Exploring the Modulatory Effect of High-Fat Nutrition on Lipopolysaccharide-Induced Acute Lung Injury in Vagotomized Rats and the Role of the Vagus Nerve
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
2.3. Experimental Models
2.3.1. Model A: Effect of Selectively Sparing Vagus Nerve on Lung Injury and Function
2.3.2. Model B: Further Exploring the Role of the α7nAChR
2.3.3. Model C: Vagus Nerve Stimulation through High-Fat Enteral Nutrition
2.4. Pulmonary Function Measurement
2.5. Bronchoalveolar Lavage (BAL)
2.6. Preparation of Lung Homogenates
2.7. Lung Histology
2.8. ELISA
2.9. Statistics
3. Results
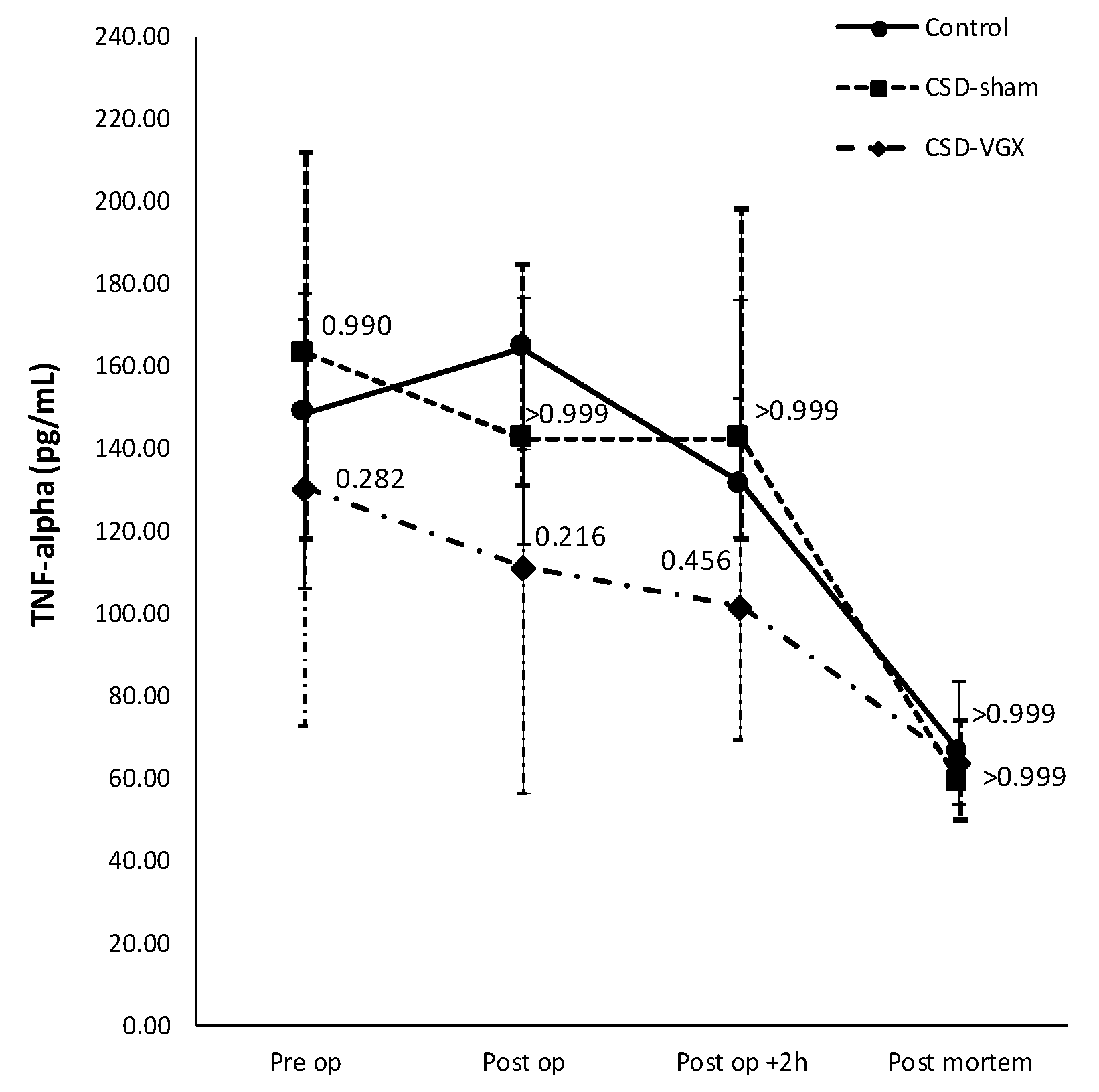
3.1. Model A: Selectively Sparing Vagus Nerve
3.2. Model B: Role of the α7nACh Receptor
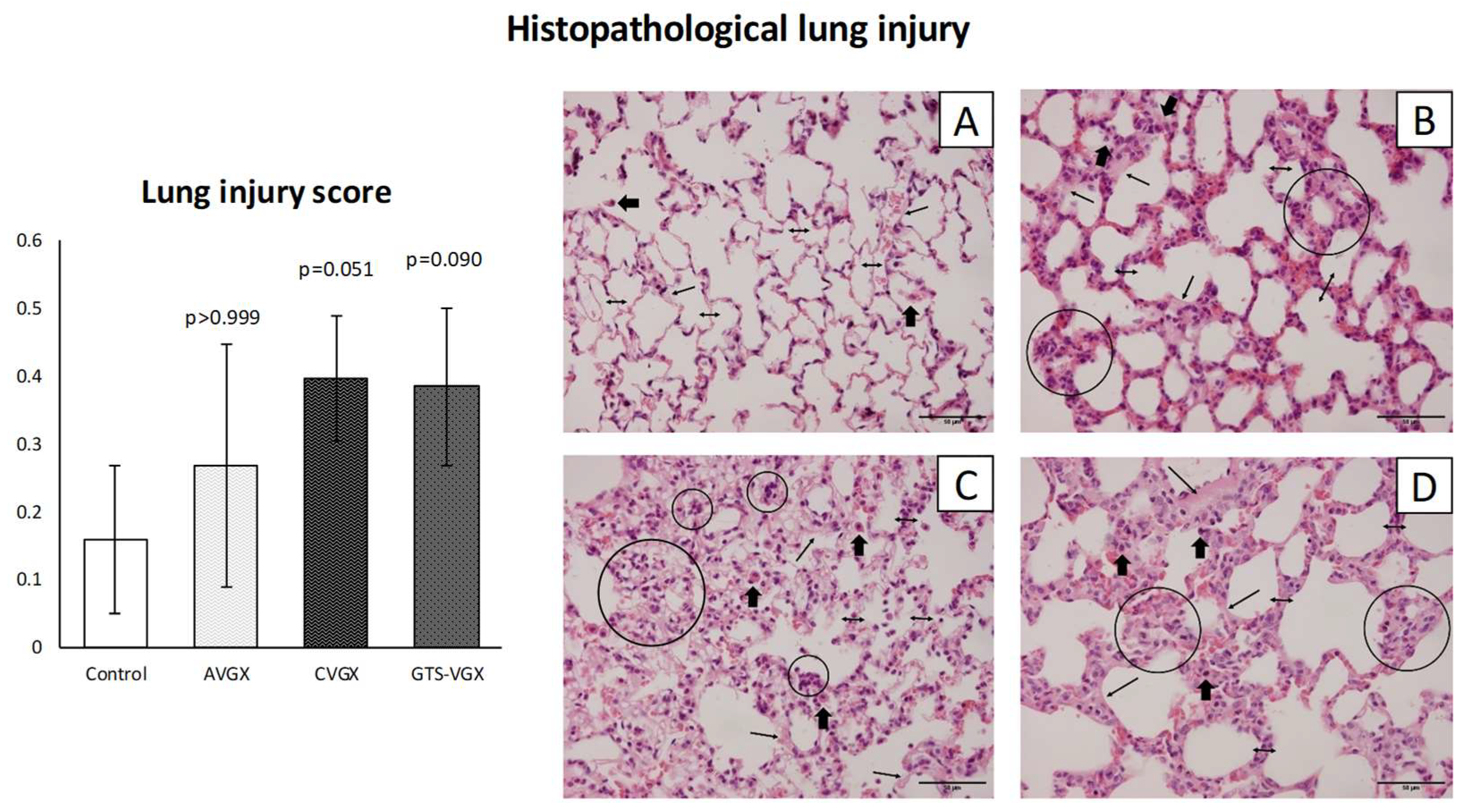
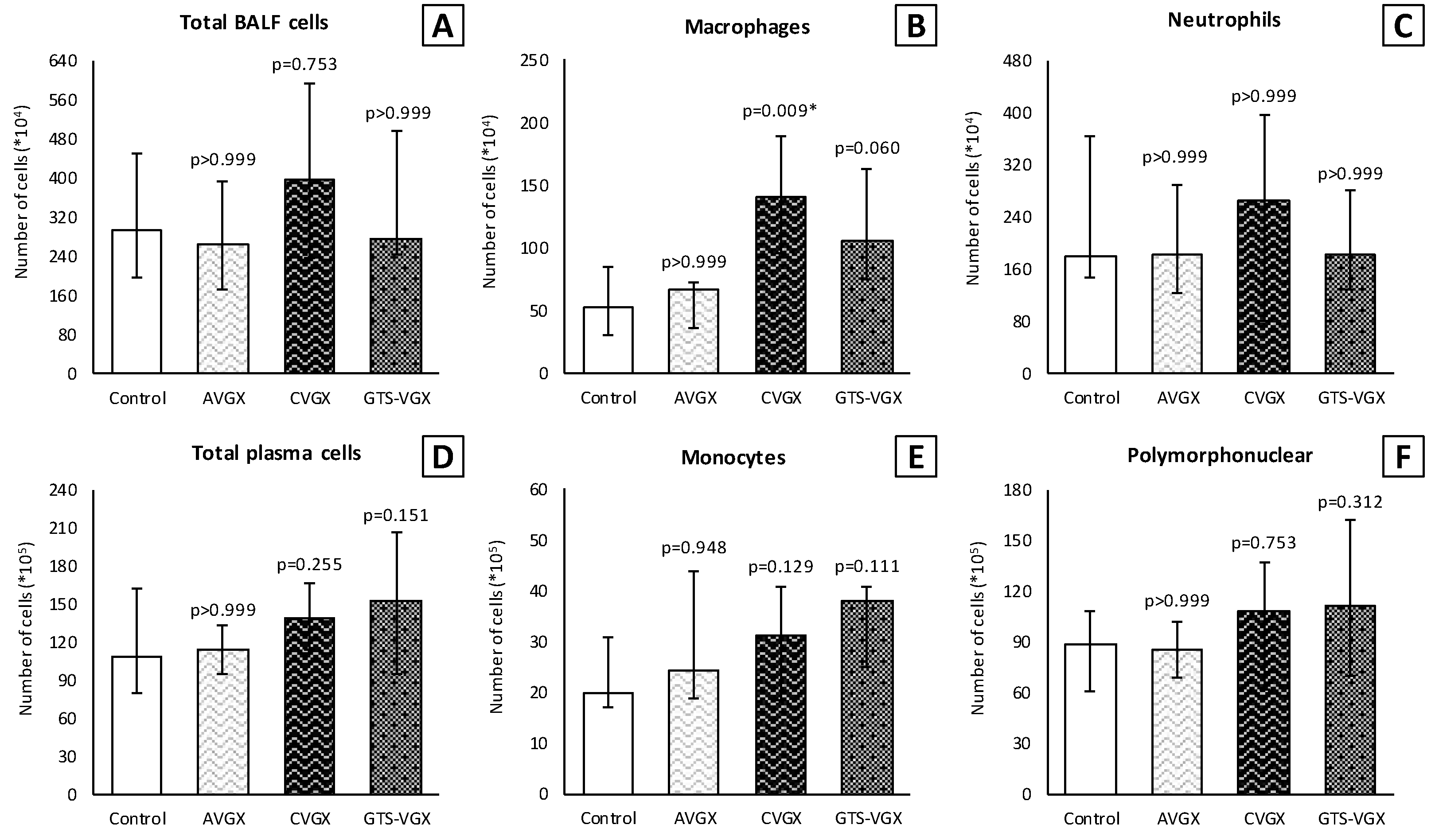
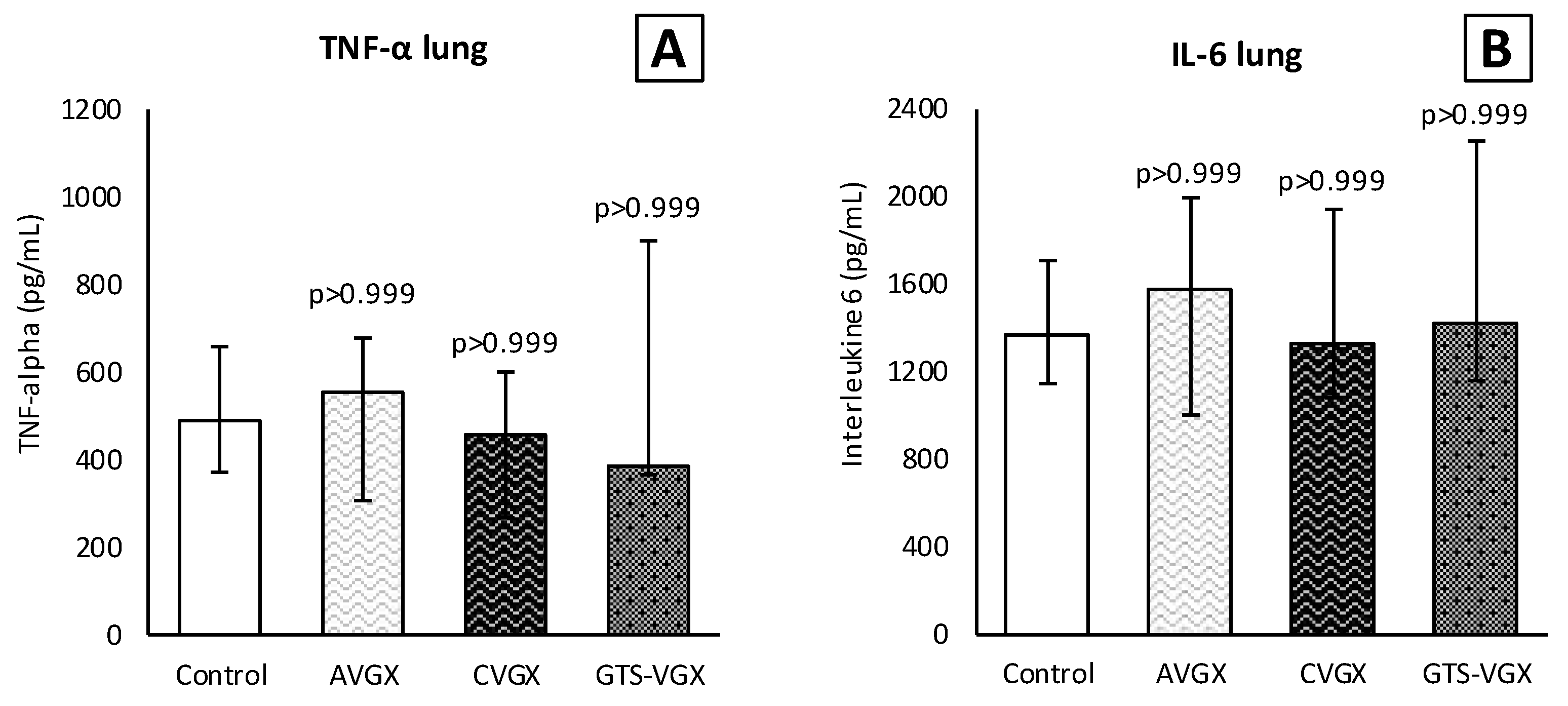
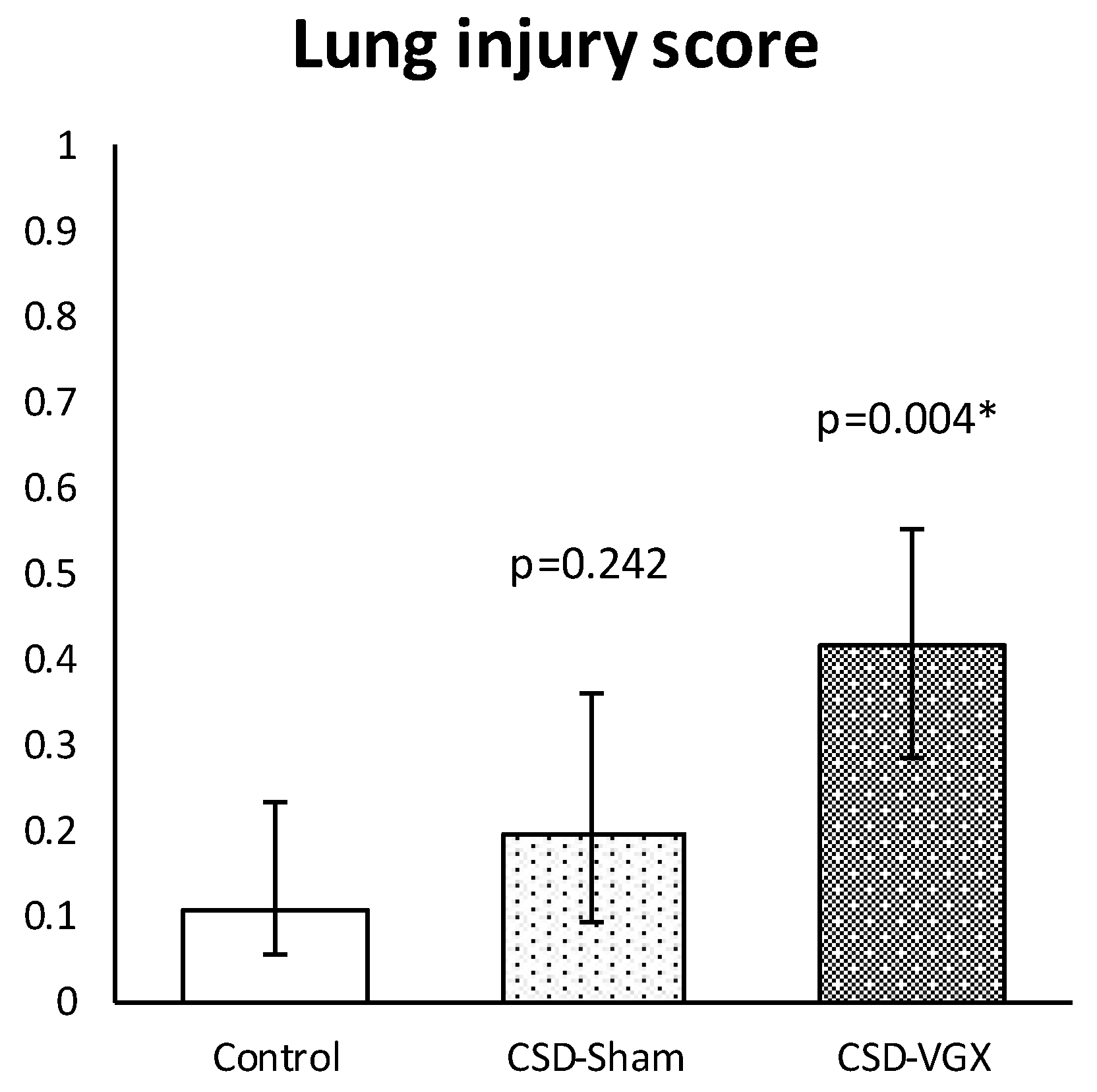
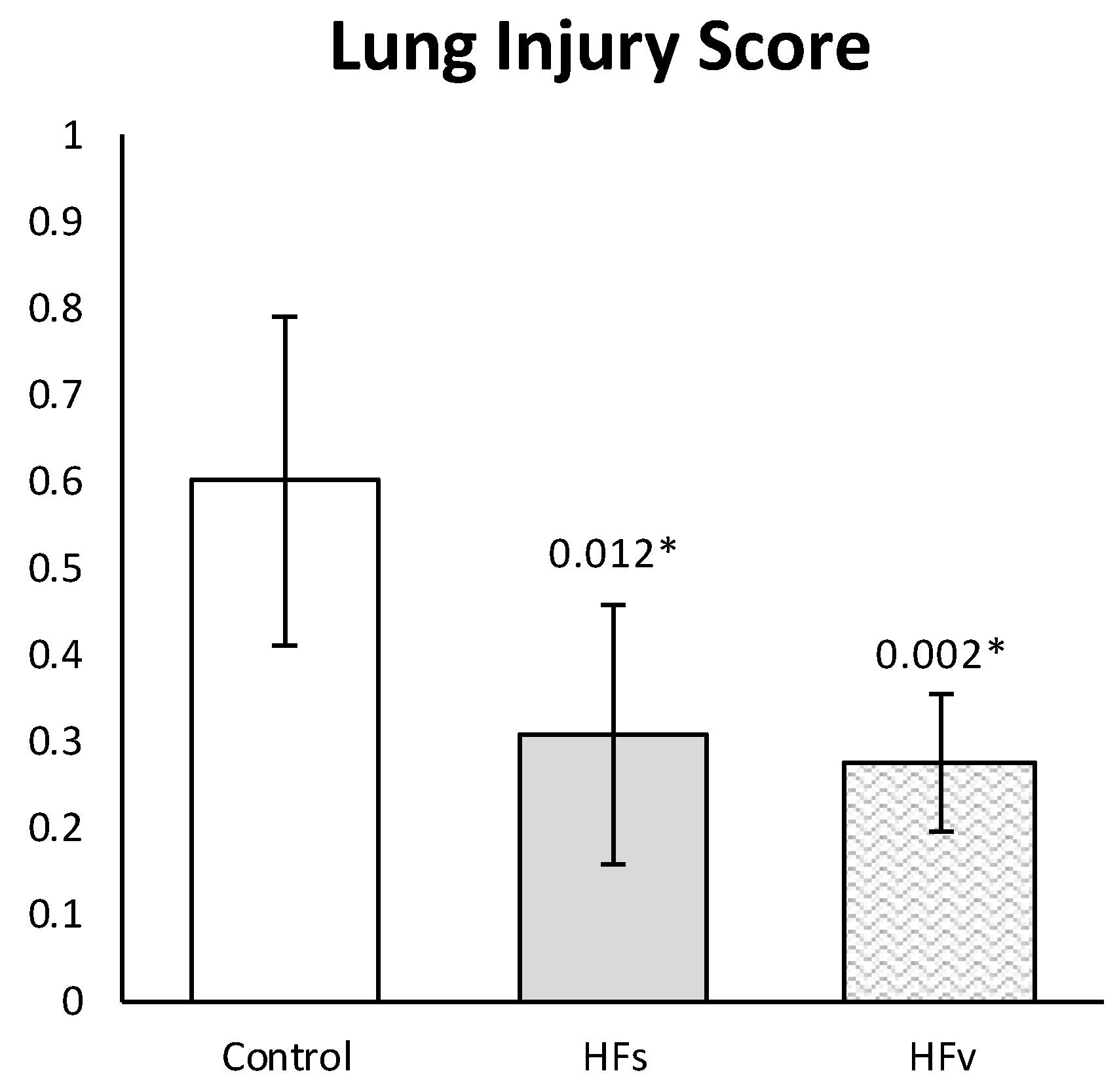
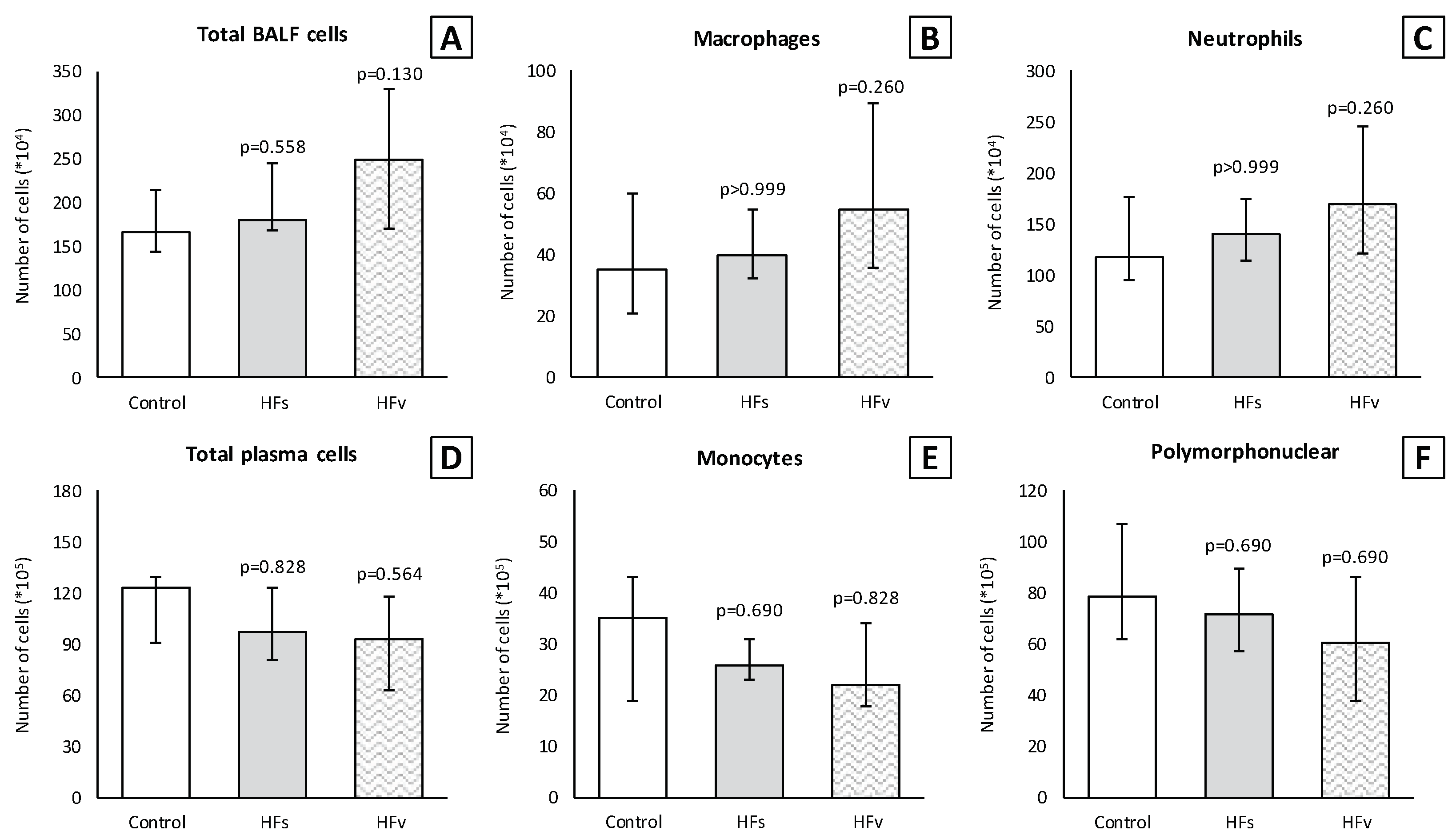
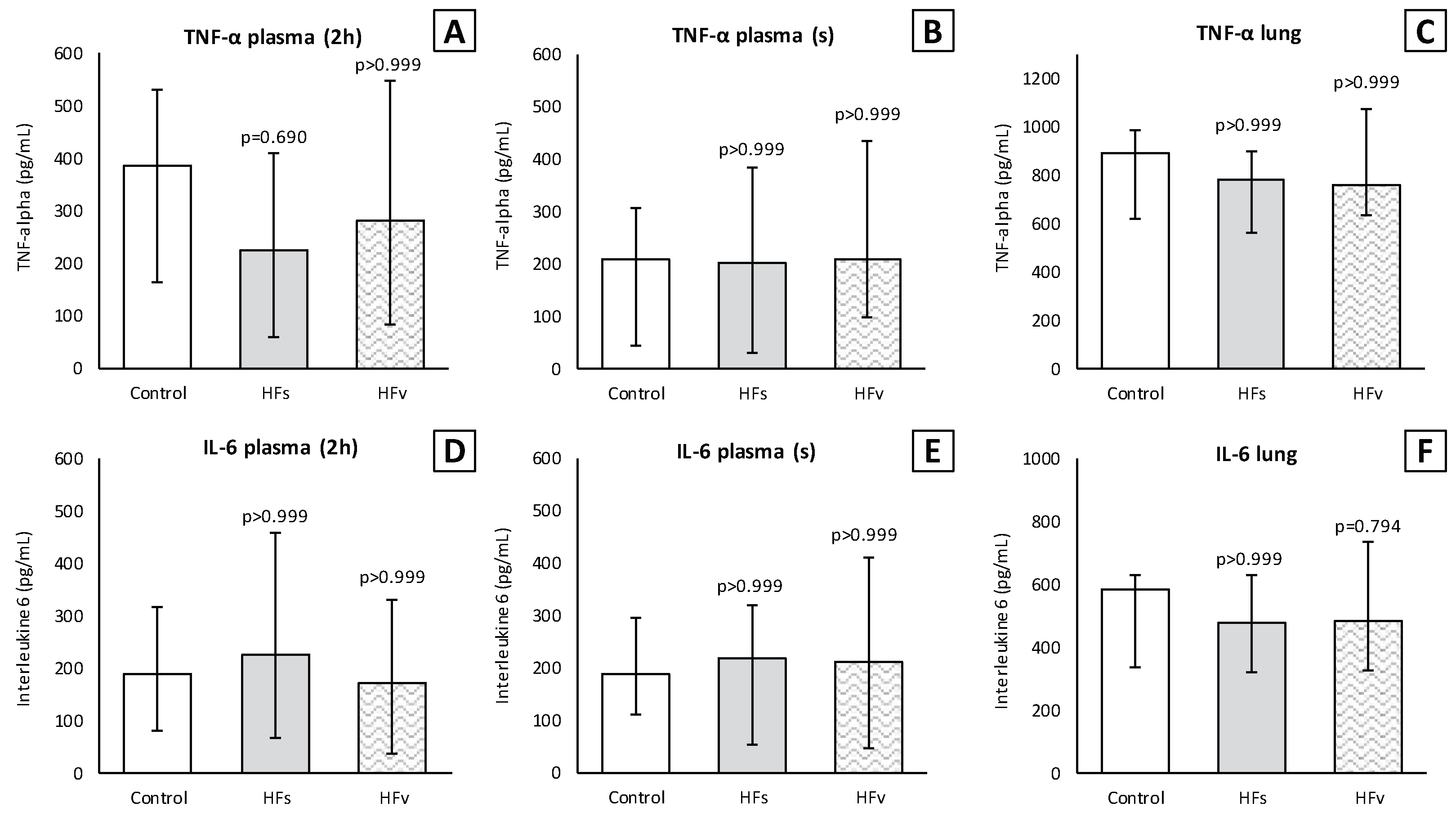
3.3. Model C: Effect of High-Fat Enteral Nutrition on LPS-Induced Lung Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Giebelen, I.A.; van Westerloo, D.J.; LaRosa, G.J.; de Vos, A.F.; van der Poll, T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock 2007, 28, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Luyer, M.D.; Greve, J.W.; Hadfoune, M.; Jacobs, J.A.; Dejong, C.H.; Buurman, W.A. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 2005, 202, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Matsumoto, N.; Imamura, Y.; Muroya, T.; Yamada, T.; Nakagawa, J.; Shimazaki, J.; Ogura, H.; Kuwagata, Y.; Shimazu, T. Electrical vagus nerve stimulation attenuates systemic inflammation and improves survival in a rat heatstroke model. PLoS ONE 2013, 8, e56728. