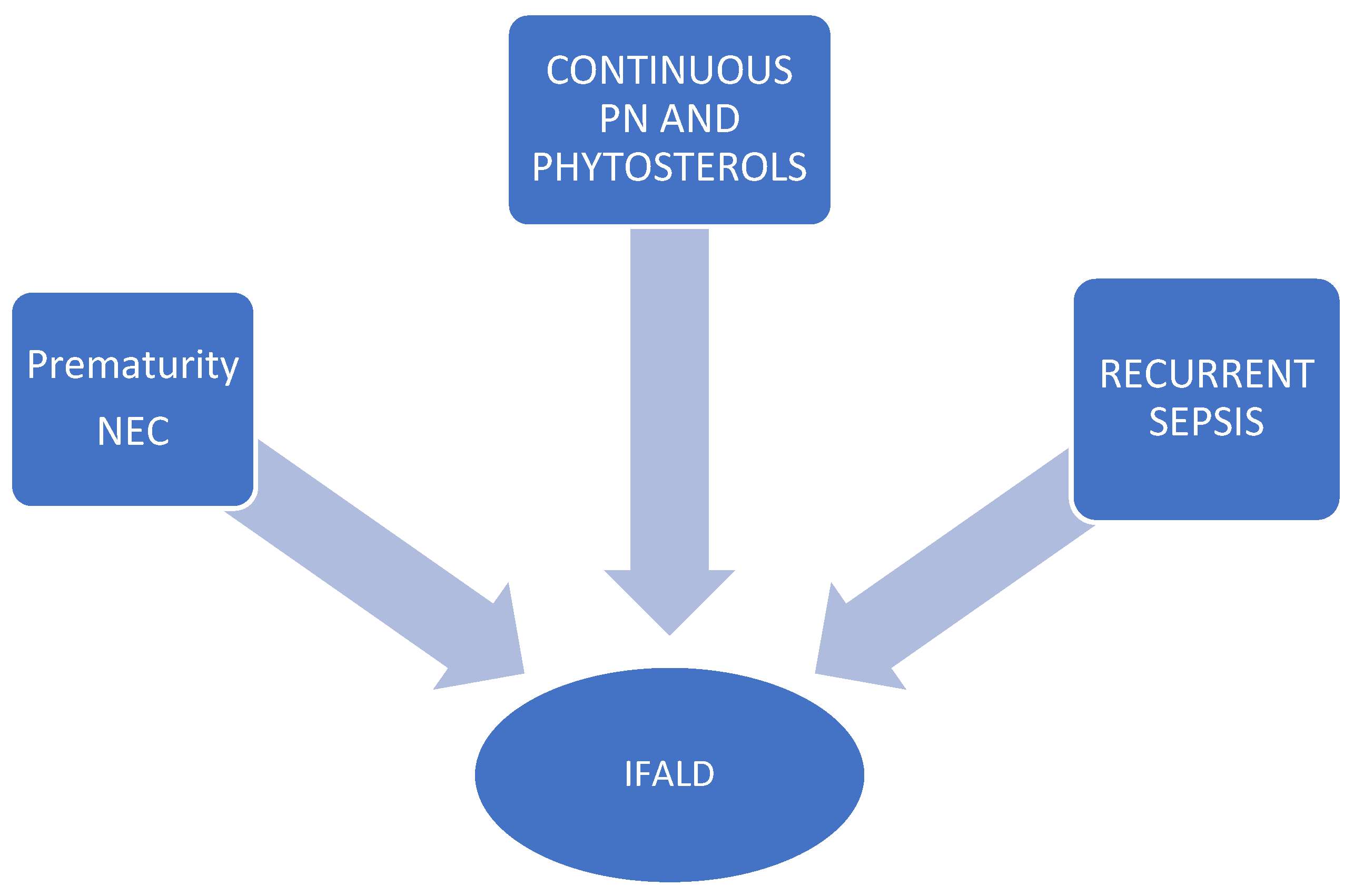
7.4. Intestinal Failure-Associated Liver Disease (IFALD)
7.5. Other Complications
8. Nutritional Therapies
8.1. Enteral Nutrition (EN)
8.2. Micronutrient Supplementation
8.3. Parental Nutrition (PN)
9. Pharmacotherapeutic Options
9.1. Antimotility Agents and Bile Acid Binding Resins
9.2. Proton Pump Inhibitors (PPIs)
9.3. Antibiotics
9.4. Hormonal Pharmacologic Agents
9.5. Preparations Based on Bile Acids
9.6. Probiotics
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shakhsheer, B.A.; Warner, B.W. Short bowel syndrome. Curr. Treat Options Ped. 2019, 5, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.H.; Duggan, C.; Teitelbaum, D.H.; Wales, P.W.; Balint, J.; Venick, R.; Rhee, S.; Sudan, D.; Mercer, D.; Martinez, J.A.; et al. Natural history of pediatric intestinal failure: Initial report from the Pediatric Intestinal Failure Consortium. J. Pediatr. 2012, 161, 723–728. [Google Scholar] [CrossRef]
- Chandra, R.; Kesavan, A. Current treatment paradigms in pediatric short bowel syndrome. Clin. J. Gastroenterol. 2018, 11, 103–112. [Google Scholar] [CrossRef]
- Duggan, C.P.; Jaksic, T. Pediatric intestinal failure. N. Engl. J. Med. 2017, 377, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S.; Rochling, F.A.; Weseman, R.A.; Mercer, D.F. Current management of short bowel syndrome. Curr. Probl. Surg. 2012, 49, 52–115. [Google Scholar] [CrossRef]
- Batra, A.; Keys, S.C.; Johnson, M.J.; Wheeler, R.A.; Beattie, R.M. Epidemiology, management and outcome of ultrashort bowel syndrome in infancy. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F551–F556. [Google Scholar] [CrossRef]
- Goulet, O.; Abi Nader, E.; Pigneur, B.; Lambe, C. Short Bowel Syndrome as the Leading Cause of Intestinal Failure in Early Life: Some Insights into the Management. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 303–329. [Google Scholar] [CrossRef]
- Cole, C.R.; Hansen, N.I.; Higgins, R.D.; Ziegler, T.R.; Stoll, B.J.; Kennedy for the Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short bowel syndrome: Incidence, morbidity and mortality and growth outcomes at 18 to 22 months. Pediatrics 2008, 122, e573–e582. [Google Scholar] [CrossRef]
- Salvia, G.; Guarino, A.; Terrin, G.; Cascioli, C.; Paludetto, R.; Indrio, F.; Lega, L.; Fanaro, S.; Stronati, M.; Corvaglia, L.; et al. Neonatal onset intestinal failure: An Italian multicenter study. J. Pediatr. 2008, 153, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Wales, P.W.; de Silva, N.; Kim, J.; Lecce, L.; To, T.; Moore, A. Neonatal short bowel syndrome: Population-based estimates of incidence and mortality rates. J. Pediatr. Surg. 2004, 39, 690–695. [Google Scholar] [CrossRef]
- Hollwarth, M.E. Surgical strategies in short bowel syndrome. Pediatr. Surg. Int. 2017, 33, 413–419. [Google Scholar] [CrossRef]
- Merritt, R.J.; Cohran, V.; Raphael, B.P. Clinical report: Intestinal rehabilitation programs in the management of pediatric intestinal failure and short bowel syndrome. J. Pediatr. Gastr Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef]
- Batra, A.; Beattie, R.M. Management of short bowel syndrome in infancy. Early Hum. Dev. 2013, 89, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Wales, P.W. Etiology and prognosis of pediatric short bowel syndrome. Semin. Pediatr. Surg. 2018, 27, 209–217. [Google Scholar] [CrossRef]
- Amin, S.C.; Pappas, C.; Iyengar, H.; Maheshwari, A. Short bowel syndrome in the NICU. Clin. Perinatol. 2013, 40, 53–68. [Google Scholar] [CrossRef]
- Demehri, F.R.; Stephens, L.; Herrman, E.; West, B.; Mehringer, A.; Arnold, M.A.; Brown, P.I.; Teitelbaum, D.H. Enteral autonomy in pediatric short bowel syndrome: Predictive factors one year after diagnosis. J. Pediatr. Surg. 2015, 50, 131–135. [Google Scholar] [CrossRef]
- Infantino, B.J.; Mercer, D.F.; Hobson, B.D.; Fischer, R.T.; Gerhardt, B.K.; Grant, W.J.; Langnas, A.N.; Quiros-Tejeira, R.E. Successful rehabilitation in pediatric ultrashort small bowel syndrome. J. Pediatr. 2013, 163, 1361. [Google Scholar] [CrossRef] [PubMed]
- Fallon, E.M.; Mitchell, P.D.; Nehra, D. Neonates with short bowel syndrome: An optimistic future for parenteral nutrition independence. JAMA Surg. 2014, 149, 663. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.A.; Ba’ath, M.E.; Aziz, A. Intestinal rehabilitation and bowel reconstructive surgery: Improved outcomes in children with short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 505. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Kaji, T.; Onishi, S.; Yano, K.; Yamada, W.; Ieiri, S. An overview of the current management of short-bowel syndrome in pediatric patients. Surg. Today 2022, 52, 12–21. [Google Scholar] [CrossRef]
- Sigalet, D.; Boctor, D.; Brindle, M.; Lam, V.; Robertson, M. Elements of successful intestinal rehabilitation. J. Pediatr. Surg. 2011, 46, 150–156. [Google Scholar] [CrossRef]
