Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis
Abstract
1. Introduction
2. Material and Methods
2.1. Primary Rat Hepatocytes
2.2. Animal Study
2.3. Exploration of the Insulin Signaling Pathway
2.4. In Situ Proximity Ligation Assay (PLA)
2.5. Triglyceride and Glycogen Assay
2.6. Western Blot
2.7. Gene Expression
2.8. Mice Liver and Muscle Endometabolome Analyses through 1 H-NMR Spectroscopy
2.9. Statistical Analysis
3. Results
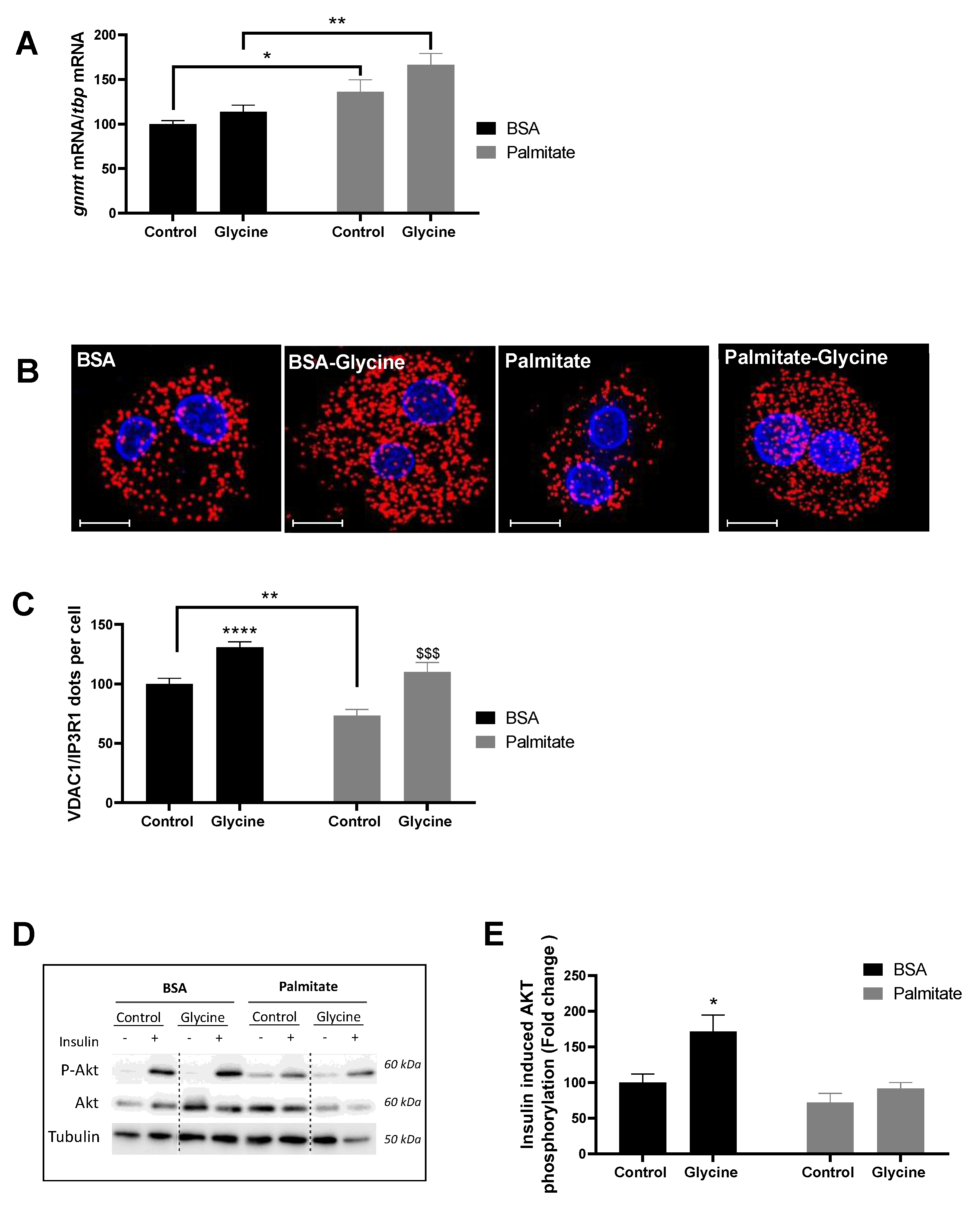
3.1. Glycine Enhances MAM Contact Sites and Insulin Response in Primary Rat Hepatocytes
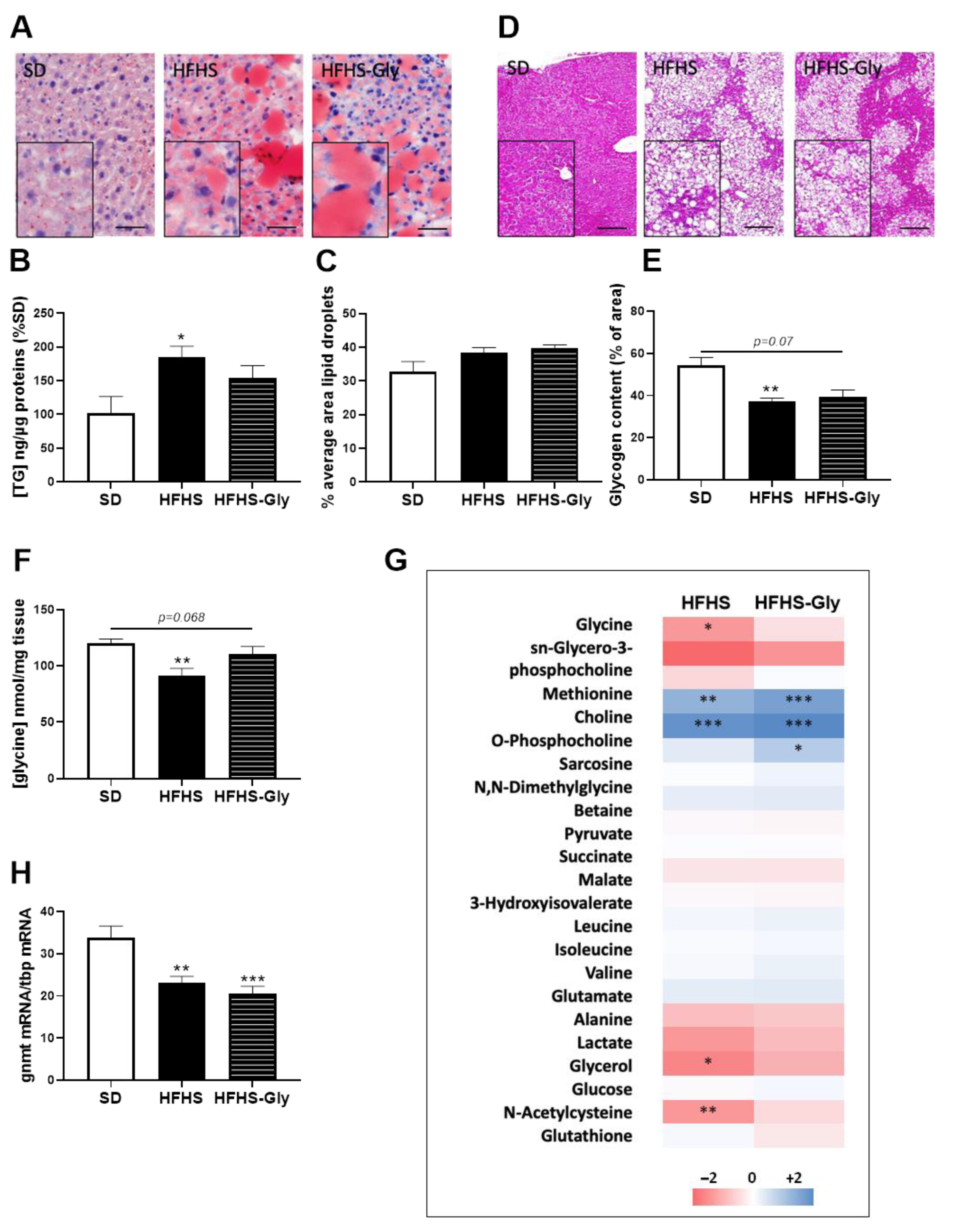
3.2. Characteristics of Diet-Induced Obese Mice
3.3. Glycine Supplementation Does Not Influence Liver MAM Integrity and Insulin Response in Diet-Induced Obese Mice
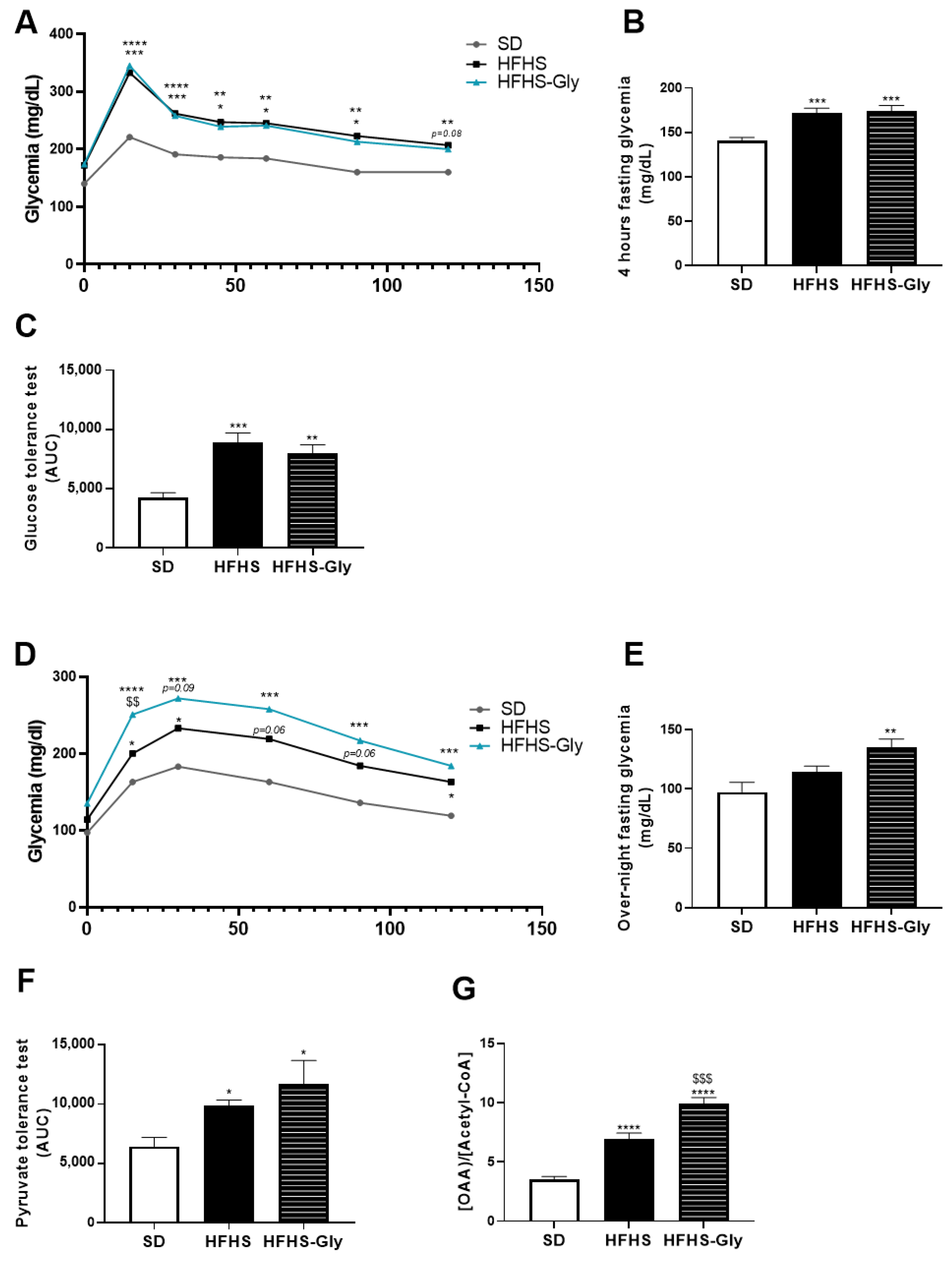
3.4. Glycine Supplementation Worsens Glucose Homeostasis of Diet-Induced Obese Mice
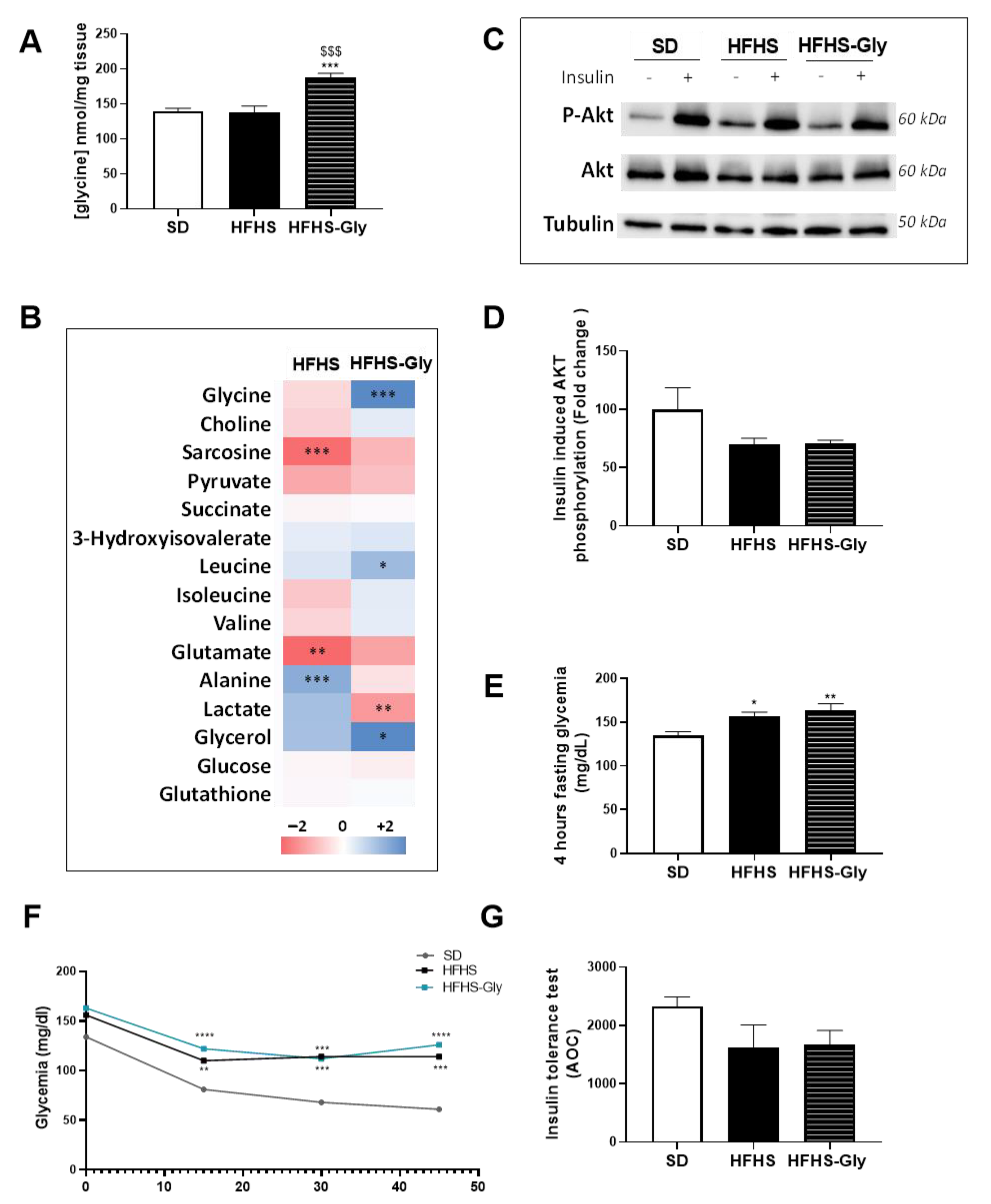
