Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota
Abstract
1. Introduction
2. Dyslipidemia and Gut Microbiota
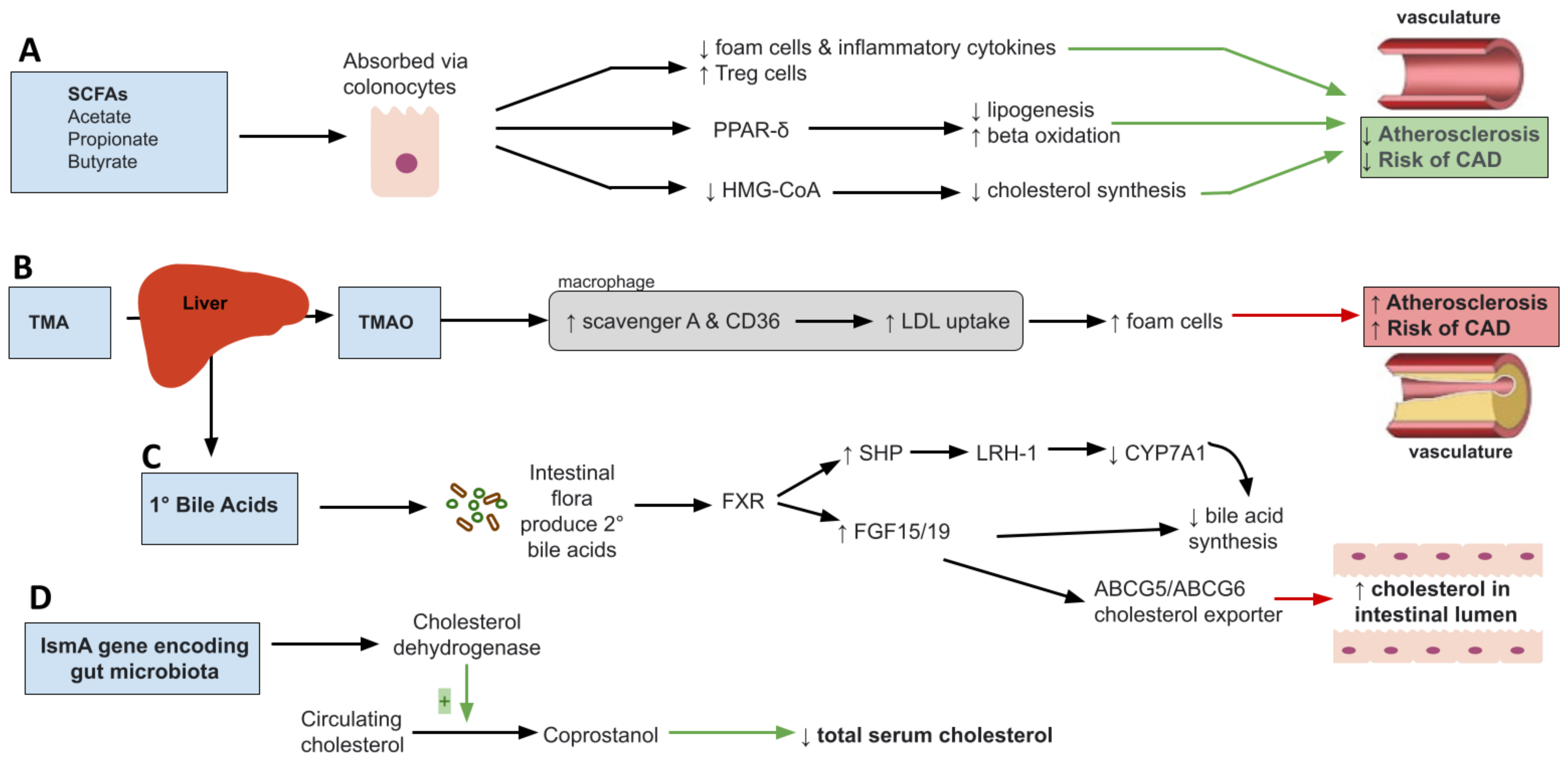
2.1. Short Chain Fatty Acids and Dyslipidemia
2.2. Trimethylamine N-Oxide (TMAO)
2.3. Primary Bile Acids
2.4. Microbial Cholesterol Dehydrogenases
3. Diet and Its Effects on Gut Microbiota and Dyslipidemia
3.1. High Fat Diet, Gut Microbiota and Dyslipidemia
3.2. Mediterranean Diet, Gut Microbiota and Dyslipidemia
3.3. Current Pharmacologic Treatments for Dyslipidemia and Relations to Gut Microbiota
4. Targeted Microbiota Therapies
4.1. Prebiotics
| Targeted Microbiota Therapy Method | Study Period | Species Involved/Outcome Measured | Results/Implications | Subject Type | Reference |
|---|---|---|---|---|---|
| Prebiotic—Beta-glucans | Bifidobacterium, Lactobacillus | Increased SCFA production Decreased cholesterol biosynthesis | Mice | [147] | |
| Prebiotic—Beta-glucans (oat and tartary buckwheat) | Bacteroidetes/Firmicutes ratio | Increased SCFA production Reduction of plasma lipids Increased fecal bile acid concentration | Rodent | [131] | |
| Prebiotic—Beta-glucans | Strong immunomodulary effects Reduced serum cholesterol levels | [152] | |||
| Prebiotic—Oatmeal | 45-day follow-up | Akkermansia, Dialister, Faecalibacterium, Barnesiella, Agathobacter, Lactobacillus Ruminococcaceae-MK4A214 | Increased Akkermansia, Dialister, Faecalibacterium, Barnesiella, Agathobacter, Lactobacillus Decreased Ruminococcaceae-MK4A214 Decreased serum TC, LDL, and non-HDL cholesterol Increased serum total antioxidant capacity Increased SCFA production | Human | [128] |
| Flavonoids from whole-grain oat | Akkermansia, Blautia Lachnoclostridium, Colidextribacter, and Desulfovibrio | Improved serum lipid profiles Decreased body weight Decreased lipid deposition Increased Akkermansia Decreased Lachnoclostridium, Blautia, Colidextribacter, and Desulfovibrio | Mice | [132] | |
| Prebiotic—Wheat bread and barley beta glucans | 4 weeks | Akkermansia muciniphila & Bifidobacterium were elevated pre-intervention in cholesterol-responsive group | Decreased abdominal circumference Decreased total cholesterol Increased fecal propionic acid Decreased Clostridium leptum by 25% and Collinsella aerofaciens, a species that thrives within inflamed gut tissues | Human | [146] |
| Prebiotic—Oat beta-glucans | 8 weeks | Serum lipids | Reduced LDL, TC, and non-HDL in mildly hypercholesterolemic patients | Human | [148] |
| 4 weeks | Serum lipids | Reduced LDL by 6% 8% reduction in CVD risk | Human | [149] | |
| 4 weeks | Reduced serum TC and LDL | Human | [150] | ||
| Lowered markers of inflammation in heart/liver/kidney/spleen/colon in obese mice fed high-cholesterol diets | Mice | [151] | |||
| 30 days | Acetic acid Propionic acid Hydroxybutyric acid | Reduction in mucosal damage—Increased fecal concentrations of acetic acid, propionic acid, and hydroxybutyric acid Decreased serum CRP | Human | [153] | |
| Prebiotic—Psyllium (plantago ovata) fiber | Meta analysis of 28 trials greater than or equal to 3 weeks | N/A | Significant reduction in LDL cholesterol, non-HDL cholesterol, and apoB lipoproteins | Human | [155] |
| Prebiotic—Psyllium husk | 7 days | Roseburia, Lachnospira, and Faecalibacterium | Increased concentrations of Lachnospira, Faecalibacterium, Phascolartobaceterium, Veillonella, and Sutterella Increased fecal water content associated with increased butyrate-producing strains (Lachnospira, Roseburia, and Faecalibacterium) | Human | [156] |
| Roseburia Bacteroides, Faecalibacterium, Coprobacillus, and Akkernansia | Greater reduction in cholesterol and TGs compared to Orlistat | Mice | [157] | ||