- Moon, J.; Iyer, K. Intestinal rehabilitation and transplantation for intestinal failure. Mt. Sinai J. Med. 2012, 79, 256–266. [Google Scholar] [CrossRef]
- Stanger, J.D.; Oliveira, C.; Blackmore, C.; Avitzur, Y.; Wales, P.W. The impact of multidisciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: A systematic review and meta-analysis. J. Pediatr. Surg. 2013, 48, 983–992. [Google Scholar] [CrossRef]
- Avitzur, Y.; Wang, J.Y.; de Silva, N.T.; Burghardt, K.M.; DeAngelis, M.; Grant, D.; Ng, V.L.; Jones, N.; Wales, P.W. Impact of intestinal rehabilitation program and its innovative therapies on the outcome of intestinal transplant candidates. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wales, P.W.; Allen, N.; Worthington, P.; George, D.; Compher, C.; American Society for Parenteral and Enteral Nutrition; Teitelbaum, D. A.S.P.E.N. clinical guidelines: Support of pediatric patients with intestinal failure at risk of parenteral nutrition-associated liver disease. J. Parenter. Enter. Nutr. 2014, 38, 538–557. [Google Scholar] [CrossRef]
- Warner, B.W. The pathogenesis of resection-associated intestinal adaptation. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 429–438. [Google Scholar] [CrossRef]
- McMellen, M.E.; Wakeman, D.; Erwin, C.R.; Guo, J.; Warner, B.W. Epidermal growth factor receptor signaling modulates chemokine (CXC) ligand 5 expression and is associated with villus angiogenesis after small bowel resection. Surgery 2010, 148, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Rowland, K.J.; McMellen, M.E.; Wakeman, D.; Wandu, W.S.; Erwin, C.R.; Warner, B.W. Enterocyte expression of epidermal growth factor receptor is not required for intestinal adaptation in response to massive small bowel resection. J. Pediatr. Surg. 2012, 47, 1748–1753. [Google Scholar] [CrossRef]
- Martin, C.A.; Perrone, E.E.; Longshore, S.W.; Toste, P.; Bitter, K.; Nair, R.; Guo, J.; Erwin, C.R.; Warner, B.W. Intestinal resection induces angiogenesis within adapting intestinal villi. J. Pediatr. Surg. 2009, 44, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Miron, J.; Sun, R.; Choi, P.; Sommovilla, J.; Guo, J.; Erwin, C.R.; Mei, J.; Scott Worthen, G.; Warner, B.W. The effect of impaired angiogenesis on intestinal function following massive small bowel resection. J. Pediatr. Surg. 2015, 50, 948–953. [Google Scholar] [CrossRef]
- Onufer, E.J.; Aladegbami, B.; Imai, T.; Seiler, K.; Bajinting, A.; Courtney, C.; Sutton, S.; Bustos, A.; Yao, J.; Yeh, C.H.; et al. EGFR in enterocytes & endothelium and HIF1α in enterocytes are dispensable for massive small bowel resection induced angiogenesis. PLoS ONE 2020, 15, e0236964. [Google Scholar]
- Longshore, S.W.; Wakeman, D.; McMellen, M.; Warner, B.W. Bowel resection induced intestinal adaptation: Progress from bench to bedside. Minerva Pediatr. 2009, 61, 239–251. [Google Scholar]
- McDuffie, L.A.; Bucher, B.T.; Erwin, C.R.; Wakeman, D.; White, F.V.; Warner, B.W. Intestinal adaptation after small bowel resection in human infants. J. Pediatr. Surg. 2011, 46, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.M.; Waye, S.E.; Kong, W.; Kamimoto, K.; Bajinting, A.; Goo, W.H.; Onufer, E.J.; Courtney, C.; Guo, J.; Warner, B.W.; et al. Single-cell analysis reveals regional reprogramming during adaptation to massive small bowel resection in mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 10, 407–426. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Young, R.J.; Vanderhoof, J.A. Intestinal rehabilitation and the short bowel syndrome: Part 1. Am. J. Gastroenterol. 2004, 99, 1386–1395. [Google Scholar] [CrossRef]
- Seetharam, P.; Rodrigues, G. Short bowel syndrome: A review of management options. Saudi J. Gastroenterol. 2011, 17, 229–235. [Google Scholar]
- Miller, M.; Burjonrappa, S. A review of enteral strategies in infant short bowel syndrome: Evidence-based or NICU culture? J. Pediatr. Surg. 2013, 48, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’keefe, S.J.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012, 143, 1473–1481.e3. [Google Scholar] [CrossRef]
- Kelly, D.G.; Tappenden, K.A.; Winkler, M.F. Short bowel syndrome: Highlights of patient management, quality of life, and survival. JPEN J. Parenter. Enter. Nutr. 2014, 38, 427–437. [Google Scholar] [CrossRef]
- Mutanen, A.; Kosola, S.; Merras-Salmio, L.; Kolho, K.-L.; Pakarinen, M.P. Long-term health-related quality of life of patients with pediatric onset intestinal failure. J. Pediatr. Surg. 2015, 50, 1854–1858. [Google Scholar] [CrossRef]
- Struijs, M.C.; Diamond, I.R.; de Silva, N.; Wales, P.W. Establishing norms for intestinal length in children. J. Pediatr. Surg. 2009, 44, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Sudan, D.; Vanderhoof, J.; Grant, W.; Botha, J.; Raynor, S.; Langnas, A. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 204–212. [Google Scholar] [CrossRef]