3.5. The Impact of Glycine Supplementation on the Skeletal Muscle of Diet-Induced Obese Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, R.J.; Liu, B.; Bhuket, T. Significant Burden of Nonalcoholic Fatty Liver Disease with Advanced Fibrosis in the US: A Cross-Sectional Analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2017, 46, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal Circulating Amino Acid Profiles in Multiple Metabolic Disorders. Diabetes Res. Clin. Pr. 2017, 132, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Padberg, I.; Rein, D.; Ecker, J.; Höfle, A.S.; Spanier, B.; Daniel, H. Metabolite Profiling in Plasma and Tissues of Ob/Ob and Db/Db Mice Identifies Novel Markers of Obesity and Type 2 Diabetes. Diabetologia 2015, 58, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the Plasma Amino Acid Profile, Obesity, and Glucose Metabolism in Japanese Adults with Normal Glucose Tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel Biomarkers for Pre-diabetes Identified by Metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of Serum Metabolites Associated With Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Palmer, N.D.; Stevens, R.D.; Antinozzi, P.A.; Anderson, A.; Bergman, R.N.; Wagenknecht, L.E.; Newgard, C.B.; Bowden, D.W. Metabolomic Profile Associated With Insulin Resistance and Conversion to Diabetes in the Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2015, 100, E463–E468. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Rieusset, J. Endoplasmic Reticulum-Mitochondria Calcium Signaling in Hepatic Metabolic Diseases. Biochim. Et Biophys. Acta Mol. Cell Res. 2017, 1864, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Theurey, P.; Tubbs, E.; Vial, G.; Jacquemetton, J.; Bendridi, N.; Chauvin, M.