| Prebiotic- Inulin-type fructans | Bifidobacterium, Faecalibacterium, Lactobacillus | Increased insulin sensitivity Increased gut barrier function Improved lipid profiles | [158] | ||
| Bifidobacterium, Anaerostipes, Bilophila | Increased Bifidobacterium and Anaerostipes Decreased Bilophila | Human | [159] | ||
| 6 weeks | Bifidobacterium Acetic acid, propionic acid, butyric acid | Significantly increased Bifidobacterium Increased total fecal SCFA, acetic acid and propionic acid in Type 2 DM patients | Human | [160] | |
| Dietary glycan—Seaweed Polysaccharide | 6 weeks and 12 weeks | Bifidobacteria, Akkermansia, Pseudobutyrivibrio, Clostridium, Bilophila | Significantly reduced non-HDL cholesterol Increased Bifidobacteria, Akkermansia, Pseudobutyrivibrio and Clostridium Decreased Bilophila | [162] | |
| Probiotic- Lactobacillus, Bifidobacterium, Streptococcus | 6 weeks | Lactobacillus, Bifidobacterium and Streptococcus | Decreased fasting plasma glucose versus control group Increased serum HDL versus control group | Human | [163] |
| Probiotic—Lactic acid producing strains | Lactobacillus casei, Lactobacillus paracasei, Lactobacillus plantarum, Enterococcus faecium, Enterococcus lactis | Incorporation of probiotics into foods containing dairy reduced reduced serum cholesterol | [164] | ||
| Probiotic—Bifidobacterium bifidum | 3 weeks | Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, Fusobacteria, Dorea, Lachnospira | Increased Firmicutes, Bacteroides and Actinobacteria Decreased in Firmicutes, Bacteroides and Actinobacteria Decreased in total cholesterol and LDL cholesterol | Human | [165] |
| Probiotic milk—Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium lactis | 10 weeks supplement plus 2 weeks follow-up | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium lactis | Improved fecal weight Decreased fecal passing time Increased biodiversity of Lactobacillus and Bifidobacterium spp. Improved lag-time of LDL oxidation Decreased serum cholesterol | Human | [166] |
| Probiotic—Bifidobacterium animalis subsp. lactis | 6 months | Lactobacillus and Akkermansia | Significantly increased fecal Bifidobacterium, Akkermansia, and Streptococcus in supplemented group Decreased glycocholic acid, glycoursodeoxycholic acid, taurohyodeoxycholic acid, and tauroursodeoxycholic acid | Human | [167] |
| Synbiotic—xylo-oligosaccharides (XOS) + Bifidobacterium animalis lactis | 3 weeks | XOS + Bifidobacterium animalis lactis | Increased host Th1 responses, increase in HDL, increased Bifidobacterium count | Human | [168] |
| Synbiotic—xylo-oligosaccharides (XOS) + Bacillus licheniformis | XOS + Bacillus licheniformis | Reduction in serum LPS, decreased body weight, decreased serum total cholesterol | Mice | [169] | |
| Folate | Reduced body weight gain, adipocyte size and dysbiosis Down-regulated lipid-metabolism genes | Mice | [170] | ||
| Lower serum folate levels were associated with increased carotid intima-media thickness | Human | [171] | |||
| Porphyromonadaceae | Low folate diet resulted in higher amounts of Porphyromonadaceae and associated NAFLD | Mice | [172] | ||
| Fecal Microbiota Transplant | Bifidobacterium, Lactobacillus, Bilophila and Desulfovibrio | Increases in Bifidobacterium and Lactobacillus Decreased Bilophila and Desulfovibrio | Human | [173] | |
| 24 weeks | Bifidobacterium and Lactobacillus | Increases in butyrate-producing bacteria Improvements in total cholesterol and LDL | [174] | ||
| 12 weeks | Fecal bacteria Bile acids | Decreased taurocholic acid versus baseline Bile acid profile shifts towards that of the donor | [175] | ||