- Khan, F.A.; Squires, R.H.; Litman, H.J.; Balint, J.; Carter, B.A.; Fisher, J.G.; Horslen, S.P.; Jaksic, T.; Kocoshis, S.; Martinez, J.A.; et al. Pediatric intestinal failure consortium. Predictors of enteral autonomy in children with intestinal failure: A multicenter cohort study. J. Pediatr. 2015, 167, 29–34.e1. [Google Scholar] [CrossRef]
- Belza, C.; Fitzgerald, K.; de Silva, N.; Avitzur, Y.; Steinberg, K.; Courtney-Martin, G.; Wales, P.W. Predicting intestinal adaptation in pediatric intestinal failure: A retrospective cohort study. Ann. Surg. 2017, 269, 988–993. [Google Scholar] [CrossRef]
- Goulet, O.; Baglin-Gobet, S.; Talbotec, C.; Fourcade, L.; Colomb, V.; Sauvat, F.; Jais, J.-P.; Michel, J.-L.; Jan, D.; Ricour, C. Outcome and long-term growth after extensive small bowel resection in the neonatal period: A survey of 87 children. Eur. J. Pediatr. Surg. 2005, 15, 95–101. [Google Scholar] [CrossRef]
- Diamond, I.R.; de Silva, N.T.; Tomlinson, G.A.; Pencharz, P.B.; Feldman, B.M.; Moore, A.M.; Ling, S.C.; Wales, P.W. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: A multiple-variable analysis. JPEN J. Parenter. Enter. Nutr. 2011, 35, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, J.; Prajapati, P.; Ogola, G.; Nguyen, V.; Channabasappa, N.; Piper, H.G. A comparison of lipid minimization strategies in children with intestinal failure. J. Pediatr. Surg. 2017, 53, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, B.S.; Hong, C.R.; Jaksic, T. Long-term outcomes of pediatric intestinal failure. Semin. Pediatr. Surg. 2017, 26, 328–335. [Google Scholar] [CrossRef]
- Mohammed, A.; Grant, F.K.; Zhao, V.M.; Shane, A.L.; Ziegler, T.R.; Cole, C.R. Characterization of posthospital bloodstream infections in children requiring home parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2011, 35, 581–587. [Google Scholar] [CrossRef]
- Rahhal, R.; Abu-El-Haija, M.A.; Fei, L.; Ebach, D.; Orkin, S.; Kiscaden, E.; Cole, C.R. Systematic Review and Meta-Analysis of the Utilization of Ethanol Locks in Pediatric Patients with Intestinal Failure. JPEN J. Parenter. Enter. Nutr. 2018, 42, 690–701. [Google Scholar] [CrossRef]
- Oliveira, C.; Nasr, A.; Brindle, M.; Wales, P.W. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: A meta-analysis. Pediatrics 2012, 129, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, A.A.; Haddad, I.; Ament, M.E.; Buchman, A.L.; Reyen, L.; Maggioni, A.; Baron, H.I.; Vargas, J. 230 patient years of experience with home long-term parenteral nutrition in childhood: Natural history and life of central venous catheters. J. Pediatr. Surg. 1994, 29, 1323–1327. [Google Scholar] [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. Intestinal failure-associated liver disease: A position paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef]
- Bradshaw, J.H.; Puntis, J.W. Taurolidine and catheter-related bloodstream infection: A systematic review of the literature. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 179–186. [Google Scholar] [CrossRef]
- McKee, R.; Dunsmuir, R.; Whitby, M.; Garden, O.J. Does antibiotic prophylaxis at the time of catheter insertion reduce the incidence of catheter-related sepsis in intravenous nutrition? J. Hosp. Infect. 1985, 6, 419–425. [Google Scholar] [CrossRef]
- Ralls, M.W.; Blackwood, R.A.; Arnold, M.A.; Partipilo, M.L.; Dimond, J.; Teitelbaum, D.H. Drug shortage-associated increase in catheter-related blood stream infection in children. Pediatrics 2012, 130, e1369–e1373. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Kaji, T.; Onishi, S.; Yamada, K.; Yamada, W.; Nakame, K.; Mukai, M.; Ieiri, S. Efficacy of ethanol locks to reduce the incidence of catheter-related bloodstream infections for home parenteral nutrition pediatric patients: Comparison of therapeutic treatment with prophylactic treatment. Pediatr. Surg. Int. 2016, 32, 863–867. [Google Scholar] [CrossRef]
- Klek, S.; Szczepanek, K.; Hermanowicz, A.; Galas, A. Taurolidine lock in home parenteral nutrition in adults: Results from an open-label randomized controlled clinical trial. JPEN J. Parenter. Enter. Nutr. 2015, 39, 331–335. [Google Scholar] [CrossRef]
- Tribler, S.; Brandt, C.F.; Petersen, A.H.; Petersen, J.H.; Fuglsang, K.A.; Staun, M.; Broebech, P.; Moser, C.E.; Jeppesen, P.B. Taurolidine-citrate-heparin lock reduces catheter-related bloodstream infections in intestinal failure patients dependent on home parenteral support: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2017, 106, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Morel, H.R.; Sanchez-Payá, J.; García-Shimizu, P.; Mendoza-García, J.L.; Tenza-Iglesias, I.; Rodríguez-Díaz, J.C.; Merino-DE-Lucas, E.; Nolasco, A. Effectiveness of a programme to reduce the burden of catheter-related bloodstream infections in a tertiary hospital. Epidemiol. Infect. 2016, 144, 2011–2017. [Google Scholar] [CrossRef]