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Du, Z.; Zhang, M.; Niu, L.; Wang, Y.; Li, J. Vagal efferent fiber stimulation ameliorates pulmonary microvascular endothelial cell injury by downregulating inflammatory responses. Inflammation 2013, 36, 1567–1575. [Google Scholar] [CrossRef]
- Reys, L.G.; Ortiz-Pomales, Y.T.; Lopez, N.; Cheadle, G.; de Oliveira, P.G.; Eliceiri, B.; Bansal, V.; Costantini, T.W.; Coimbra, R. Uncovering the neuroenteric-pulmonary axis: Vagal nerve stimulation prevents acute lung injury following hemorrhagic shock. Life Sci. 2013, 92, 783–792. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Di Giovangiulio, M.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W.; et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Su, X. Vagus nerve through alpha7 nAChR modulates lung infection and inflammation: Models, cells, and signals. Biomed. Res. Int. 2014, 2014, 283525. [Google Scholar] [CrossRef]
- Costello, R.W.; Jacoby, D.B.; Fryer, A.D. Pulmonary neuronal M2 muscarinic receptor function in asthma and animal models of hyperreactivity. Thorax 1998, 53, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, S.B.; Undem, B.J. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef] [PubMed]
- Weijs, T.J.; Ruurda, J.P.; Luyer, M.D.; Nieuwenhuijzen, G.A.; van der Horst, S.; Bleys, R.L.; van Hillegersberg, R. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg. Endosc. 2016, 30, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, J.F.; Roberts, A.M.; Collins, L.C.; Fletcher, E.C. Pulmonary rapidly adapting receptor stimulation does not increase airway resistance in anesthetized rabbits. Am. J. Respir. Crit. Care Med. 1999, 160, 906–912. [Google Scholar] [CrossRef]