| Akkermansia muciniphila | Akkermansia muciniphila | Significant positive correlation with PUFA/SFA ratio Negatively correlated with onset of dyslipidemia Reduced body fat mass and insulin resistance Increased tight junction proteins, zonulin-1 and occludin Increased IL-10 Degradation of host mucin lining | Human | [176] | |
| Akkermansia muciniphila | Improved gut barrier function via interactions with TLR-2 | Mice | [129] | ||
| Akkermansia muciniphila and Periplaneta americana extract (PAE) | PAE pretreatment greatly increased amount of Akkermansia muciniphila versus control facing diquat-induced oxidative stress | Mice | [130] | ||
| Akkermansia mucinophila | Increased therapeutic effect of the novel anti-hyperlipidemic plant-alkaloid, Nuciferine, via enrichment with Akkermansia mucinophila | Mice | [177] | ||
| Akkermansia mucinophila | Increased Akkermansia muciniphila was associated with decreased risk of metabolic syndrome once A. muciniphila comprised 0.2% of total microbiome | Human | [178] | ||
| Faecalibacterium prausnitzii | Faecalibacterium prausnitzii | Mononuclear cell stimulation of Faecalibacterium prausnitzii lowered IL-12 and IFN-gamma production Increased secretion of IL-10 Displayed anti-inflammatory effects including blocking NF-KB and IL-8 production | [179] | ||
| Faecalibacterium prausnitzii | Produced butyrate thereby inhibiting NF-KB, and downregulating TLR-3/TLR-4 Stimulated mucin secretion, improving gut barrier functionality | [180] | |||
| Faecalibacterium prausnitzii | Decreased abundance of the species in obese individuals Exhibited anti-inflammatory effects Produced butyrate | [181] | |||
| 13 weeks | Faecalibacterium prausnitzii | Decreased adipose tissue inflammation Lowered AST/ALT Increased fatty acid oxidation Improved intestinal integrity | Mice | [182] |
4.2. Probiotics
4.3. Synbiotics
4.4. Gut Microbiota, Folate and Dyslipidemia
4.5. Fecal Microbiota Transplantation in Restoring Dyslipidemia
4.6. The Use of Faecalibacterium prausnitzii and Akkermansia muciniphila as Next-Generation Probiotics
4.6.1. Akkermansia muciniphila
4.6.2. Faecalibacterium prausnitzii
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Patel, K.; Patel, A.; Hawes, D.; Shah, J.; Shah, K. Faecal microbiota transplantation: Looking beyond clostridium difficile infection at inflammatory bowel disease. Gastroenterol. Hepatol. Bed Bench 2018, 11, 1–8. [Google Scholar]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar]
- Clifton, P.M. Diet, exercise and weight loss and dyslipidaemia. Pathology 2019, 51, 222–226. [Google Scholar] [CrossRef]
- Zwartjes, M.S.Z.; Gerdes, V.E.A.; Nieuwdorp, M. The Role of Gut Microbiota and Its Produced Metabolites in Obesity, Dyslipidemia, Adipocyte Dysfunction, and Its Interventions. Metabolites 2021, 11, 531. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, Itc81–Itc96. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Rämö, J.T.; Ripatti, P.; Tabassum, R.; Söderlund, S.; Matikainen, N.; Gerl, M.J.; Klose, C.; Surma, M.A.; Stitziel, N.O.; Havulinna, A.S.; et al. Coronary Artery Disease Risk and Lipidomic Profiles Are Similar in Hyperlipidemias With Family History and Population-Ascertained Hyperlipidemias. J. Am. Heart Assoc. 2019, 8, e012415. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barceló, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lu, X.; Deng, Z.; Zhong, H.J.; Zhang, W.; Li, Q.; Zhou, H.H.; Liou, Y.L.; He, X.X. Effect of Washed Microbiota Transplantation on Patients With Dyslipidemia in South China. Front. Endocrinol. 2022, 13, 827107. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e455. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Rebolledo, C.; Cuevas, A.; Zambrano, T.; Acuña, J.J.; Jorquera, M.A.; Saavedra, K.; Martínez, C.; Lanas, F.; Serón, P.; Salazar, L.A.; et al. Bacterial Community Profile of the Gut Microbiota Differs between Hypercholesterolemic Subjects and Controls. BioMed Res. Int. 2017, 2017, 8127814. [Google Scholar] [CrossRef]
