Depletion of Zinc Causes Osteoblast Apoptosis with Elevation of Leptin Secretion and Phosphorylation of JAK2/STAT3
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Zn Treatment
2.2. Cell Viability
2.3. Alkaline Phosphatase (ALP) Activity Assay
2.4. Leptin Quntification
2.5. Transmission Electron Microscopy
2.6. Quantitative Real-Time qPCR Analysis and RT-PCR Analysis
2.7. Western Blotting Analysis
2.8. Fluorescent Microscopy
2.9. Statistical Analysis
3. Results
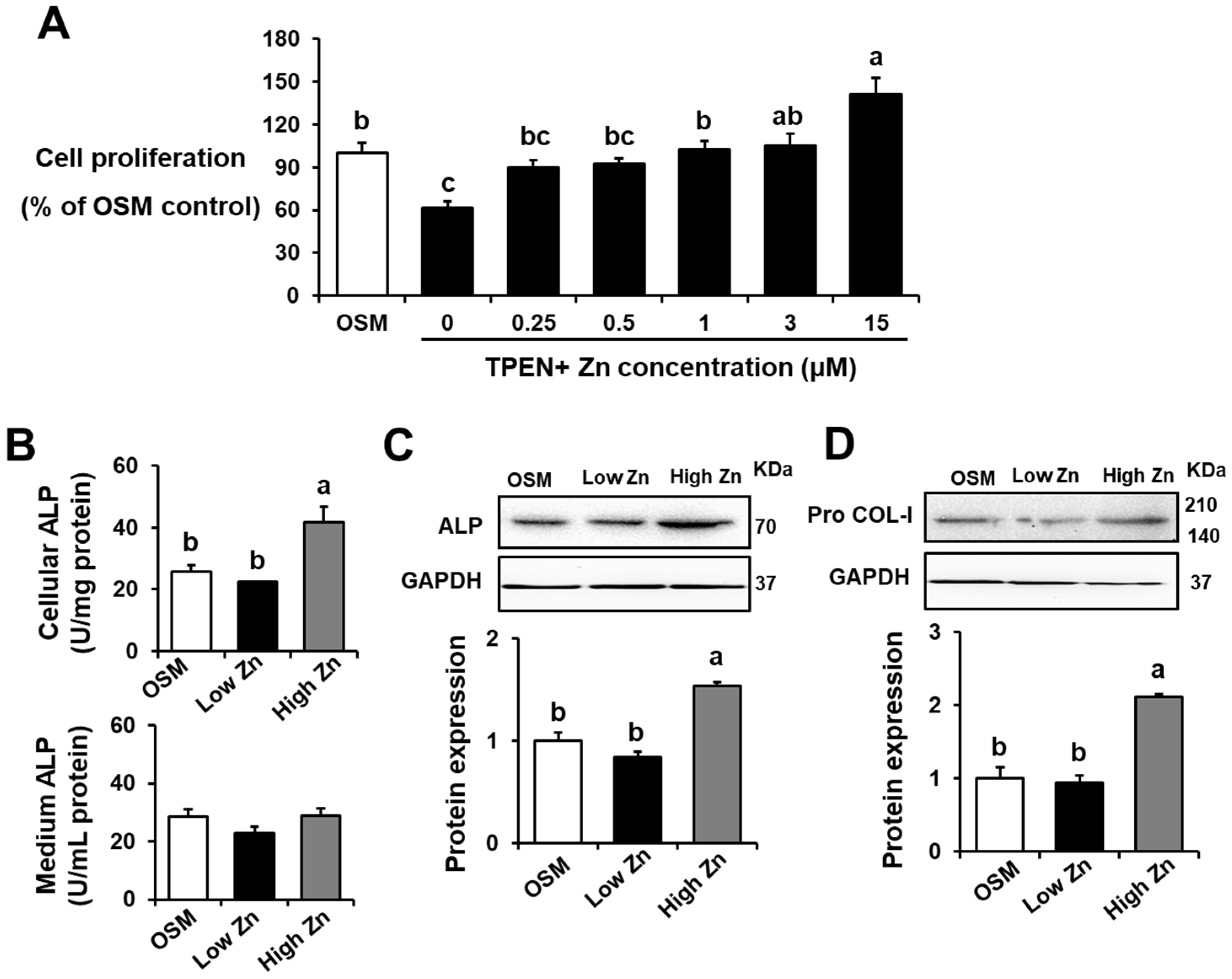
3.1. Low Zn Decreased Cell Viability, ALP Activity, and Bone-Related Protein Expression in MC3T3-E1 Cells
3.2. Low Zn Increased Leptin Secretion and Leptin mRNA and Protein Expression in MC3T3-E1 Cells
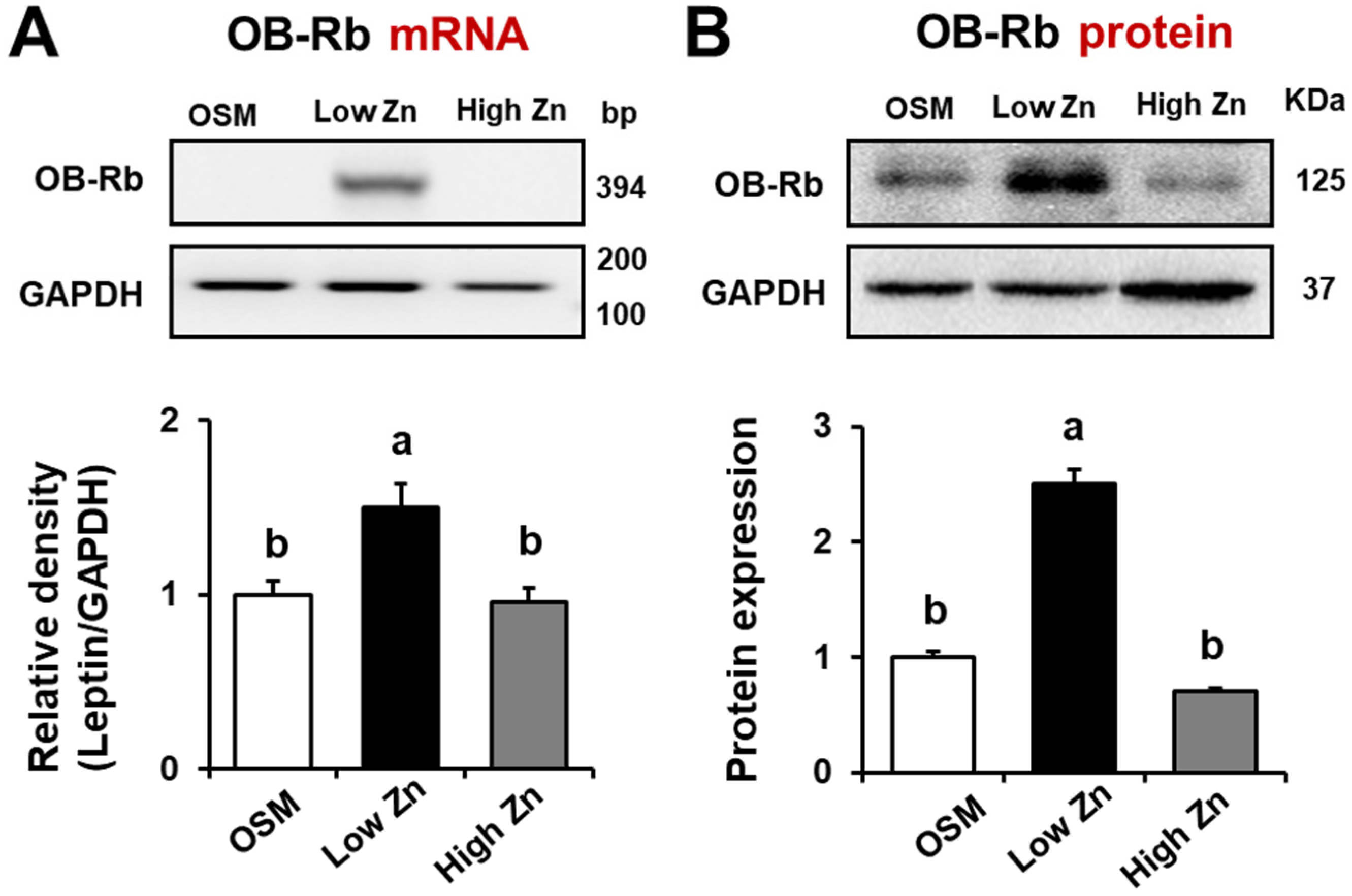
3.3. Leptin Receptor (OB-Rb) mRNA and Protein Expression by Low Zn in MC3T3-E1 Cells
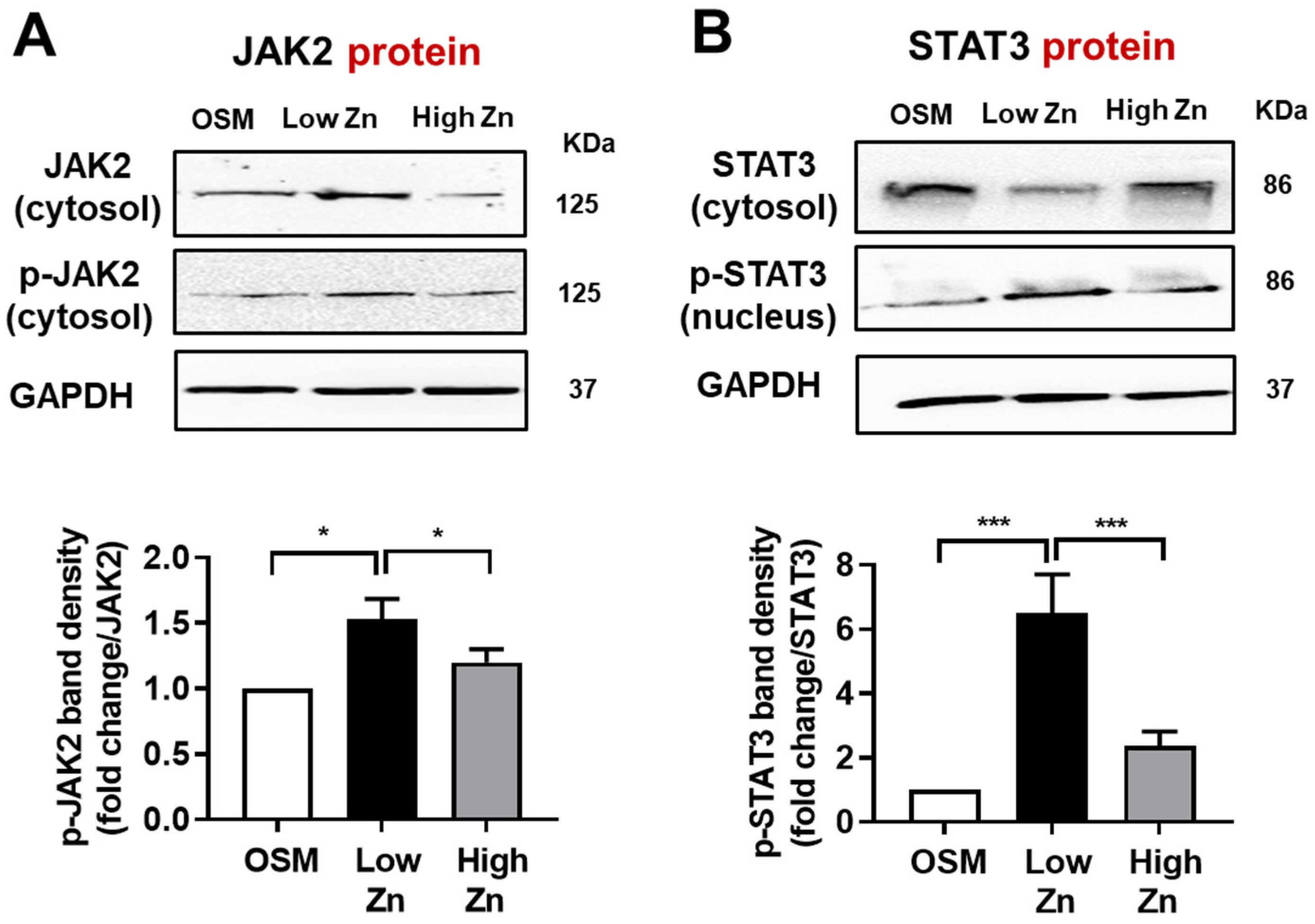
3.4. JAK/p-STAT Protein Expression by Low Zn in MC3T3-E1 Cells
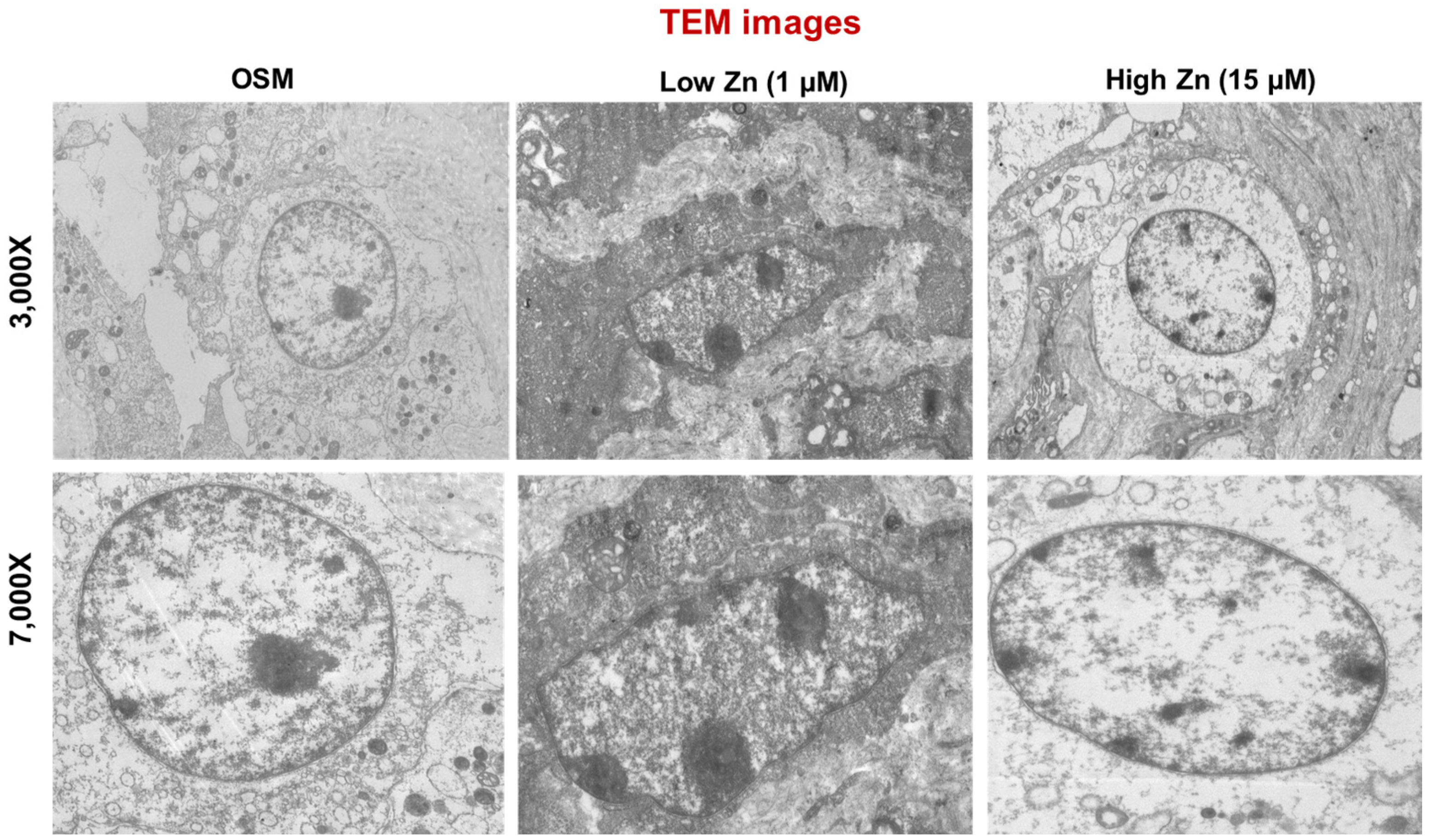
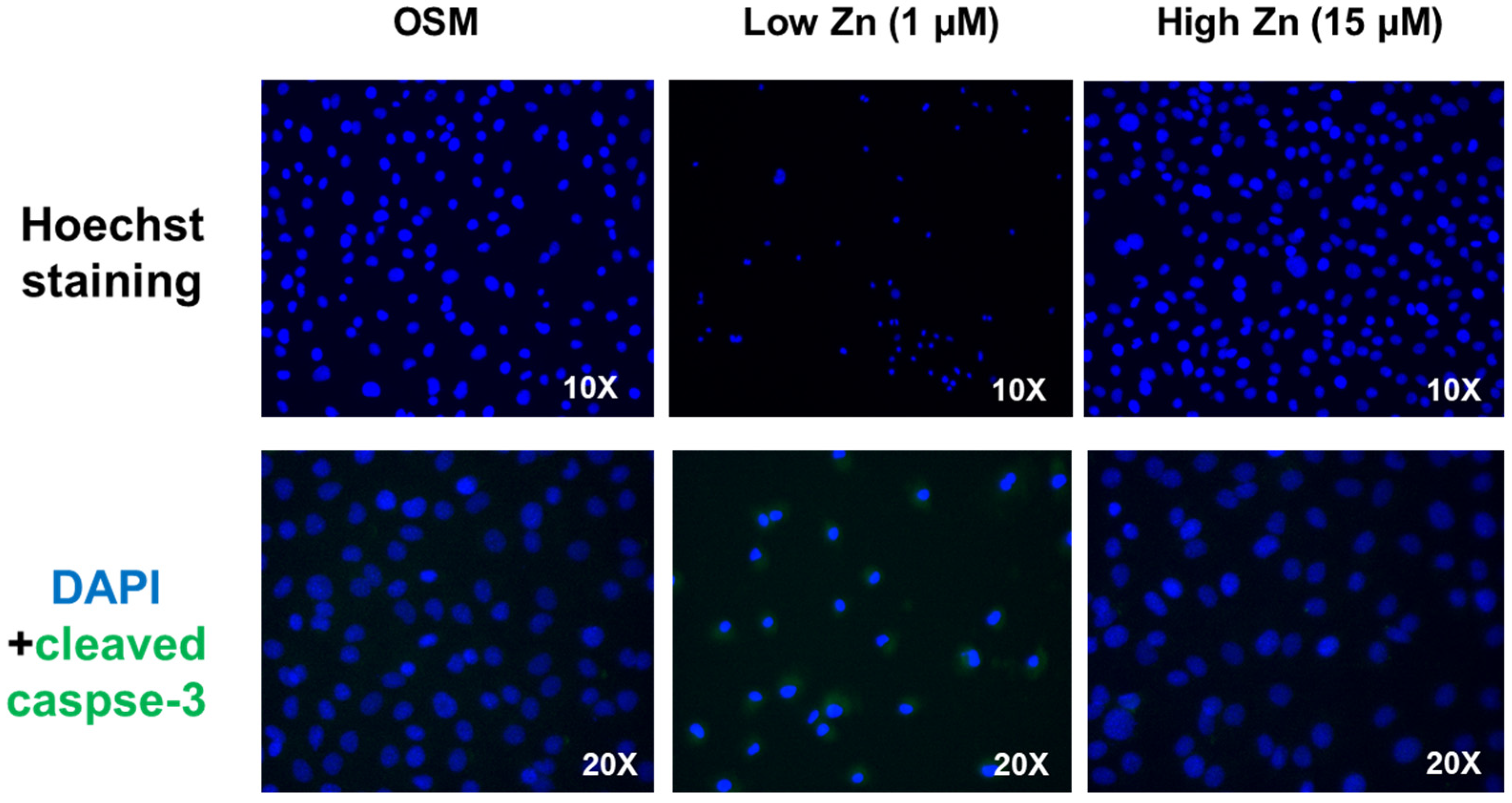
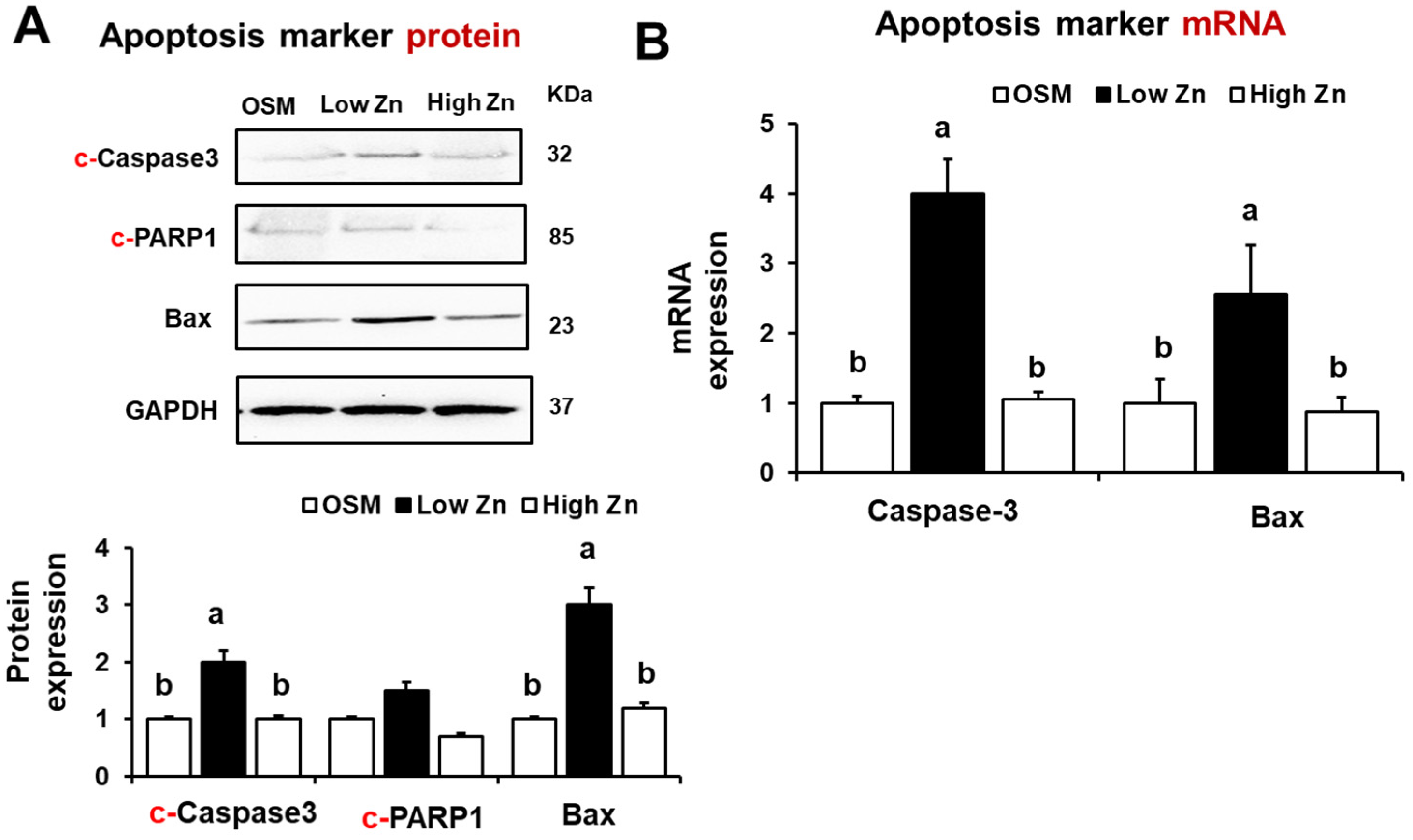
3.5. Low Zn Increased Apoptosis Signals in MC3T3-E1 Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A mechanism involved in exercise-induced bone remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, S.E. The impact of trace minerals on bone metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjila, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a therapeutic agent in bone regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Gao, Y.H. Potent effect of zinc acexamate on bone components in the femoral-metaphyseal tissues of elderly female rats. Gen. Pharmacol. Vasc. Syst. 1998, 30, 423–427. [Google Scholar] [CrossRef]
- Hie, M.; Tsukamoto, I. Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur. J. Pharmacol. 2011, 668, 140–146. [Google Scholar]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Sohn, H.Y.; Shin, H.I.; Beattie, J.H.; Kwun, I.S. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr. Res. Pract. 2007, 1, 113–119. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, M.Y.; Son, K.H.; Kim, E.C.; Kwun, I.S.; Shin, H.I. Yam (Dioscorea batatas) root and bark extracts stimulate osteoblast mineralization by increasing Ca and P accumulation and alkaline phosphatase activity. Prev. Nutr. Food Sci. 2014, 19, 194–203. [Google Scholar] [CrossRef]
- Kwun, I.S.; Cho, Y.E.; Lomeda, R.A.R.; Shin, H.I.; Choi, J.Y.; Kang, Y.H.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 6, 732–741. [Google Scholar] [CrossRef]
- Geiser, J.; Venken, K.J.; De, L.R.C.; Andrews, G.K. A mouse model of acrodermatitis enteropathica: Loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 2012, 8, e1002766. [Google Scholar] [CrossRef]
