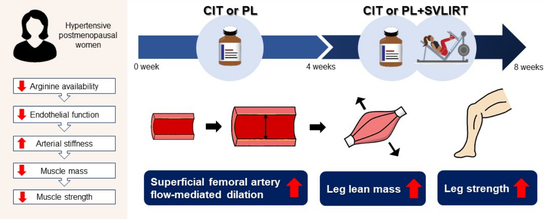
Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
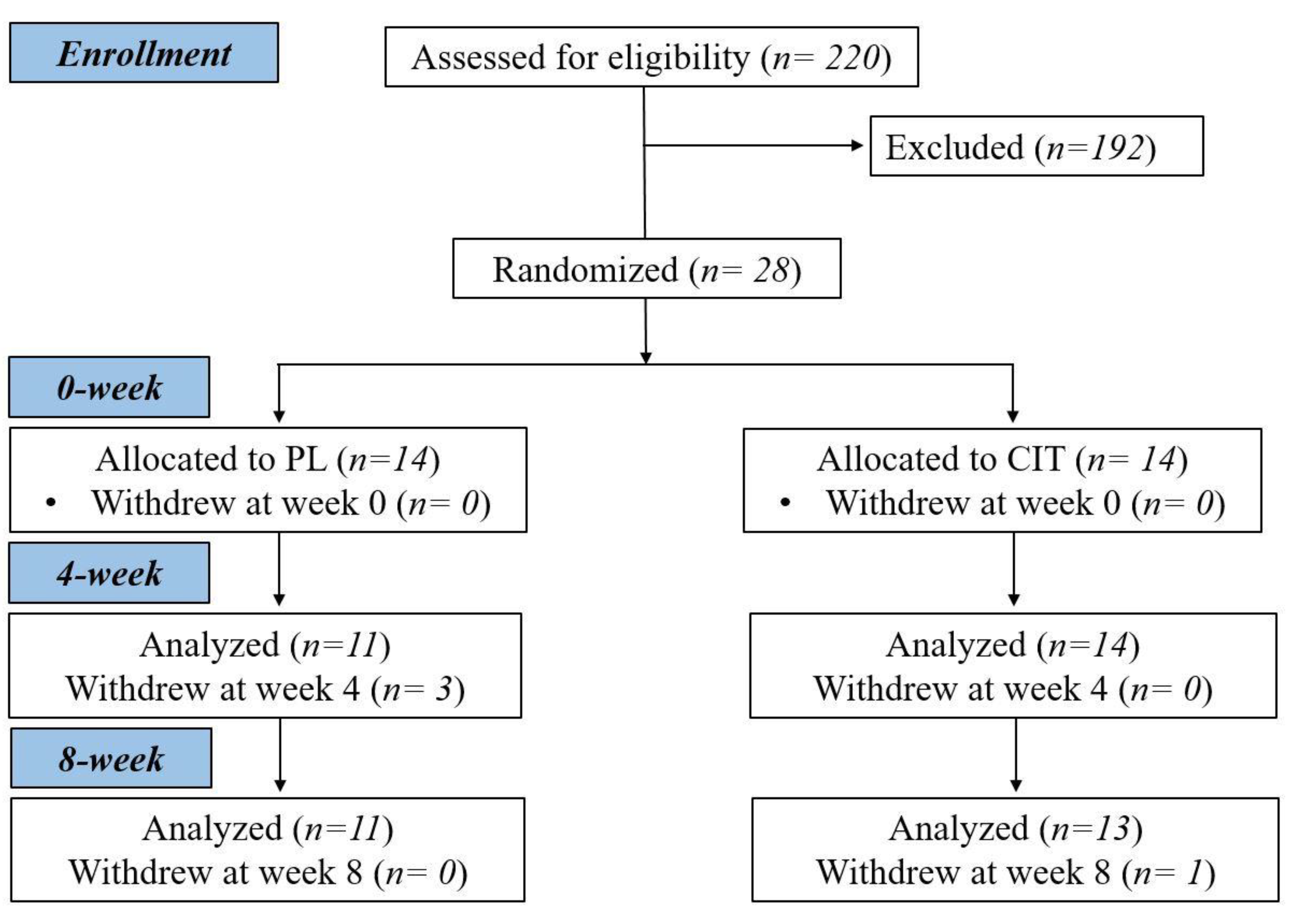
2.1. Participants
2.2. Study Protocol
2.3. Anthropometrics and Body Composition
2.4. Measurement of Resting BP and faPWV
2.5. Measurement of sfemFMD
2.6. Measurement of Leg Muscle Strength
2.7. CIT Supplementation and SVLIRT
2.8. Statistical Analysis
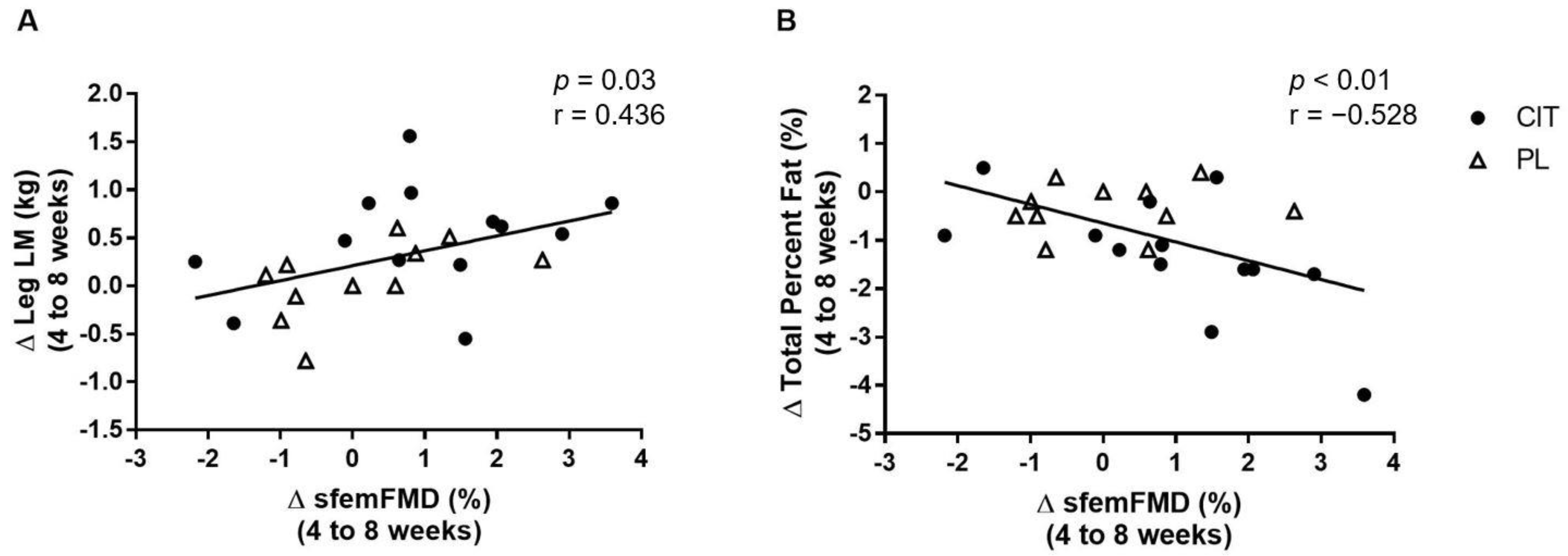
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex Differences in Arterial Stiffness and Ventricular-Arterial Interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef] [PubMed]
- Beijers, H.J.; Ferreira, I.; Bravenboer, B.; Henry, R.M.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D. Higher central fat mass and lower peripheral lean mass are independent determinants of endothelial dys-function in the elderly: The Hoorn study. Atherosclerosis 2014, 233, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-I.; Kim, M.-J.; Na, J.-B.; Chun, Y.-H.; Park, Y.-J.; Park, Y.; Hah, Y.-S.; Ha, Y.-C.; Park, K.S. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachex- Sarcopenia Muscle 2018, 9, 1034–1041. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyai, N.; Abe, K.; Utsumi, M.; Uematsu, Y.; Terada, K.; Nakatani, T.; Takeshita, T.; Arita, M. Muscle mass reduction, low muscle strength, and their combination are associated with arterial stiffness in community-dwelling elderly population: The Wakayama Study. J. Hum. Hypertens. 2020, 35, 446–454. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Klich-Rączka, A.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6477. [Google Scholar] [CrossRef]
- Kohara, K.; Okada, Y.; Ochi, M.; Ohara, M.; Nagai, T.; Tabara, Y.; Igase, M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J. Cachex- Sarcopenia Muscle 2017, 8, 557–566. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 715. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ladeiras, D.; Yu, Y.; Ming, X.-F.; Yang, Z. Detrimental Effects of Chronic L-Arginine Rich Food on Aging Kidney. Front. Pharmacol. 