- Gargari, G.; Deon, V.; Taverniti, V.; Gardana, C.; Denina, M.; Riso, P.; Guardamagna, O.; Guglielmetti, S. Evidence of dysbiosis in the intestinal microbial ecosystem of children and adolescents with primary hyperlipidemia and the potential role of regular hazelnut intake. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Korecka, A.; de Wouters, T.; Cultrone, A.; Lapaque, N.; Pettersson, S.; Doré, J.; Blottière, H.M.; Arulampalam, V. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. Am. J Physiol. Gastrointest. Liver Physiol. 2013, 304, G1025–G1037. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Demigné, C.; Morand, C.; Levrat, M.A.; Besson, C.; Moundras, C.; Rémésy, C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [Google Scholar] [CrossRef]
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D.J. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Hamamah, S.; Covasa, M. Gut Microbiota Restores Central Neuropeptide Deficits in Germ-Free Mice. Int. J. Mol. Sci. 2022, 23, 11756. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Zhou, T.; Wang, X.A.; Tong, X.H.; Ding, J.W. Histone deacetylases and atherosclerosis. Atherosclerosis 2015, 240, 355–366. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Wada, E.; Koyanagi, S.; Kusunose, N.; Akamine, T.; Masui, H.; Hashimoto, H.; Matsunaga, N.; Ohdo, S. Modulation of peroxisome proliferator-activated receptor-α activity by bile acids causes circadian changes in the intestinal expression of Octn1/Slc22a4 in mice. Mol. Pharmacol. 2015, 87, 314–322. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Mechanism of Butyrate Stimulation of Triglyceride Storage and Adipokine Expression during Adipogenic Differentiation of Porcine Stromovascular Cells. PLoS ONE 2015, 10, e0145940. [Google Scholar] [CrossRef] [PubMed]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A Necessary but Harmful Strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef] [PubMed]
- Arkenberg, A.; Runkel, S.; Richardson, D.J.; Rowley, G. The production and detoxification of a potent cytotoxin, nitric oxide, by pathogenic enteric bacteria. Biochem. Soc. Trans. 2011, 39, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hao, Z.; Zhou, W.; Zhang, S.; Sun, M.; Li, H.; Hou, N.; Jing, C.; Zhao, M. Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPAR-γ signaling based on network pharmacology and experimental validation. Bioengineered 2021, 12, 4887–4898. [Google Scholar] [CrossRef]
- Hiben, M.G.; de Haan, L.; Spenkelink, B.; Wesseling, S.; Vervoort, J.; Rietjens, I. Induction of peroxisome proliferator activated receptor γ (PPARγ) mediated gene expression and inhibition of induced nitric oxide production by Maerua subcordata (Gilg) DeWolf. BMC Complement. Med. Ther. 2020, 20, 80. [Google Scholar] [CrossRef]
- Tian, Q.; Leung, F.P.; Chen, F.M.; Tian, X.Y.; Chen, Z.; Tse, G.; Ma, S.; Wong, W.T. Butyrate protects endothelial function through PPARδ/miR-181b signaling. Pharmacol. Res. 2021, 169, 105681. [Google Scholar] [CrossRef]