- Gutierrez, I.M.; Kang, K.H.; Calvert, C.E.; Johnson, V.M.; Zurakowski, D.; Kamin, D.; Jaksic, T.; Duggan, C. Risk factors for small bowel bacterial overgrowth and diagnostic yield of duodenal aspirates in children with intestinal failure: A retrospective review. J. Pediatr. Surg. 2012, 47, 1150–1154. [Google Scholar] [CrossRef]
- Bohm, M.; Siwiec, R.M.; Wo, J.M. Diagnosis and management of small intestinal bacterial overgrowth. Nutr. Clin. Pract. 2013, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Toskes, P.P. Small bowel bacterial overgrowth: Presentation, diagnosis, and treatment. Curr. Gastroenterol. Rep. 2003, 5, 365–372. [Google Scholar] [CrossRef]
- Cole, C.R.; Frem, J.C.; Schmotzer, B.; Gewirtz, A.T.; Meddings, J.B.; Gold, B.D.; Ziegler, T.R. The rate of bloodstream infection is high in infants with short bowel syndrome: Relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J. Pediatr. 2010, 156, 941–947.e1. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.; Tang, J.; Pyleris, E.; Pistiki, A.; Barbatzas, C.; Brown, J.; Lee, C.C.; Harkins, T.T.; Kim, G.; Weitsman, S.; et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand. J. Gastroenterol. 2015, 50, 1076–1087. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Anderson, A.L.; Devereaux, M.W.; Fillon, S.A.; Harris, J.K.; Lovell, M.A.; Finegold, M.J.; Sokol, R.J. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 2012, 55, 1518–1528. [Google Scholar] [CrossRef]
- Kowlgi, N.G.; Chhabra, L. D-lactic acidosis: An underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015, 2015, 476215. [Google Scholar] [CrossRef]
- Petersen, C. D-lactic acidosis. Nutr. Clin. Pract. 2005, 20, 634–645. [Google Scholar] [CrossRef]
- Uchida, H.; Yamamoto, H.; Kisaki, Y.; Fujino, J.; Ishimaru, Y.; Ikeda, H. D-lactic acidosis in short-bowel syndrome managed with antibiotics and probiotics. J. Pediatr. Surg. 2004, 39, 634–636. [Google Scholar] [CrossRef]
- Takahashi, K.; Terashima, H.; Kohno, K.; Ohkohchi, N. A stand-alone synbiotic treatment for the prevention of D-lactic acidosis in short bowel syndrome. Int. Surg. 2013, 98, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Knell, J.; Henry, O.; Hong, C.R.; Han, G.Y.; Staffa, S.J.; Modi, B.P.; Jaksic, T. Long-Term Outcomes and Disease Burden of Neonatal Onset Short Bowel Syndrome. J. Pediatr. Surg. 2020, 55, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Joly, F.; Corriol, O.; Colomb-Jung, V. Some new insights in intestinal failure-associated liver disease. Curr. Opin. Organ. Transplant. 2009, 14, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Pichler, J.; Horn, V.; Macdonald, S.; Hill, S. Intestinal failure-associated liver disease in hospitalised children. Arch. Dis. Child. 2012, 97, 211–214. [Google Scholar] [CrossRef]
- Fullerton, B.S.; Sparks, E.A.; Hall, A.M.; Duggan, C.; Jaksic, T.; Modi, B.P. Enteral autonomy, cirrhosis, and long term transplant-free survival in pediatric intestinal failure patients. J. Pediatr. Surg. 2016, 51, 96–100. [Google Scholar] [CrossRef]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130 (Suppl. S1), S16–S28. [Google Scholar] [CrossRef]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R.; Parenteral Nutrition Guidelines Working Group; European Society for Clinical Nutrition and Metabolism; European Society of Paediatric Gastroenterology; Hepatology and Nutrition (ESPGHAN); European Society of Paediatric Research (ESPR). 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. S2), S1–S87. [Google Scholar]
- Duro, D.; Mitchell, P.D.; Kalish, L.A.; Martin, C.; McCarthy, M.; Jaksic, T.; Dunn, J.; Brandt, M.L.; Nobuhara, K.K.; Sylvester, K.G.; et al. Risk factors for parenteral nutrition–associated liver disease following surgical therapy for necrotizing enterocolitis: A Glaser Pediatric Research Network Study [corrected]. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 595–600, Erratum in J. Pediatr. Gastroenterol. Nutr. 2011, 53, 583. [Google Scholar] [CrossRef]
- Btaiche, I.F.; Khalidi, N. Parenteral nutrition-associated liver complications in children. Pharmacotherapy 2002, 22, 188–211. [Google Scholar] [CrossRef]
- Beath, S.V.; Davies, P.; Papadopoulou, A.; Khan, A.R.; Buick, R.G.; Corkery, J.J.; Gornall, P.; Booth, I.W. Parenteral nutrition-related cholestasis in postsurgical neonates: Multivariate analysis of risk factors. J. Pediatr. Surg. 1996, 31, 604–606. [Google Scholar] [CrossRef]