2021, 11, 582155. [Google Scholar] [CrossRef] [PubMed]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline Supplementation Increases Plasma Nitric Oxide Levels and Reduces Arginase Activity in Patients with Type 2 Diabetes. Front. Pharmacol. 2020, 11, 584669. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K.; Miyakex, T. Effects of Oral L-Citrulline Supplementation on Lipoprotein Oxidation and Endothelial Dysfunction in Humans with Vasospastic Angina. Immunol. Endocr. Metab. Agents Med. Chem. (Formerly Curr. Med. Chem. Immunol. Endocr. Metab. Agents) 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Ormsbee, M.J.; Madzima, T.A.; Campbell, J.C.; Wong, A. Impact of l-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp. Gerontol. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Rowley, N.; Padilla, J.; Simmons, G.H.; Laughlin, M.H.; Whyte, G.; Cable, N.T.; Green, D.J. Relationship between upper and lower limb conduit artery vasodilator function in humans. J. Appl. Physiol. 2011, 111, 244–250. [Google Scholar] [CrossRef]
- Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Aging affects vascular structure and function in a limb-specific manner. J. Appl. Physiol. 2008, 105, 1661–1670. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Jourdan, M.; Nair, K.S.; Carter, R.E.; Schimke, J.; Ford, G.C.; Marc, J.; Aussel, C.; Cynober, L. Citrulline stimulates muscle protein synthesis in the post-absorptive state in healthy people fed a low-protein diet—A pilot study. Clin. Nutr. 2014, 34, 449–456. [Google Scholar] [CrossRef]
- Bouillanne, O.; Melchior, J.-C.; Faure, C.; Paul, M.; Canoui-Poitrine, F.; Boirie, Y.; Chevenne, D.; Forasassi, C.; Guery, E.; Herbaud, S.; et al. Impact of 3-week citrulline supplementation on postprandial protein metabolism in malnourished older patients: The Ciproage randomized controlled trial. Clin. Nutr. 2018, 38, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Breen, L.; Von Allmen, M.; MacDonald, M.J.; Moore, D.R.; Offord, E.A.; Horcajada, M.