- Rocha, É.D.; de Brito, N.J.; Dantas, M.M.; Silva Ade, A.; Almeida, M.d.; Brandão-Neto, J. Effect of zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non-zinc-deficient children. J. Am. Coll. Nutr. 2015, 34, 290–299. [Google Scholar] [CrossRef]
- Auwerx, J.; Staels, B. Leptin. Lancet 1998, 351, 737–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Mercer, J.G.; Hoggard, N.; Williams, L.M.; Lawrence, C.B.; Hannah, L.T.; Trayhurn, P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996, 387, 113–116. [Google Scholar] [CrossRef]
- Mutabaruka, M.S.; Aoulad, A.M.; Delalandre, A.; Lavigne, M.; Lajeunesse, D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res. Ther. 2010, 12, R20. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Driessler, F.; Baldock, P.A. Hypothalamic regulation of bone. J. Mol. Endocrinol. 2010, 45, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Reseland, J.E.; Syversen, U.; Bakke, I.; Qvigstad, G.; Eide, L.G.; Hjertner, O.; Gordeladze, J.O.; Drevon, C.A. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J. Bone Miner. Res. 2011, 16, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Satomura, K.; Nishisho, S.; Kitaoka, E.; Yamanouchi, K.; Tobiume, S.; Nagayama, M. Potential role of leptin in endochondral ossification. J. Histochem. Cytochem. 