- Rangel, S.J.; Calkins, C.M.; Cowles, R.A.; Barnhart, D.C.; Huang, E.Y.; Abdullah, F.; Arca, M.J.; Teitelbaum, D.H.; 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Parenteral nutrition-associated cholestasis: An American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J. Pediatr. Surg. 2012, 47, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.G.; Wales, P.W. Prevention of catheter-related blood stream infections in children with intestinal failure. Curr. Opin. Gastroenterol. 2013, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andorsky, D.J.; Lund, D.P.; Lillehei, C.W.; Jaksic, T.; Dicanzio, J.; Richardson, D.S.; Collier, S.B.; Lo, C.; Duggan, C. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J. Pediatr. 2001, 139, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Nissinen, M.J.; Lohi, J.; Heikkilä, P.; Gylling, H.; Pakarinen, M.P. Serum plant sterols, cholestanol, and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 2014, 100, 1085–1094. [Google Scholar] [CrossRef]
- Li, S.; Nussbaum, M.S.; Teague, D.; Gapen, C.L.; Dayal, R.; Fischer, J.E. Increasing dextrose concentrations in total parenteral nutrition (TPN) causes alterations in hepatic morphology and plasma levels of insulin and glucagon in rats. J. Surg. Res. 1988, 44, 639–648. [Google Scholar] [CrossRef]
- Nehra, D.; Fallon, E.M.; Puder, M. The prevention and treatment of intestinal failure-associated liver disease in neonates and children. Surg. Clin. N. Am. 2011, 91, 543–563. [Google Scholar] [CrossRef]
- Diamanti, A.; Bizzarri, C.; Basso, M.S.; Gambarara, M.; Cappa, M.; Daniele, A.; Noto, C.; Castro, M. How does long-term parenteral nutrition impact the bone mineral status of children with intestinal failure? J. Bone Miner. Metab. 2010, 28, 351–358. [Google Scholar] [CrossRef]
- Mutanen, A.; Mäkitie, O.; Pakarinen, M.P. Risk of metabolic bone disease is increased both during and after weaning off parenteral nutrition in pediatric intestinal failure. Horm. Res. Paediatr. 2013, 79, 227–235. [Google Scholar] [CrossRef]
- Derepas, C.; Kosar, C.; Avitzur, Y.; Wales, P.W.; Courtney-Martin, G. Decreased bone turnover markers in children on long-term parenteral nutrition (PN) for intestinal failure (IF). JPEN J. Parenter. Enter. Nutr. 2015, 39, 85–94. [Google Scholar] [CrossRef]
- Ylinen, E.; Merras-Salmio, L.; Gunnar, R.; Jahnukainen, T.; Pakarinen, M.P. Intestinal failure as a significant risk factor for renal impairment in children. Nutrition 2018, 45, 90–93. [Google Scholar] [CrossRef]
- Pironi, L.; Lauro, A.; Soverini, V.; Zanfi, C.; Agostini, F.; Guidetti, M.; Pazzeschi, C.; Pinna, A.D. Renal function in patients on long-term home parenteral nutrition and in intestinal transplant recipients. Nutrition 2014, 30, 1011–1014. [Google Scholar] [CrossRef]
- Billing, H.; Traunspurger, A.; Sturm, E.; Busch, A. High Incidence of Proteinuria in Children with Chronic Intestinal Failure Under Long-term Parenteral Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Kosar, C.; De Silva, N.; Avitzur, Y.; Steinberg, K.; Courtney-Martin, G.; Chambers, K.; Fitzgerald, K.; Harvey, E.; Wales, P.W. Prevalence of renal abnormality in pediatric intestinal failure. J. Pediatr. Surg. 2016, 51, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.M.; Pierro, A.; Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F193–F198. [Google Scholar] [CrossRef] [PubMed]
- Hintz, S.R.; Kendrick, D.E.; Stoll, B.J.; Vohr, B.R.; Fanaroff, A.A.; Donovan, E.F.; Poole, W.K.; Blakely, M.L.; Wright, L.; Higgins, R.; et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005, 115, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Dammann, O.; Allred, E.N.; Patel, S.; O’Shea, T.M.; Kuban, K.C.; Leviton, A. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J. Pediatr. 2010, 157, 751–756.e1. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.P.; Gura, K.M.; Jaksic, T. (Eds.) Clinical Management of Intestinal Failure; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Buccigrossi, V.; Armellino, C.; Tozzi, A.; Nicastro, E.; Esposito, C.; Alicchio, F.; Cozzolino, S.; Guarino, A. Time- and segment-related changes of postresected intestine: A 4-dimensional model of intestinal adaptation. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 40–45. [Google Scholar] [CrossRef]
- Courtney, C.M.; Warner, B.W. Pediatric intestinal failure-associated liver disease. Curr. Opin. Pediatr. 2017, 29, 363–370. [Google Scholar] [CrossRef]
- Sondheimer, J.M.; Cadnapaphornchai, M.; Sontag, M.; Zerbe, G.O. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J. Pediatr. 1998, 132, 80–84. [Google Scholar] [CrossRef]
- Goulet, O.; Olieman, J.; Ksiazyk, J.; Spolidoro, J.; Tibboe, D.; Köhler, H.; Yagci, R.V.; Falconer, J.; Grimble, G.; Beattie, R.M. Neonatal short bowel syndrome as a model of intestinal failure: Physiological background for enteral feeding. Clin. Nutr. 2013, 32, 162–171. [Google Scholar] [CrossRef]