- Alvaro, A.; Solà, R.; Rosales, R.; Ribalta, J.; Anguera, A.; Masana, L.; Vallvé, J.C. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life 2008, 60, 757–764. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.H.; Yeung, C.K.; Peter, R.M.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.M.; Rettie, A.E. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Hernandez, D.; Janmohamed, A.; Chandan, P.; Phillips, I.R.; Shephard, E.A. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: Identification of novel gene and pseudogene clusters. Pharmacogenetics 2004, 14, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.C.; Buffa, J.A.; Org, E.; Wang, Z.; Levison, B.S.; Zhu, W.; Wagner, M.A.; Bennett, B.J.; Li, L.; DiDonato, J.A.; et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 2015, 290, 5647–5660. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Ress, S. Antineutrophil cytoplasmic auto-antibodies in the diagnosis and management of vasculitis. S. Afr. Med. J. 1989, 75, 41. [Google Scholar] [PubMed]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(−/−) transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Vales, C.; Lee, F.Y.; Lee, H.; Lusis, A.J.; Edwards, P.A. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2316–2321. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Fonseca, V.A. Bile acids and metabolic regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care 2009, 32 (Suppl. 2), S237–S245. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e1126. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Maruyama, T.; Tanaka, K.; Suzuki, J.; Miyoshi, H.; Harada, N.; Nakamura, T.; Miyamoto, Y.; Kanatani, A.; Tamai, Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol. 2006, 191, 197–205. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Labbé, A.; Ganopolsky, J.G.; Prakash, S.; Jones, M.L. Changes in bile acids, FGF-19 and sterol absorption in response to bile salt hydrolase active L. reuteri NCIMB 30242. Gut Microbes 2015, 6, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.; Hofmann, T.; Ahnis, A.; Elbelt, U.; Goebel-Stengel, M.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front. Neurosci. 2015, 9, 199. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Camastra, S.; Nannipieri, M.; Astiarraga, B.; Castro-Perez, J.; Xie, D.; Wang, L.; Chakravarthy, M.; Ferrannini, E. Increased Bile Acid Synthesis and Impaired Bile Acid Transport in Human Obesity. J. Clin. Endocrinol. Metab. 2016, 101, 1935–1944. [Google Scholar] [CrossRef]
- Pathak, P.; Chiang, J.Y.L. Sterol 12α-Hydroxylase Aggravates Dyslipidemia by Activating the Ceramide/mTORC1/SREBP-1C Pathway via FGF21 and FGF15. Gene Expr. 2019, 19, 161–173. [Google Scholar] [CrossRef]
- Bertaggia, E.; Jensen, K.K.; Castro-Perez, J.; Xu, Y.; Di Paolo, G.; Chan, R.B.; Wang, L.; Haeusler, R.A. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E121–E133. [Google Scholar] [CrossRef]
- Semova, I.; Levenson, A.E.; Krawczyk, J.; Bullock, K.; Gearing, M.E.; Ling, A.V.; Williams, K.A.; Miao, J.; Adamson, S.S.; Shin, D.J.; et al. Insulin Prevents Hypercholesterolemia by Suppressing 12α-Hydroxylated Bile Acids. Circulation 2022, 145, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e246. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Li, L.; Schwabacher, A.W.; Young, J.W.; Beitz, D.C. Mechanism of cholesterol reduction to coprostanol by Eubacterium coprostanoligenes ATCC 51222. Steroids 1996, 61, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.H.; O’Flaherty, S.; Allen, G.; Rivera, A.J.; Stewart, A.K.; Barrangou, R.; Theriot, C.M. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl. Acad. Sci. USA 2021, 118, e2017709118. [Google Scholar] [CrossRef]
- Long, S.L.; Gahan, C.G.M.; Joyce, S.A. Interactions between gut bacteria and bile in health and disease. Mol. Aspects Med. 2017, 56, 54–65. [Google Scholar] [CrossRef]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.B.; Ducroc, R.; Regnault, B.; Kun Tan, C.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef]