-N.; Breuillé, D.; Phillips, S.M. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol. Rep. 2015, 3, e12493. [Google Scholar] [CrossRef]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.P.; Marcangeli, V.; Boutros, G.E.H.; Dulac, M.; Noirez, P.; Morais, J.A.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of High-Intensity Interval Training Combined with L-Citrulline Supplementation on Functional Capacities and Muscle Function in Dynapenic-Obese Older Adults. J. Clin. Med. 2018, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Boeno, F.P.; Ramis, T.R.; Munhoz, S.V.; Farinha, J.B.; Moritz, C.E.; Leal-Menezes, R.; Ribeiro, J.L.; Christou, D.D.; Reischak-Oliveira, A. Effect of aerobic and resistance exercise training on inflammation, endothelial function and ambulatory blood pressure in middle-aged hypertensive patients. J. Hypertens. 2020, 38, 2501–2509. [Google Scholar] [CrossRef]
- Marshall, R.N.; Smeuninx, B.; Morgan, P.T.; Breen, L. Nutritional Strategies to Offset Disuse-Induced Skeletal Muscle Atrophy and Anabolic Resistance in Older Adults: From Whole-Foods to Isolated Ingredients. Nutrients 2020, 12, 1533. [Google Scholar] [CrossRef]
- Ubolsakka-Jones, C.; Sangthong, B.; Aueyingsak, S.; Jones, D.A. Older Women with Controlled Isolated Systolic Hypertension: Exercise and Blood Pressure. Med. Sci. Sport. Exerc. 2016, 48, 983–989. [Google Scholar] [CrossRef]
- Tanimoto, M.; Ishii, N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J. Appl. Physiol. 2006, 100, 1150–1157. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of low-intensity resistance training with slow lifting and lowering on vascular function. J. Hum. Hypertens. 2008, 22, 509–511. [Google Scholar] [CrossRef]
- Hvid, L.; Aagaard, P.; Justesen, L.; Bayer, M.L.; Andersen, J.L.; Ørtenblad, N.; Kjaer, M.; Suetta, C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J. Appl. Physiol. 2010, 109, 1628–1634. [Google Scholar] [CrossRef]