- Norsa, L.; Nicastro, E.; Di Giorgio, A.; Lacaille, F.; D’Antiga, L. Prevention and Treatment of Intestinal Failure-Associated Liver Disease in Children. Nutrients 2018, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fantini, P.M.; Thomas, S.L.; Taylor, R.G.; Nagy, E.; Sourial, M.; Fuller, P.J.; Bines, J.E. Colostrum supplementation restores insulin-like growth factor -1 levels and alters muscle morphology following massive small bowel resection. JPEN J. Parenter. Enter. Nutr. 2008, 32, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Olieman, J.F.; Penning, C.; Ijsselstijn, H.; Escher, J.C.; Joosten, K.F.; Hulst, J.M.; Tibboel, D. Enteral nutrition in children with short-bowel syndrome: Current evidence and recommendations for the clinician. J. Am. Diet. Assoc. 2010, 110, 420–426. [Google Scholar] [CrossRef]
- Kulkarni, S.; Mercado, V.; Rios, M.; Arboleda, R.; Gomara, R.; Muinos, W.; Reeves-Garcia, J.; Hernandez, E. Breast milk is better than formula milk in preventing parenteral nutrition-associated liver disease in infants receiving prolonged parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 383–388. [Google Scholar] [CrossRef]
- Parker, P.; Stroop, S.; Greene, H. A controlled comparison of continuous versus intermittent feeding in the treatment of infants with intestinal disease. J. Pediatr. 1981, 99, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Aynsley-Green, A.; Adrian, T.E.; Bloom, S.R. Feeding and the development of enteroinsular hormone secretion in the preterm infant: Effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatr. Scand. 1982, 71, 379–383. [Google Scholar] [CrossRef]
- Gosselin, K.B.; Duggan, C. Enteral nutrition in the management of pediatric intestinal failure. J. Pediatr. 2014, 165, 1085–1090. [Google Scholar] [CrossRef]
- Goulet, O.; Ruemmele, F.; Lacaille, F.; Colomb, V. Irreversible intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 250–269. [Google Scholar] [CrossRef]
- Yang, C.F.; Duro, D.; Zurakowski, D.; Lee, M.; Jaksic, T.; Duggan, C. High prevalence of multiple micronutrient deficiencies in children with intestinal failure: A longitudinal study. J. Pediatr. 2011, 159, 39–44.e1. [Google Scholar] [CrossRef]
- Ubesie, A.C.; Heubi, J.E.; Kocoshis, S.A.; Henderson, C.J.; Mezoff, A.G.; Rao, M.B.; Cole, C.R. Vitamin D deficiency and low bone mineral density in pediatric and young adult intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 372–376. [Google Scholar] [CrossRef]
- Wessel, J.J.; Kocoshis, S.A. Nutritional management of infants with short bowel syndrome. Semin. Perinatol. 2007, 31, 104–111. [Google Scholar] [CrossRef]
- Joly, F.; Mayeur, C.; Messing, B.; Lavergne-Slove, A.; Cazals-Hatem, D.; Noordine, M.L. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G116–G123. [Google Scholar] [CrossRef]
- Nader, E.A.; Lambe, C.; Talbotec, C.; Pigneur, B.; Lacaille, F.; Garnier-Lengliné, H.; Petit, L.-M.; Poisson, C.; Rocha, A.; Corriol, O.; et al. Outcome of home parenteral nutrition in 251 children over a 14-y period: Report of a single center. Am. J. Clin. Nutr. 2016, 103, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Colomb, V.; Dabbas-Tyan, M.; Taupin, P.; Talbotec, C.; Révillon, Y.; Jan, D.; De Potter, S.; Gorski-Colin, A.-M.; Lamor, M.; Herreman, K.; et al. Long-term outcome of children receiving home parenteral nutrition: A 20-year single-center experience in 302 patients. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 347–353. [Google Scholar] [CrossRef] [PubMed]
- D’Antiga, L.; Goulet, O. Intestinal failure in children: The European view. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 118–126. [Google Scholar] [CrossRef]
- Ganousse-Mazeron, S.; Lacaille, F.; Colomb-Jung, V.; Talbotec, C.; Ruemmele, F.; Sauvat, F.; Chardot, C.; Canioni, D.; Jan, D.; Revillon, Y.; et al. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clin. Nutr. 2015, 34, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef]
- Colomb, V.; Jobert-Giraud, A.; Lacaille, F.; Goulet, O.; Fournet, J.C.; Ricour, C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J. Parenter. Enter. Nutr. 2000, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Cavicchi, M.; Beau, P.; Crenn, P.; Degott, C.; Messing, B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann. Intern. Med. 2000, 132, 525–532. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Bersani, G.; Tala, S.; Grossi, G.; Puggioli, C.; Masi, M. The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids 2010, 45, 63–71. [Google Scholar] [CrossRef]
- Wanten, G.; Beunk, J.; Naber, A.; Swinkels, D. Tocopherol isoforms in parenteral lipid emulsions and neutrophil activation. Clin. Nutr. 2002, 21, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Lambe, C. Intravenous lipid emulsions in pediatric patients with intestinal failure. Curr. Opin. Organ Transplant. 2017, 22, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Hoffmann, J.; Lienhard, S.; Jauch, K.W.; Wolfram, G. Antioxidant status of surgical patients receiving TPN with an omega-3-fatty acid-containing lipid emulsion supplemented with alphatocopherol. Clin. Nutr. 2000, 19, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Puder, M.; Valim, C.; Meisel, J.A.; Le, H.D.; de Meijer, V.E.; Robinson, E.M.; Zhou, J.; Duggan, C.; Gura, K.M. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 2009, 250, 395–402. [Google Scholar] [CrossRef]