- Määttä, A.M.; Salminen, A.; Pietiäinen, M.; Leskelä, J.; Palviainen, T.; Sattler, W.; Sinisalo, J.; Salomaa, V.; Kaprio, J.; Pussinen, P.J. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. 2021, 27, 3–14. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Xu, R.; Li, S.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PLoS ONE 2014, 9, e115148. [Google Scholar] [CrossRef]
- Haneklaus, M.; O’Neill, L.A. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev. 2015, 265, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chi, Z.; Jiang, D.; Xu, T.; Yu, W.; Wang, Z.; Chen, S.; Zhang, L.; Liu, Q.; Guo, X.; et al. Cholesterol Homeostatic Regulator SCAP-SREBP2 Integrates NLRP3 Inflammasome Activation and Cholesterol Biosynthetic Signaling in Macrophages. Immunity 2018, 49, 842–856.e847. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Peng, M.; Xu, P.; Huang, F.; Xie, Y.; Li, J.; Hong, Y.; Guo, H.; Liu, Q.; Zhu, W. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J. Neuroinflammation 2020, 17, 330. [Google Scholar] [CrossRef]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Lopez-Moreno, J.; Garcia-Carpintero, S.; Gomez-Delgado, F.; Jimenez-Lucena, R.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Yubero-Serrano, E.M.; Malagon, M.M.; et al. Endotoxemia is modulated by quantity and quality of dietary fat in older adults. Exp. Gerontol. 2018, 109, 119–125. [Google Scholar] [CrossRef]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Juste, C.; Gérard, P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms 2021, 9, 1881. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Zhang, S.; Bao, L.; Chen, B.; Gong, H.; Zhao, Y.; Qi, R. Resveratrol Confers Vascular Protection by Suppressing TLR4/Syk/NLRP3 Signaling in Oxidized Low-Density Lipoprotein-Activated Platelets. Oxid. Med. Cell. Longev. 2021, 2021, 8819231. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, Y.; Wang, D.; Zhao, W.; Hu, X.; Chen, F.; Zhao, X. Protective Effects of Dietary Resveratrol against Chronic Low-Grade Inflammation Mediated through the Gut Microbiota in High-Fat Diet Mice. Nutrients 2022, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Ranaivo, H.; Thirion, F.; Béra-Maillet, C.; Guilly, S.; Simon, C.; Sothier, M.; Van Den Berghe, L.; Feugier-Favier, N.; Lambert-Porcheron, S.; Dussous, I.; et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 2022, 14, 2044722. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Sun, L.; Liu, X.; Zeng, R.; Lin, X.; Li, Y. Effects of gut microbiota and fatty acid metabolism on dyslipidemia following weight-loss diets in women: Results from a randomized controlled trial. Clin. Nutr. 2021, 40, 5511–5520. [Google Scholar] [CrossRef]
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 2021, 476, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.R.X.; Park, M.A.; Wong, L.H.; Haldar, S.; Lim, K.J.; Nagarajan, N.; Henry, C.J.; Jiang, Y.R.; Moskvin, O.V. Gut microbiome responses to dietary intervention with hypocholesterolemic vegetable oils. NPJ Biofilms Microbiomes 2022, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Maukonen, J.; Makinen, M.; Niemi, P.; Niiranen, L.; Hibberd, A.A.; Poutanen, K.; Buchert, J.; Herzig, K.H. Hypocholesterolemic Effect of the Lignin-Rich Insoluble Residue of Brewer’s Spent Grain in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 1104–1114. [Google Scholar] [CrossRef]