- Jaime, S.J.; Maharaj, A.; Alvarez-Alvarado, S.; Figueroa, A. Impact of low-intensity resistance and whole-body vibration training on aortic hemodynamics and vascular function in postmenopausal women. Hypertens. Res. 2019, 42, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Arjmandi, B.H.; Wong, A.; Sanchez-Gonzalez, M.A.; Simonavice, E.; Daggy, B. Effects of hypocaloric diet, low-intensity resistance exercise with slow movement, or both on aortic hemodynamics and muscle mass in obese postmenopausal women. Menopause 2013, 20, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Matsukawa, T.; Isoda, H. l-Citrulline Supplementation-Increased Skeletal Muscle PGC-1α Expression Is Associated with Exercise Performance and Increased Skeletal Muscle Weight. Mol. Nutr. Food Res. 2018, 62, 1701043. [Google Scholar] [CrossRef]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. -Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Heesterbeek, P.; VAN Kuppevelt, D.J.M.; Duysens, J.; Hopman, M.T.E. Local Vascular Adaptations after Hybrid Training in Spinal Cord–Injured Subjects. Med. Sci. Sports Exerc. 2005, 37, 1112–1118. [Google Scholar] [CrossRef]
- Kooijman, M.; Thijssen, D.H.J.; De Groot, P.C.E.; Bleeker, M.W.P.; Van Kuppevelt, H.J.M.; Green, D.J.; Rongen, G.A.; Smits, P.; Hopman, M.T.E. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J. Physiol. 2008, 586, 1137–1145. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral l-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 89, 77–84. [Google Scholar] [CrossRef]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.P.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Bailey, T.G.; McIlvenna, L.; Allen, J.D.; Green, D.J.; Askew, C.D. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients 2019, 11, 954. [Google Scholar] [CrossRef]
- Figueroa, A.; Jaime, S.J.; Morita, M.; Gonzales, J.U.; Moinard, C. l-Citrulline Supports Vascular and Muscular Benefits of Exercise Training in Older Adults. Exerc. Sport Sci. Rev. 2020, 48, 133–139. [Google Scholar] [CrossRef]