2002, 50, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of leptin on the skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Seo, H.J.; Jeong, J.B.; Cho, Y.E.; Kwun, I.S. Zinc modulation of osterix in MC3T3-E1 cells. J. Nutr. Health 2020, 53, 347. [Google Scholar] [CrossRef]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. The role of zinc in genomic stability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2012, 733, 111–121. [Google Scholar] [CrossRef]
- Ghilardi, N.; Skoda, R.C. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol. Endocrinol. 1997, 11, 393–399. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Harada, S.I.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Speakman, J.R.; Stubbs, R.J.; Mercer, J.G. Does body mass play a role in the regulation of food intake? Proc. Nutr. Soc. 2002, 61, 473–487. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J. Endocrinol. Metab. 2012, 16, S611–S616. [Google Scholar] [CrossRef]
- Grunfeld, C.; Zhao, C.; Fuller, J.; Pollack, A.; Moser, A.; Friedman, J.; Feingold, K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Investig. 1996, 97, 2152–2157. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Prasad, A.S.; Beck, F.W.; Grabowski, S.; Kaplan, J.; Adair, C.; Brewer, G.J. Zinc may regulate serum leptin concentrations in humans. J. Am. Coll. Nutr. 1998, 17, 270–275. [Google Scholar] [CrossRef]