- Xu, D.; Pan, D.; Liu, H.; Yang, C.; Yang, X.; Wang, X.; Liu, F.; Feng, M.; Wu, Q.; Shen, Y.; et al. Improvement in cardiometabolic risk markers following an oatmeal diet is associated with gut microbiota in mildly hypercholesterolemic individuals. Food Res. Int. 2022, 160, 111701. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef]
- Sun, N.X.; Tong, L.T.; Liang, T.T.; Wang, L.L.; Liu, L.Y.; Zhou, X.R.; Zhou, S.M. Effect of Oat and Tartary Buckwheat—Based Food on Cholesterol—Lowering and Gut Microbiota in Hypercholesterolemic Hamsters. J. Oleo Sci. 2019, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nagasawa, M.; Omae, N.; Tsunoda, M.; Ishiyama, J.; Ide, T.; Akasaka, Y.; Murakami, K. A novel JNK2/SREBP-1c pathway involved in insulin-induced fatty acid synthesis in human adipocytes. J. Lipid Res. 2013, 54, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Zhao, X.; Zhou, R.; Liu, H.; Sun, Y.; Fan, Y.; Shi, Y.; Qiao, S.; Liu, S.; et al. Multi-omics study reveals that statin therapy is associated with restoration of gut microbiota homeostasis and improvement in outcomes in patients with acute coronary syndrome. Theranostics 2021, 11, 5778–5793. [Google Scholar] [CrossRef]
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D.; et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 2020, 581, 310–315. [Google Scholar] [CrossRef]
- Sun, B.; Li, L.; Zhou, X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J. Microbiol. 2018, 56, 886–892. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Yue, Y.; Wu, X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int. J. Mol. Sci. 2022, 23, 5935. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Chen, Y.; Wang, Q.; Yan, L.; Wang, R.; Wei, Y.; You, Z.; Li, Y.; Miao, Q.; et al. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary Cholangitis. Gut Microbes 2021, 13, 1946366. [Google Scholar] [CrossRef]
- Jena, P.K.; Setayesh, T.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Wan, Y.Y. Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline. Cells 2022, 11, 504. [Google Scholar] [CrossRef]
- Lin, S.H.; Cheng, P.C.; Tu, S.T.; Hsu, S.R.; Cheng, Y.C.; Liu, Y.H. Effect of metformin monotherapy on serum lipid profile in statin-naïve individuals with newly diagnosed type 2 diabetes mellitus: A cohort study. PeerJ 2018, 6, e4578. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu , S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-e17. [Google Scholar]
- Ermolenko, E.; Simanenkova, A.; Voropaeva, L.; Lavrenova, N.; Kotyleva, M.; Minasian, S.; Chernikova, A.; Timkina, N.; Gladyshev, N.; Dmitriev, A.; et al. Metformin Influence on the Intestinal Microbiota and Organism of Rats with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6837. [Google Scholar] [CrossRef] [PubMed]
- Basavaiah, R.; Gurudutt, P.S. Prebiotic Carbohydrates for Therapeutics. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell. Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef]
- Ms Wolever, T.; Rahn, M.; Dioum, E.; Spruill, S.E.; Ezatagha, A.; Campbell, J.E.; Jenkins, A.L.; Chu, Y. An Oat β-Glucan Beverage Reduces LDL Cholesterol and Cardiovascular Disease Risk in Men and Women with Borderline High Cholesterol: A Double-Blind, Randomized, Controlled Clinical Trial. J. Nutr. 2021, 151, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of soluble fiber from oats on lipid levels of Asian Indians—A randomized controlled, parallel arm study. Lipids Health Dis. 2017, 16, 71. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H.; Chen, S.N. Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Gudej, S.; Filip, R.; Harasym, J.; Wilczak, J.; Dziendzikowska, K.; Oczkowski, M.; Jałosińska, M.; Juszczak, M.; Lange, E.; Gromadzka-Ostrowska, J. Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients. Nutrients 2021, 13, 2791. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar]