-A.; Alam, M.R.; Le Romancer, M.; Vidal, H.; Rieusset, J. Mitochondria-Associated Endoplasmic Reticulum Membranes Allow Adaptation of Mitochondrial Metabolism to Glucose Availability in the Liver. J. Mol. Cell Biol. 2016, 8, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.-A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Is Required for Insulin Signaling and Is Implicated in Hepatic Insulin Resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, E.; Axelsson, A.S.; Vial, G.; Wollheim, C.B.; Rieusset, J.; Rosengren, A.H. Sulforaphane Improves Disrupted ER-Mitochondria Interactions and Suppresses Exaggerated Hepatic Glucose Production. Mol. Cell. Endocrinol. 2018, 461, 205–214. [Google Scholar] [CrossRef]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.-A.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; et al. Disruption of Calcium Transfer from ER to Mitochondria Links Alterations of Mitochondria-Associated ER Membrane Integrity to Hepatic Insulin Resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef]
- Bassot, A.; Chauvin, M.-A.; Bendridi, N.; Ji-Cao, J.; Vial, G.; Monnier, L.; Bartosch, B.; Alves, A.; Cottet-Rousselle, C.; Gouriou, Y.; et al. Regulation of Mitochondria-Associated Membranes (MAMs) by NO/SGC/PKG Participates in the Control of Hepatic Insulin Response. Cells 2019, 8, 1319. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-Yield Preparation of Isolated Rat Liver Parenchymal Cells. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
- Groen, A.K.; Sips, H.J.; Vervoorn, R.C.; Tager, J.M. Intracellular Compartment Ation and Control of Alanine Metabolism in Rat Liver Parenchymal Cells. Eur. J. Biochem. 1982, 122, 87–93. [Google Scholar] [CrossRef]
- International Association for the Study of Pain. IASP Guidelines for the Use of Animals in Research. Available online: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1217 (accessed on 15 January 2020).