- Beck, D.; Casey, D.; Martin, J.S.; Emerson, B.D.; Braith, R.W. Exercise training improves endothelial function in young prehypertensives. Exp. Biol. Med. 2013, 238, 433–441. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Madzima, T.A.; Wong, A. Whole-body vibration exercise training reduces arterial stiffness in postmenopausal women with prehy-pertension and hypertension. Menopause 2014, 21, 131–136. [Google Scholar] [CrossRef]
- Macedo, F.N.; Mesquita, T.; Melo, V.U.; Mota, M.M.; Silva, T.L.T.B.; Santana, M.N.; Oliveira, L.R.; Santos, R.V.; dos Santos, R.M.; Lauton-Santos, S.; et al. Increased Nitric Oxide Bioavailability and Decreased Sympathetic Modulation Are Involved in Vascular Adjustments Induced by Low-Intensity Resistance Training. Front. Physiol. 2016, 7, 265. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.A.; Tanaka, H. Brachial–ankle pulse wave velocity: An index of central arterial stiffness? J. Hum. Hypertens. 2005, 19, 401–406. [Google Scholar] [CrossRef]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two Weeks of Reduced Activity Decreases Leg Lean Mass and Induces “Anabolic Resistance” of Myofibrillar Protein Synthesis in Healthy Elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Singh, M.A.F. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J. Gerontol. Ser. A 2001, 56, B209–B217. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.-J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary l-Arginine Supplementation Reduces White Fat Gain and Enhances Skeletal Muscle and Brown Fat Masses in Diet-Induced Obese Rats. J. Nutr. 2008, 139, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Pons-Biescas, A.; Noriega, D.C.; Roche, E. L-Arginine and beetroot extract supplementation in the prevention of sarcopenia. Pharmaceuticals 2022, 15, 290. [Google Scholar] [CrossRef]
- Osowska, S.; Duchemann, T.; Walrand, S.; Paillard, A.; Boirie, Y.; Cynober, L.; Moinard, C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am. J. Physiol. Metab. 2006, 291, E582–E586. [Google Scholar] [CrossRef]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2015, 115, 399–404. [Google Scholar] [CrossRef][Green Version]