- Kageyama-Yahara, N.; Suehiro, Y.; Yamamoto, T.; Kadowaki, M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem. Biophys. Res. Commun. 2008, 377, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.; Barnes, P.J.; Urban, L.; Dray, A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J. Physiol. 1993, 469, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Coles, S.K.; McCrimmon, D.R. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J. Neurosci. 1996, 16, 6526–6536. [Google Scholar] [CrossRef]
- Pack, A.I.; Ogilvie, M.D.; Davies, R.O.; Galante, R.J. Responses of pulmonary stretch receptors during ramp inflations of the lung. J. Appl. Physiol. 1986, 61, 344–352. [Google Scholar] [CrossRef]
- Cong, B.; Li, S.J.; Ling, Y.L.; Yao, Y.X.; Gu, Z.Y.; Wang, J.X.; You, H.Y. Expression and cell-specific localization of cholecystokinin receptors in rat lung. World J. Gastroenterol. 2003, 9, 1273–1277. [Google Scholar] [CrossRef]
- Yu, J.; Lin, S.; Zhang, J.; Otmishi, P.; Guardiola, J.J. Airway nociceptors activated by pro-inflammatory cytokines. Respir. Physiol. Neurobiol. 2007, 156, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; van Berge Henegouwen, M.I.; Dutch Upper Gastrointestinal Cancer Audit, G. Outcomes of Esophagogastric Cancer Surgery During Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Muller-Stich, B.P.; Probst, P.; Nienhuser, H.; Fazeli, S.; Senft, J.; Kalkum, E.; Heger, P.; Warschkow, R.; Nickel, F.; Billeter, A.T.; et al. Meta-analysis of randomized controlled trials and individual patient data comparing minimally invasive with open oesophagectomy for cancer. Br. J. Surg. 2021, 108, 1026–1033. [Google Scholar] [CrossRef]
- Janssen, T.; Fransen, L.F.C.; Heesakkers, F.; Dolmans-Zwartjes, A.C.P.; Moorthy, K.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Effect of a multimodal prehabilitation program on postoperative recovery and morbidity in patients undergoing a totally minimally invasive esophagectomy. Dis. Esophagus 2021, 35, doab082. [Google Scholar] [CrossRef] [PubMed]