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C.C.; et al. Endoplasmic Reticulum-Mitochondria Miscommunication Is an Early and Causal Trigger of Hepatic Insulin Resistance and Steatosis. J. Hepatol. 2022, 77, 710–722. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction: Twenty-Something Years On. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Bassot, A.; Prip-Buus, C.; Alves, A.; Berdeaux, O.; Perrier, J.; Lenoir, V.; Ji-Cao, J.; Berger, M.-A.; Loizon, E.; Cabaret, S.; et al. Loss and Gain of Function of Grp75 or Mitofusin 2 Distinctly Alter Cholesterol Metabolism, but All Promote Triglyceride Accumulation in Hepatocytes. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159030. [Google Scholar] [CrossRef] [PubMed]
- Rautureau, G.J.P.; Morio, B.; Guibert, S.; Lefevre, C.; Perrier, J.; Alves, A.; Chauvin, M.A.; Pinteur, C.; Monet, M.A.; Godet, M.; et al. Dietary Obesity in Mice Is Associated with Lipid Deposition and Metabolic Shifts in the Lungs Sharing Features with the Liver. Sci. Rep. 2021, 11, 8712. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Hyeon, J.-S.; Hwang, G.-S. Software-Assisted Serum Metabolite Quantification Using NMR. Anal. Chim. Acta 2016, 934, 194–202. [Google Scholar] [CrossRef]
- White, P.J.; Lapworth, A.L.; McGarrah, R.W.; Kwee, L.C.; Crown, S.B.; Ilkayeva, O.; An, J.; Carson, M.W.; Christopher, B.A.; Ball, J.R.; et al. Muscle-Liver Trafficking of BCAA-Derived Nitrogen Underlies Obesity-Related Glycine Depletion. Cell Rep. 2020, 33, 108375. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes. Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, M.-T.; Lin, Y.-J.; Tang, F.-Y.; Ko, H.-A.; Chiang, E.-P. Regulation of Folate-Mediated One-Carbon Metabolism by Glycine N-Methyltransferase (GNMT) and Methylenetetrahydrofolate Reductase (MTHFR). J. Nutr. Sci. Vitaminol. 2015, 61, S148–S150. [Google Scholar] [CrossRef]
- Shinjo, S.; Jiang, S.; Nameta, M.; Suzuki, T.; Kanai, M.; Nomura, Y.; Goda, N. Disruption of the Mitochondria-Associated ER Membrane (MAM) Plays a Central Role in Palmitic Acid–Induced Insulin Resistance. Exp. Cell Res. 2017, 359, 86–93. [Google Scholar] [CrossRef]
- Nguyen, D.; Hsu Jean, W.; Jahoor, F.; Sekhar Rajagopal, V. Effect of Increasing Glutathione With Cysteine and Glycine Supplementation on Mitochondrial Fuel Oxidation, Insulin Sensitivity, and Body Composition in Older HIV-Infected Patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Xu, R.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. Glycine Protects against High Sucrose and High Fat-Induced Non-Alcoholic Steatohepatitis in Rats. Oncotarget 2016, 7, 80223–80237. [Google Scholar] [CrossRef] [PubMed]
- Verne, J.; Hebert, S.; Bescol Liversac, J.; Graf, B. Analysis of neoglucogenesis in hepatocyte culture by the use of C-14-glycine. Comptes Rendus des Séances de la Société de Biologie et de Ses Filiales 1966, 160, 1148–1150. [Google Scholar] [PubMed]

| SD (n = 10) | HFHS (n = 10) | HFHS-Gly (n = 10) | |
|---|---|---|---|
| Body weight (g) | 28.8 ± 0.5 | 41.5 ± 1.3 *** | 43.8 ± 1.0 *** |
| Liver weight (g) | 1.19 ± 0.03 | 1.85 ± 0.19 ** | 1.91 ± 0.16 ** |
| Gastrocnemius muscles (mg) | 399 ± 12 | 399 ± 12 | 413 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.; Lamarche, F.; Lefebvre, R.; Drevet Mulard, E.; Bassot, A.; Chanon, S.; Loizon, E.; Pinteur, C.; Bloise, A.M.N.d.L.G.; Godet, M.; et al. Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis. Nutrients 2023, 15, 96. https://doi.org/10.3390/nu15010096
Alves A, Lamarche F, Lefebvre R, Drevet Mulard E, Bassot A, Chanon S, Loizon E, Pinteur C, Bloise AMNdLG, Godet M, et al. Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis. Nutrients. 2023; 15(1):96. https://doi.org/10.3390/nu15010096
Chicago/Turabian StyleAlves, Anaïs, Frédéric Lamarche, Rémy Lefebvre, Eva Drevet Mulard, Arthur Bassot, Stéphanie Chanon, Emmanuelle Loizon, Claudie Pinteur, Aline Maria Nunes de Lira Gomes Bloise, Murielle Godet, and et al. 2023. "Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis" Nutrients 15, no. 1: 96. https://doi.org/10.3390/nu15010096
APA StyleAlves, A., Lamarche, F., Lefebvre, R., Drevet Mulard, E., Bassot, A., Chanon, S., Loizon, E., Pinteur, C., Bloise, A. M. N. d. L. G., Godet, M., Rautureau, G. J. P., Panthu, B., & Morio, B. (2023). Glycine Supplementation in Obesity Worsens Glucose Intolerance through Enhanced Liver Gluconeogenesis. Nutrients, 15(1), 96. https://doi.org/10.3390/nu15010096