- Nandivada, P.; Fell, G.L.; Gura, K.M.; Puder, M. Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am. J. Clin. Nutr. 2016, 103, 629S–634S. [Google Scholar] [CrossRef]
- Gura, K.M.; Duggan, C.P.; Collier, S.B.; Jennings, R.W.; Folkman, J.; Bistrian, B.R.; Puder, M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: Implications for future management. Pediatrics 2006, 118, e197–e201. [Google Scholar] [CrossRef]
- Cowles, R.A.; Ventura, K.A.; Martinez, M.; Lobritto, S.J.; Harren, P.A.; Brodlie, S. Reversal of intestinal failureassociated liver disease in infants and children on parenteral nutrition: Experience with 93 patients at a referral center for intestinal rehabilitation. J. Pediatr. Surg. 2010, 45, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Anez-Bustillos, L.; Dao, D.T.; Fell, G.L.; Baker, M.A.; Gura, K.M.; Bistrian, B.R.; Puder, M. Redefining essential fatty acids in the era of novel intravenous lipid emulsions. Clin. Nutr. 2018, 37, 784–789. [Google Scholar] [CrossRef]
- Cober, M.P.; Killu, G.; Brattain, A.; Welch, K.B.; Kunisaki, S.M.; Teitelbaum, D.H. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J. Pediatr. 2012, 160, 421–427. [Google Scholar] [CrossRef]
- Rollins, M.D.; Ward, R.M.; Jackson, W.D.; Mulroy, C.W.; Spencer, C.P.; Ying, J.; Greene, T.; Book, L.S. Effect of decreased parenteral soybean lipid emulsion on hepatic function in infants at risk for parenteral nutrition associated liver disease: A pilot study. J. Pediatr. Surg. 2013, 48, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Sokol, R.J. Intestinal Microbiota, Lipids, and the Pathogenesis of Intestinal Failure-Associated Liver Disease. J. Pediatr. 2015, 167, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B. ESPGHAN/ESPEN/ ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, J.; Gates, A.; Parish, A. Medical management of short gut syndrome. J. Perinatol. 2010, 30, S2–S5. [Google Scholar] [CrossRef]
- Duro, D.; Kamin, D.; Duggan, C. Overview of pediatric short bowel syndrome. J. Pediatr. Gastr Nutr. 2008, 47 (Suppl. S1), S33–S36. [Google Scholar] [CrossRef]
- Kumpf, V.J. Pharmacologic management of diarrhea in patients with short bowel syndrome. J. Parenter. Enter. Nutr. 2014, 38, 38S–44S. [Google Scholar] [CrossRef]
- Neelis, E.G.; Olieman, J.F.; Hulst, J.M.; de Koning, B.; Wijnen, R.; Rings, E. Promoting intestinal adaptation by nutrition and medication. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 249. [Google Scholar] [CrossRef]
- Canani, R.B.; Cirillo, P.; Roggero, P.; Romano, C.; Malamisura, B.; Terrin, G.; Passariello, A.; Manguso, F.; Morelli, L.; Guarino, A. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics 2006, 117, e817–e820. [Google Scholar] [CrossRef]
- Shah, S.C.; Day, L.W.; Somsouk, M.; Sewell, J.L. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2013, 38, 925–933. [Google Scholar] [CrossRef]
- Gatta, L.; Scarpignato, C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; DuPont, H.L. Rifaximin: In vitro and in vivo antibacterial activity—A review. Chemotherapy 2005, 51 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- Lauritano, E.C.; Gabrielli, M.; Lupascu, A.; Santoliquido, A.; Nucera, G.; Scarpellini, E.; Vincenti, F.; Cammarota, G.; Flore, R.; Pola, P.; et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2005, 22, 31–35. [Google Scholar] [CrossRef]
- Cuoco, L.; Salvagnini, M. Small intestine bacterial overgrowth in irritable bowel syndrome: A retrospective study with rifaximin. Minerva Gastroenterol. Dietol. 2006, 52, 89–95. [Google Scholar]
- Di Stefano, M.; Malservisi, S.; Veneto, G.; Ferrieri, A.; Corazza, G.R. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2000, 14, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Miceli, E.; Missanelli, A.; Mazzocchi, S.; Corazza, G.R. Absorbable vs. non-absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind-loop syndrome. Aliment. Pharmacol. Ther. 2005, 21, 985–992. [Google Scholar] [CrossRef]
- Attar, A.; Flourié, B.; Rambaud, J.C.; Franchisseur, C.; Ruszniewski, P.; Bouhnik, Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: A crossover, randomized trial. Gastroenterology 1999, 117, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Rispo, A.; Di Girolamo, E.; Cozzolino, A.; Manguso, F.; Grassia, R.; Mazzacca, G. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 18, 1107–1112. [Google Scholar] [CrossRef]
- Carter, B.A.; Cohran, V.C.; Cole, C.R.; Corkins, M.R.; Dimmitt, R.A.; Duggan, C.; Hill, S.; Horslen, S.; Lim, J.D.; Mercer, D.F.; et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J. Pediatr. 2017, 181, 102–111. [Google Scholar] [CrossRef]
- Jeppesen, P.B. Clinical significance of GLP-2 in short-bowel syndrome. J. Nutr. 2003, 133, 3721–3724. [Google Scholar] [CrossRef]