- Fransen, L.F.C.; Janssen, T.; Aarnoudse, M.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Direct Oral Feeding After a Minimally Invasive Esophagectomy: A Single-Center Prospective Cohort Study. Ann. Surg. 2022, 275, 919–923. [Google Scholar] [CrossRef]
- Puccetti, F.; Wijnhoven, B.P.L.; Kuppusamy, M.; Hubka, M.; Low, D.E. Impact of standardized clinical pathways on esophagectomy: A systematic review and meta-analysis. Dis. Esophagus 2022, 35, doab027. [Google Scholar] [CrossRef]
- Okamura, A.; Takeuchi, H.; Matsuda, S.; Ogura, M.; Miyasho, T.; Nakamura, R.; Takahashi, T.; Wada, N.; Kawakubo, H.; Saikawa, Y.; et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann. Surg. Oncol. 2015, 22, 3130–3135. [Google Scholar] [CrossRef]
- Shinozaki, H.; Matsuoka, T.; Ozawa, S. Pharmacological treatment to reduce pulmonary morbidity after esophagectomy. Ann. Gastroenterol. Surg. 2021, 5, 614–622. [Google Scholar] [CrossRef]
- D’Journo, X.B.; Michelet, P.; Marin, V.; Diesnis, I.; Blayac, D.; Doddoli, C.; Bongrand, P.; Thomas, P.A. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur. J. Cardiothorac. Surg. 2010, 37, 1144–1151. [Google Scholar] [CrossRef]
- Markar, S.; Gronnier, C.; Duhamel, A.; Bigourdan, J.M.; Badic, B.; du Rieu, M.C.; Lefevre, J.H.; Turner, K.; Luc, G.; Mariette, C. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann. Surg. Oncol. 2015, 22, 2615–2623. [Google Scholar] [CrossRef]
- Seesing, M.F.J.; Kingma, B.F.; Weijs, T.J.; Ruurda, J.P.; van Hillegersberg, R. Reducing pulmonary complications after esophagectomy for cancer. J. Thorac. Dis. 2019, 11, S794–S798. [Google Scholar] [CrossRef] [PubMed]