- Mangian, H.F.; Lee, R.G.; Paul, G.L.; Emmert, J.L.; Shay, N.F. Zinc deficiency suppresses plasma leptin concentrations in rats. J. Nutr. Biochem. 1998, 9, 47–51. [Google Scholar] [CrossRef]
- Salas, S.; Jiguet-Jiglaire, C.; Campion, L.; Bartoli, C.; Frassineti, F.; Deville, J.-L.; De Paula, A.M.; Forest, F.; Jézéquel, P.; Gentet, J.C.; et al. Correlation between ERK1 and STAT3 expression and chemoresistance in patients with conventional osteosarcoma. BMC Cancer 2014, 14, 606. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Aksoy, I.; Gonnot, F.; Osteil, P.; Aubry, M.; Hamela, C.; Rognard, C.; Hochard, A.; Voisin, S.; Fontaine, E.; et al. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat. Commun. 2015, 6, 7095. [Google Scholar] [CrossRef]
- Vaisse, C.; Halaas, J.L.; Horvath, C.M.; Darnell, J.E.; Stoffel, M.; Friedman, J.M. Leptin activation of Stat3 in the hypothalamus of wild–type and ob/ob mice but not db/db mice. Nat. Genet. 1996, 14, 95–97. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, J.H.; Ju, S.K.; You, K.H.; Ko, J.S.; Kim, H.M. Leptin receptor isoform expression in rat osteoblasts and their functional analysis. FEBS Lett. 2002, 528, 43–47. [Google Scholar] [CrossRef]