- Dillon, E.L.; Casperson, S.L.; Durham, W.J.; Randolph, K.M.; Urban, R.J.; Volpi, E.; Ahmad, M.; Kinsky, M.P.; Sheffield-Moore, M. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO-induced hyperemia. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1408–R1417. [Google Scholar] [CrossRef]
- Youdas, J.W.; Hollman, J.H.; Hitchcock, J.R.; Hoyme, G.J.; Johnsen, J.J. Comparison of hamstring and quadriceps femoris electromyographic activity between men and women during a single-limb squat on both a stable and labile surface. J. Strength Cond. Res. 2007, 21, 105–111. [Google Scholar] [CrossRef]
- Kuruganti, U.; Parker, P.; Rickards, J.; Tingley, M. Strength and muscle coactivation in older adults after lower limb strength training. Int. J. Ind. Ergon. 2006, 36, 761–766. [Google Scholar] [CrossRef]
- Wang, M.-X.; Murrell, D.F.; Szabo, C.; Warren, R.F.; Sarris, M.; Murrell, G.A. Nitric Oxide in Skeletal Muscle: Inhibition of Nitric Oxide Synthase Inhibits Walking Speed in Rats. Nitric Oxide 2001, 5, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Hino, K.; Ebisu, G.; Fujita, S. Oral administration of l-citrulline alters the vascular delivery of substances to rat skeletal muscles. Biochem. Biophys. Rep. 2021, 28, 101149. [Google Scholar] [CrossRef] [PubMed]
- Le Plénier, S.; Goron, A.; Sotiropoulos, A.; Archambault, E.; Guihenneuc, C.; Walrand, S.; Salles, J.; Jourdan, M.; Neveux, N.; Cynober, L.; et al. Citrulline directly modulates muscle protein synthesis via the PI3K/MAPK/4E-BP1 pathway in a mal-nourished state: Evidence from in vivo, ex vivo, and in vitro studies. Am. J. Physiol. —Endocrinol. Metab. 2017, 312, E27–E36. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | PL (n = 11) | CIT (n = 13) | p |
|---|---|---|---|
| Age (years) | 63 ± 1 | 62 ± 2 | 0.74 |
| Height (m) | 1.57 ± 0.02 | 1.56 ± 0.02 | 0.74 |
| Weight (kg) | 70.8 ± 3.8 | 72.3 ± 2.8 | 0.75 |