- Weijs, T.J.; Ruurda, J.P.; Luyer, M.D.; Nieuwenhuijzen, G.A.; van Hillegersberg, R.; Bleys, R.L. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J. Anat. 2015, 227, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; Acute Lung Injury in Animals Study, G. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. Neural circuitry and immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Hansen, M.K.; Nguyen, K.T.; Fleshner, M.; Goehler, L.E.; Gaykema, R.P.; Maier, S.F.; Watkins, L.R. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R331–R336. [Google Scholar] [CrossRef]
- Kox, M.; Vaneker, M.; van der Hoeven, J.G.; Scheffer, G.J.; Hoedemaekers, C.W.; Pickkers, P. Effects of vagus nerve stimulation and vagotomy on systemic and pulmonary inflammation in a two-hit model in rats. PLoS ONE 2012, 7, e34431. [Google Scholar] [CrossRef]
- Van Westerloo, D.J.; Giebelen, I.A.; Florquin, S.; Daalhuisen, J.; Bruno, M.J.; de Vos, A.F.; Tracey, K.J.; van der Poll, T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 2005, 191, 2138–2148. [Google Scholar] [CrossRef]
- Pack, A.I.; DeLaney, R.G.; Fishman, A.P. Augmentation of phrenic neural activity by increased rates of lung inflation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 50, 149–161. [Google Scholar] [CrossRef]
- Rieger-Fackeldey, E.; Sindelar, R.; Sedin, G.; Jonzon, A. Bronchopulmonary C-fibers modulate the breathing pattern in surfactant-depleted juvenile cats. Respir. Physiol. Neurobiol. 2008, 160, 341–349. [Google Scholar] [CrossRef]
- Malik, A.B. Mechanisms of neurogenic pulmonary edema. Circ. Res. 1985, 57, 1–18. [Google Scholar] [CrossRef]
- Garg, B.K.; Loring, R.H. GTS-21 has cell-specific anti-inflammatory effects independent of alpha7 nicotinic acetylcholine receptors. PLoS ONE 2019, 14, e0214942. [Google Scholar] [CrossRef] [PubMed]