- Iwamoto, I.; Fujino, T.; Douchi, T. The leptin receptor in human osteoblasts and the direct effect of leptin on bone metabolism. Gynecol. Endocrinol. 2004, 19, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Armour, K.J.; Armour, K.E.; Van’t Hof, R.J.; Reid, D.M.; Wei, X.Q.; Liew, F.Y.; Ralston, S.H. Activation of the inducible nitric oxide synthase pathway contributes to inflammation-induced osteoporosis by suppressing bone formation and causing osteoblast apoptosis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2001, 44, 2790–2796. [Google Scholar] [CrossRef]
- Guo, B.; Yang, M.; Liang, D.; Yang, L.; Cao, J.; Zhang, L. Cell apoptosis induced by zinc deficiency in osteoblastic MC3T3-E1 cells via a mitochondrial-mediated pathway. Mol. Cell. Biochem. 2012, 361, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Grgurevic, S.; Faderl, S.; Ferrajoli, A.; Wierda, W.G.; Martinez, M.; et al. At high levels, constitutively activated STAT3 induces apoptosis of chronic lymphocytic leukemia cells. J. Immunol. 2016, 196, 4400–4409. [Google Scholar] [CrossRef]
- Faderl, S.; Harris, D.; Van, Q.; Kantarjian, H.M.; Talpaz, M.; Estrov, Z. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces antiapoptotic and proapoptotic signals in acute myeloid leukemia. Blood 2003, 102, 630–637. [Google Scholar] [CrossRef]
- Gross, A.; Yin, X.M.; Wang, K.; Wei, M.C.; Jockel, J.; Milliman, C.; Erdjument, B.H.; Tempst, P.; Korsmeyer, S.J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 1999, 274, 1156–1163. [Google Scholar] [CrossRef]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef]