| BMI (kg/m2) | 29.2 ± 1.7 | 29.6 ± 1.1 | 0.85 |
| Waist Circumference (cm) | 93.6 ± 4.7 | 91.5 ± 2.9 | 0.70 |
| Fasting Blood Glucose (mg/dL) | 98.0 ± 4.7 | 95.0 ± 2.8 | 0.58 |
| Resting SBP (mmHg) | 135 ± 5 | 136 ± 5 | 0.85 |
| Resting DBP (mmHg) | 79 ± 3 | 77 ± 4 | 0.76 |
| Resting MAP (mmHg) | 98 ± 4 | 97 ± 4 | 0.89 |
| Hormone Replacement Therapy, n | |||
| Estrogen | 2 | 4 | |
| Progesterone | 1 | 0 | |
| Anti-hypertensive medications, n | |||
| Diuretic | 1 | 0 | |
| ACE Inhibitor | 2 | 2 | |
| Ca2+ Channel Blocker | 1 | 1 | |
| ANG II Receptor Blocker | 0 | 4 | |
| Statins | 1 | 1 | |
| Unmedicated, n | 6 | 6 |
| PL (n = 11) | CIT (n = 13) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | 0 week | 4 weeks | 8 weeks | 0 week | 4 weeks | 8 weeks | p |
| Baseline diameter (mm) | 6.1 ± 0.3 | 6.0 ± 0.3 | 6.0 ± 0.2 | 6.1 ± 0.2 | 5.9 ± 0.3 | 5.9 ± 0.2 | 0.35 |
| Peak diameter (mm) | 6.3 ± 0.3 | 6.2 ± 0.3 | 6.3 ± 0.2 | 6.3 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.2 | 0.85 |
| sfemFMD absolute (mm) | 0.26 ± 0.03 | 0.23 ± 0.02 | 0.26 ± 0.04 | 0.19 ± 0.02 | 0.28 ± 0.03 † | 0.33 ± 0.04 †# | <0.01 |
| sfemFMD (%) | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.3 ± 0.6 | 3.1 ± 0.4 | 4.8 ± 0.5 † | 5.8 ± 0.7 † | <0.001 |
| Baseline shear rate (sec−1) | 60 ± 10 | 78 ± 8 | 82 ± 12 | 66 ± 9 | 76 ± 8 | 84 ± 11 | 0.85 |
| Peak shear rate (sec−1) | 1546 ± 549 | 1834 ± 1002 | 395 ± 70 | 453 ± 505 | 1221 ± 923 | 513 ± 65 | 0.13 |
| Shear rate AUC (au) | 11567 ± 2678 | 13295 ± 2740 | 9493 ± 1903 | 9743 ± 2463 | 10062 ± 2521 | 9624 ± 1751 | 0.48 |
| Shear rate AUC peak (au) | 8376 ± 2119 | 9678 ± 2117 | 7616 ± 1488 | 6883 ± 1949 | 7552 ± 1947 | 7100 ± 1369 | 0.66 |
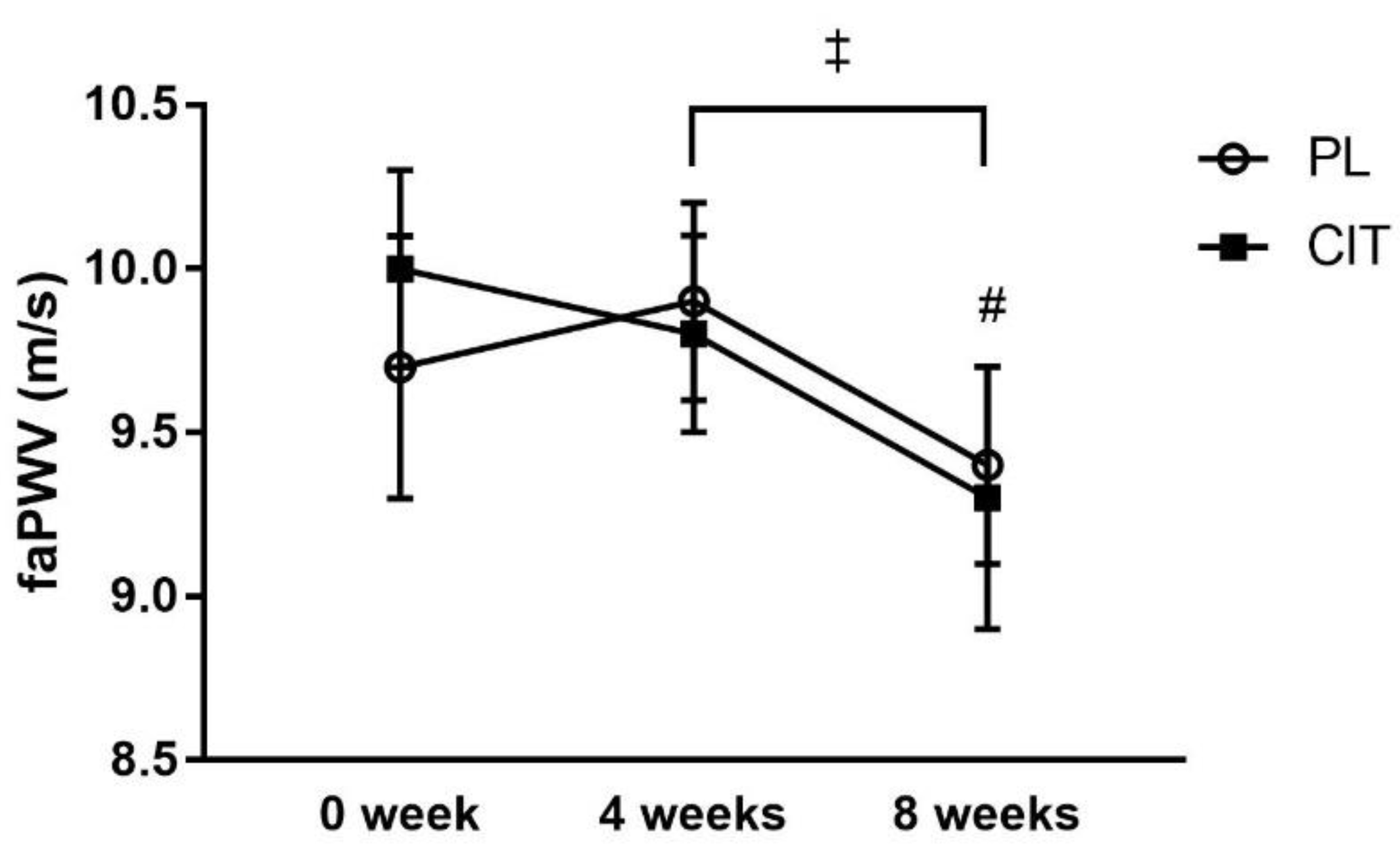
| faPWV (m/s) | 9.7 ± 0.4 | 9.9 ± 0.3 | 9.4 ± 0.3 # | 10.0 ± 0.3 | 9.8 ± 0.3 | 9.3 ± 0.4 # | 0.48 |
| PL (n = 11) | CIT (n = 13) | ||||||
|---|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 8 weeks | 0 week | 4 weeks | 8 weeks | p | |