- Giebelen, I.A.; van Westerloo, D.J.; LaRosa, G.J.; de Vos, A.F.; van der Poll, T. Stimulation of alpha 7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor alpha-independent mechanism. Shock 2007, 27, 443–447. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Peng, Z.; Zhou, W.; Hu, B.; Rao, X.; Yang, X.; Li, J. GTS-21 Reduces Inflammation in Acute Lung Injury by Regulating M1 Polarization and Function of Alveolar Macrophages. Shock 2019, 51, 389–400. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.J.; Lubbers, T.; Hadfoune, M.; Luyer, M.D.; Dejong, C.H.; Buurman, W.A.; Greve, J.W. Postshock intervention with high-lipid enteral nutrition reduces inflammation and tissue damage. Ann. Surg. 2008, 248, 842–848. [Google Scholar] [CrossRef]
- Luyer, M.D.; Jacobs, J.A.; Vreugdenhil, A.C.; Hadfoune, M.; Dejong, C.H.; Buurman, W.A.; Greve, J.W. Enteral administration of high-fat nutrition before and directly after hemorrhagic shock reduces endotoxemia and bacterial translocation. Ann. Surg. 2004, 239, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Luyer, M.D.; Derikx, J.P.; Beyaert, R.; Hadfoune, M.; van Kuppevelt, T.H.; Dejong, C.H.; Heineman, E.; Buurman, W.A.; Greve, J.W. High-fat nutrition reduces hepatic damage following exposure to bacterial DNA and hemorrhagic shock. J. Hepatol. 2009, 50, 342–350. [Google Scholar] [CrossRef]
- Lubbers, T.; de Haan, J.J.; Luyer, M.D.; Verbaeys, I.; Hadfoune, M.; Dejong, C.H.; Buurman, W.A.; Greve, J.W. Cholecystokinin/Cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann. Surg. 2010, 252, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, T.; De Haan, J.J.; Hadfoune, M.; Zhang, Y.; Luyer, M.D.; Grundy, D.; Buurman, W.A.; Greve, J.W. Lipid-enriched enteral nutrition controls the inflammatory response in murine Gram-negative sepsis. Crit. Care Med. 2010, 38, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.L.; Meng, A.H.; Zhao, X.Y.; Shan, B.E.; Zhang, J.L.; Zhang, X.P. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J. Gastroenterol. 2001, 7, 667–671. [Google Scholar] [CrossRef]