- Schreiber, V.; Hunting, D.; Trucco, C.; Gowans, B.; Grunwald, D.; De Murcia, G.; De Murcia, J.M. A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis, and sister chromatid exchange following DNA damage. Proc. Natl. Acad. Sci. USA 1995, 92, 4753–4757. [Google Scholar] [CrossRef]

| Transcript | Forward Primer | Reverse Primer |
|---|---|---|
| Leptin | GAG ACC CCT GTG TCG GTT C | CTG CGT GTG TGA AAT GTC ATT G |
| OB-Rb | GGG TAA TAC TTA AAC AGT GAC C | CTA TCT GAA AAT AAA AAC TTC ATG |
| Caspase-3 | TGG TGA TGA AGG GGT CAT TTA TG | TTC GGC TTT CCA GTC AGA CTC |
| Bax | CTA CAG GGT TTC ATC CAG | CCA GTT CAT CTC CAA TTC G |
| GAPDH | TCC ACT CAC GGC AAA TTC AAC | TAG ACT CCA CGA CAT ACT CAG C |
| Antibody | Dilution Factor | Corporation | Cat. No. | |
|---|---|---|---|---|
| Primary antibody | ALP | 1:1000 | Santa Cruz | sc-271431 |
| Pro COL-I | 1:1000 | Santa Cruz | sc-166572 | |
| Leptin | 1:1000 | Santa Cruz | sc-471278 | |
| OB-R | 1:1000 | Santa Cruz | sc-8391 | |
| JAK2 | 1:1000 | Cell signaling | #3230 | |
| p-JAK2 | 1:1000 | Cell signaling | #3774 | |
| STAT3 | 1:1000 | Cell signaling | #9139 | |
| p-STAT3 | 1:1000 | Cell signaling | #52075 | |
| c-Caspase-3 | 1:1000 | Santa Cruz | sc-7272 | |