| Height (m) | 1.57 ± 0.02 | 1.56 ± 0.02 | 0.74 | ||||
| Weight (kg) | 70.8 ± 3.8 | 72.2 ± 3.8 | 72.4 ± 3.8 | 72.3 ± 2.8 | 72.1 ± 2.9 | 72.5 ± 2.9 | 0.34 |
| BMI (kg/m2) | 29.2 ± 1.7 | 29.4 ± 1.7 | 29.4 ± 1.7 | 29.6 ± 1.1 | 29.5 ± 1.2 | 29.7 ± 1.2 | 0.76 |
| WC (cm) | 93.6 ± 4.7 | 93.7 ± 4.2 | 92.6 ± 4.6 | 91.5 ± 2.9 | 92.2 ± 2.7 | 90.8 ± 2.4 | 0.82 |
| VAT (kg) | 1.21 ± 0.23 | 1.20 ± 0.25 | 1.15 ± 0.26 | 0.84 ± 0.14 | 0.85 ± 0.14 | 0.81 ± 0.13 | 0.54 |
| Total Percent Fat (%) | 44.1 ± 2.0 | 44.6 ± 1.9 | 44.3 ± 2.0 | 42.9 ± 1.4 | 43.3 ± 1.0 | 42.0 ± 1.1 # | 0.27 |
| Arms LM (kg) | 4.14 ± 0.21 | 4.16 ± 0.23 | 4.15 ± 0.23 | 4.28 ± 0.22 | 4.31 ± 0.21 | 4.30 ± 0.22 | 0.91 |
| Legs LM (kg) | 12.6 ± 0.5 | 12.4 ± 0.5 | 12.4 ± 0.5 | 13.4 ± 0.6 | 13.5± 0.6 | 14.0 ± 0.7 †,# | <0.001 |
| Leg Press 10RM (kg) | 88.1 ± 10.5 | 93.7 ± 10.2 | 100.2 ± 9.7 †,# | 96.4 ± 9.7 | 99.4 ± 9.3 | 109.6 ± 9.0 †,# | 0.76 |
| Leg Extension 10RM (kg) | 19.6 ± 1.5 | 22.2 ± 2.4 | 27.3 ± 2.3 *,# | 20.9 ± 1.5 | 22.8 ± 1.8 | 27.7 ± 2.1 *,‡ | 0.77 |
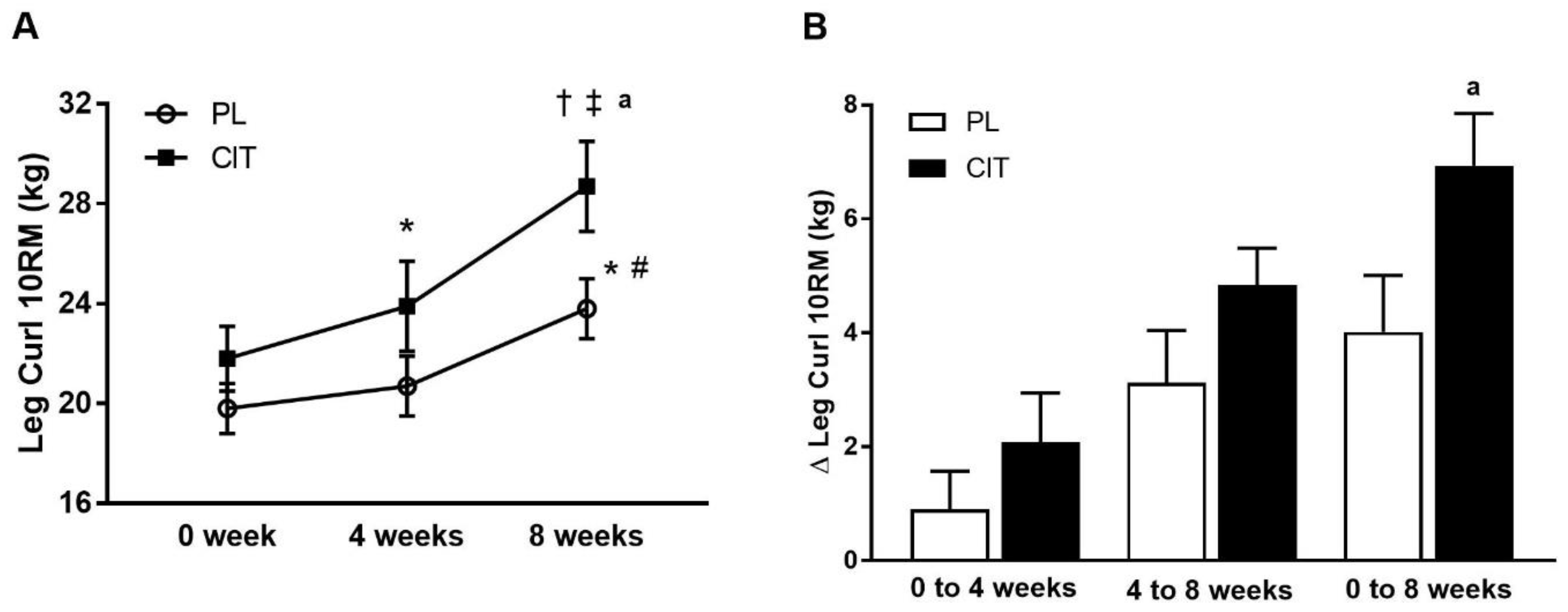
| Leg Curl 10RM (kg) | 19.8 ± 1.0 | 20.7 ± 1.2 | 23.8 ± 1.2 *,# | 21.8 ± 1.3 | 23.9 ± 1.8 * | 28.7 ± 1.8 †,‡,a | <0.05 |
| Calf Raise 10RM (kg) | 78.6 ± 5.9 | 82.6 ± 5.9 | 94.4 ± 5.1 *,# | 84.4 ± 8.3 | 90.0 ± 9.5 | 102.6 ± 10.8 *,# | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2023, 15, 74. https://doi.org/10.3390/nu15010074
Kang Y, Dillon KN, Martinez MA, Maharaj A, Fischer SM, Figueroa A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients. 2023; 15(1):74. https://doi.org/10.3390/nu15010074
Chicago/Turabian StyleKang, Yejin, Katherine N. Dillon, Mauricio A. Martinez, Arun Maharaj, Stephen M. Fischer, and Arturo Figueroa. 2023. "Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women" Nutrients 15, no. 1: 74. https://doi.org/10.3390/nu15010074
APA StyleKang, Y., Dillon, K. N., Martinez, M. A., Maharaj, A., Fischer, S. M., & Figueroa, A. (2023). Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients, 15(1), 74. https://doi.org/10.3390/nu15010074