- Meng, A.H.; Ling, Y.L.; Zhang, X.P.; Zhang, J.L. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J. Gastroenterol. 2002, 8, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Ling, Y.; Chen, Y.; Wang, Z. Effects of CCK-8 and Cystathionine gamma-Lyase/Hydrogen Sulfide System on Acute Lung Injury in Rats. Inflammation 2017, 40, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ni, Z.; Cong, B.; Gao, W.; Xu, S.; Wang, C.; Yao, Y.; Ma, C.; Ling, Y. CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock 2007, 27, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Gao, W.J.; Cong, B.; Yao, Y.X.; Gu, Z.Y. Effect of lipopolysaccharide on expression and characterization of cholecystokinin receptors in rat pulmonary interstitial macrophages. Acta Pharmacol. Sin. 2004, 25, 1347–1353. [Google Scholar]
- Monnikes, H.; Lauer, G.; Bauer, C.; Tebbe, J.; Zittel, T.T.; Arnold, R. Pathways of Fos expression in locus ceruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am. J. Physiol. 1997, 273, R2059–R2071. [Google Scholar] [CrossRef]
- Glatzle, J.; Kreis, M.E.; Kawano, K.; Raybould, H.E.; Zittel, T.T. Postprandial neuronal activation in the nucleus of the solitary tract is partly mediated by CCK-A receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R222–R229. [Google Scholar] [CrossRef]