| Bax | 1:1000 | Santa Cruz | Sc-7480 | |
| c-PARP1 | 1:1000 | Santa Cruz | sc-8007 | |
| GAPDH | 1:1000 | Cell signaling | #2118 | |
| Secondary antibody | Goat anti-mouse-HRP | 1:5000 | Santa Cruz | sc-516102 |
| Goat anti-rabbit-HRP | 1:5000 | Santa Cruz | sc-2357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Ha, J.-H.; Kim, D.-K.; Kwon, J.; Cho, Y.-E.; Kwun, I.-S. Depletion of Zinc Causes Osteoblast Apoptosis with Elevation of Leptin Secretion and Phosphorylation of JAK2/STAT3. Nutrients 2023, 15, 77. https://doi.org/10.3390/nu15010077
Lee JK, Ha J-H, Kim D-K, Kwon J, Cho Y-E, Kwun I-S. Depletion of Zinc Causes Osteoblast Apoptosis with Elevation of Leptin Secretion and Phosphorylation of JAK2/STAT3. Nutrients. 2023; 15(1):77. https://doi.org/10.3390/nu15010077
Chicago/Turabian StyleLee, Jennifer K., Jung-Heun Ha, Do-Kyun Kim, JaeHee Kwon, Young-Eun Cho, and In-Sook Kwun. 2023. "Depletion of Zinc Causes Osteoblast Apoptosis with Elevation of Leptin Secretion and Phosphorylation of JAK2/STAT3" Nutrients 15, no. 1: 77. https://doi.org/10.3390/nu15010077
APA StyleLee, J. K., Ha, J.-H., Kim, D.-K., Kwon, J., Cho, Y.-E., & Kwun, I.-S. (2023). Depletion of Zinc Causes Osteoblast Apoptosis with Elevation of Leptin Secretion and Phosphorylation of JAK2/STAT3. Nutrients, 15(1), 77. https://doi.org/10.3390/nu15010077