- Jeppesen, P.B. Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 14–20. [Google Scholar] [CrossRef]
- Lim, D.W.; Wales, P.W.; Mi, S.; Yap, J.Y.K.; Curtis, J.M.; Mager, D.R.; Mazurak, V.C.; Wizzard, P.R.; Sigalet, D.L.; Turner, J.M. Glucagon-Like Peptide-2 Alters Bile Acid Metabolism in Parenteral Nutrition—Associated Liver Disease. J. Parenter. Enter. Nutr. 2016, 40, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Lipari, M.; Kulik, J.K.; Kale-Pradhan, P.B. Teduglutide for the treatment of short bowel syndrome. Ann. Pharmacother. 2014, 48, 1209–1213. [Google Scholar] [CrossRef]
- Keam, S.J. Teduglutide: A review in short bowel syndrome. Drugs 2017, 77, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Pharmacologic options for intestinal rehabilitation in patients with short bowel syndrome. J. Parenter. Enter. Nutr. 2014, 38, 45S–52S. [Google Scholar] [CrossRef]
- Goulet, O.; Dabbas-Tyan, M.; Talbotec, C.; Kapel, N.; Rosilio, M.; Souberbielle, J.C.; Corriol, O.; Ricour, C.; Colomb, V. Effect of recombinant human growth hormone on intestinal absorption and body composition in children with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2010, 34, 513–520. [Google Scholar] [CrossRef]
- Peretti, N.; Loras-Duclaux, I.; Kassai, B.; Restier-Miron, L.; Guimber, D.; Gottrand, F. Growth hormone to improve short bowel syndrome intestinal autonomy: A pediatric randomized open-label clinical trial. JPEN J. Parenter. Enter. Nutr. 2011, 35, 723–731. [Google Scholar] [CrossRef]
- Wales, P.W.; Nasr, A.; de Silva, N.; Yamada, J. Human growth hormone and glutamine for patients with short bowel syndrome. Cochrane Database Syst. Rev. 2010, 6, CD006321. [Google Scholar] [CrossRef] [PubMed]
- Ben Lulu, S.; Coran, A.G.; Shehadeh, N.; Shamir, R.; Mogilner, J.G.; Sukhotnik, I. Oral insulin stimulates intestinal epithelial cell turnover following massive small bowel resection in a rat and a cell culture model. Pediatr. Surg. Int. 2012, 28, 179–187. [Google Scholar] [CrossRef]
- McMellen, M.E.; Wakeman, D.; Longshore, S.W.; McDuffie, L.A.; Warner, B.W. Growth factors: Possible roles for clinical management of the short bowel syndrome. Semin. Pediatr. Surg. 2010, 19, 35–43. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Gismondi, P.; Fornaroli, F.; Iuliano, S.; de’Angelis, G.L.; Esposito, S. Gut Microbiota in Celiac Disease: Is. There Any Role for Probiotics? Front. Immunol. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Puong, K.Y.; Ouwehand, A.C.; Salminen, S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 2003, 52 Pt 10, 925–930. [Google Scholar] [CrossRef]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635, Erratum in Am. J. Pathol. 2012, 180, 1324. [Google Scholar] [CrossRef] [PubMed]
- Barc, M.C.; Charrin-Sarnel, C.; Rochet, V.; Bourlioux, F.; Sandré, C.; Boureau, H.; Doré, J.; Collignon, A. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: Influence of Saccharomyces boulardii. Anaerobe 2008, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1158. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Nataro, J.P. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv. Drug Deliv. Rev. 2004, 56, 795–807. [Google Scholar] [CrossRef]
- Liévin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.R.; Servin, A.L. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef]
- Tappenden, K.A.; McBurney, M.I. Systemic short-chain fatty acids rapidly alter gastrointestinal structure, function, and expression of early response genes. Dig. Dis. Sci. 1998, 43, 1526–1536. [Google Scholar] [CrossRef]
- Bartholome, A.L.; Albin, D.M.; Baker, D.H.; Holst, J.J.; Tappenden, K.A. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J. Parenter. Enter. Nutr. 2004, 28, 210–222; discussion 222–223. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigón, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; Nanno, M.; Okumura, K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; des Robert, C.; Fang, M.; Liboni, K.; McMahon, R.; Caicedo, R.A.; Neu, J. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporilli, C.; Giannì, G.; Grassi, F.; Esposito, S. An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications. Nutrients 2023, 15, 2341. https://doi.org/10.3390/nu15102341
Caporilli C, Giannì G, Grassi F, Esposito S. An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications. Nutrients. 2023; 15(10):2341. https://doi.org/10.3390/nu15102341
Chicago/Turabian StyleCaporilli, Chiara, Giuliana Giannì, Federica Grassi, and Susanna Esposito. 2023. "An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications" Nutrients 15, no. 10: 2341. https://doi.org/10.3390/nu15102341
APA StyleCaporilli, C., Giannì, G., Grassi, F., & Esposito, S. (2023). An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications. Nutrients, 15(10), 2341. https://doi.org/10.3390/nu15102341