- Peters, E.G.; Smeets, B.J.J.; Nors, J.; Back, C.M.; Funder, J.A.; Sommer, T.; Laurberg, S.; Love, U.S.; Leclercq, W.K.G.; Slooter, G.D.; et al. Perioperative lipid-enriched enteral nutrition versus standard care in patients undergoing elective colorectal surgery (SANICS II): A multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 242–251. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seesing, M.F.J.; Janssen, H.J.B.; Geraedts, T.C.M.; Weijs, T.J.; van Ark, I.; Leusink-Muis, T.; Folkerts, G.; Garssen, J.; Ruurda, J.P.; Nieuwenhuijzen, G.A.P.; et al. Exploring the Modulatory Effect of High-Fat Nutrition on Lipopolysaccharide-Induced Acute Lung Injury in Vagotomized Rats and the Role of the Vagus Nerve. Nutrients 2023, 15, 2327. https://doi.org/10.3390/nu15102327
Seesing MFJ, Janssen HJB, Geraedts TCM, Weijs TJ, van Ark I, Leusink-Muis T, Folkerts G, Garssen J, Ruurda JP, Nieuwenhuijzen GAP, et al. Exploring the Modulatory Effect of High-Fat Nutrition on Lipopolysaccharide-Induced Acute Lung Injury in Vagotomized Rats and the Role of the Vagus Nerve. Nutrients. 2023; 15(10):2327. https://doi.org/10.3390/nu15102327
Chicago/Turabian StyleSeesing, Maarten F. J., Henricus J. B. Janssen, Tessa C. M. Geraedts, Teus J. Weijs, Ingrid van Ark, Thea Leusink-Muis, Gert Folkerts, Johan Garssen, Jelle P. Ruurda, Grard A. P. Nieuwenhuijzen, and et al. 2023. "Exploring the Modulatory Effect of High-Fat Nutrition on Lipopolysaccharide-Induced Acute Lung Injury in Vagotomized Rats and the Role of the Vagus Nerve" Nutrients 15, no. 10: 2327. https://doi.org/10.3390/nu15102327
APA StyleSeesing, M. F. J., Janssen, H. J. B., Geraedts, T. C. M., Weijs, T. J., van Ark, I., Leusink-Muis, T., Folkerts, G., Garssen, J., Ruurda, J. P., Nieuwenhuijzen, G. A. P., van Hillegersberg, R., & Luyer, M. D. P. (2023). Exploring the Modulatory Effect of High-Fat Nutrition on Lipopolysaccharide-Induced Acute Lung Injury in Vagotomized Rats and the Role of the Vagus Nerve. Nutrients, 15(10), 2327. https://doi.org/10.3390/nu15102327