- Jovanovski, E.; Yashpal, S.; Komishon, A.; Zurbau, A.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Sievenpiper, J.; Duvnjak, L.; Vuksan, V. Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 922–932. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef]
- Deng, Z.; Meng, C.; Huang, H.; Song, S.; Fu, L.; Fu, Z. The different effects of psyllium husk and orlistat on weight control, the amelioration of hypercholesterolemia and non-alcohol fatty liver disease in obese mice induced by a high-fat diet. Food Funct. 2022, 13, 8829–8849. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-type Fructans: A Systematic Review. Adv. Nutr. 2021, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Rowan, F.E.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br. J. Surg. 2009, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Roach, L.A.; Meyer, B.J.; Fitton, J.H.; Winberg, P. Improved Plasma Lipids, Anti-Inflammatory Activity, and Microbiome Shifts in Overweight Participants: Two Clinical Studies on Oral Supplementation with Algal Sulfated Polysaccharide. Mar. Drugs 2022, 20, 500. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr. 2019, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.C.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018, 101, 10807–10818. [Google Scholar] [CrossRef]
- Wang, K.; Yu, X.; Li, Y.; Guo, Y.; Ge, L.; Pu, F.; Ma, X.; Cui, W.; Marrota, F.; He, F.; et al. Bifidobacterium bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob. Proteins 2019, 11, 1182–1194. [Google Scholar] [CrossRef]
- Chiu, H.F.; Fang, C.Y.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Efficacy of Probiotic Milk Formula on Blood Lipid and Intestinal Function in Mild Hypercholesterolemic Volunteers: A Placebo-control, Randomized Clinical Trial. Probiotics Antimicrob. Proteins 2021, 13, 624–632. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Childs, C.E.; Röytiö, H.; Alhoniemi, E.; Fekete, A.A.; Forssten, S.D.; Hudjec, N.; Lim, Y.N.; Steger, C.J.; Yaqoob, P.; Tuohy, K.M.; et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: A double-blind, placebo-controlled, randomised, factorial cross-over study. Br. J. Nutr. 2014, 111, 1945–1956. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Liu, H.; Wei, X.; Su, X.; Li, M.; Yuan, J. Oral Supplements of Combined Bacillus licheniformis Zhengchangsheng® and Xylooligosaccharides Improve High-Fat Diet-Induced Obesity and Modulate the Gut Microbiota in Rats. BioMed Res. Int. 2020, 2020, 9067821. [Google Scholar] [CrossRef]
- Cheng, C.K.; Wang, C.; Shang, W.; Lau, C.W.; Luo, J.Y.; Wang, L.; Huang, Y. A high methionine and low folate diet alters glucose homeostasis and gut microbiome. Biochem. Biophys. Rep. 2021, 25, 100921. [Google Scholar] [CrossRef]
- Ding, C.; Bi, C.; Lin, T.; Hu, L.; Huang, X.; Liu, L.; Liu, C.; Song, Y.; Tang, G.; Wang, B.; et al. Serum folate modified the association between low-density lipoprotein cholesterol and carotid intima-media thickness in Chinese hypertensive adults. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2303–2311. [Google Scholar] [CrossRef]
- Chang, F.Y.; Siuti, P.; Laurent, S.; Williams, T.; Glassey, E.; Sailer, A.W.; Gordon, D.B.; Hemmerle, H.; Voigt, C.A. Gut-inhabiting Clostridia build human GPCR ligands by conjugating neurotransmitters with diet- and human-derived fatty acids. Nat. Microbiol. 2021, 6, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863.e852. [Google Scholar] [CrossRef]
- López-Montoya, P.; Cerqueda-García, D.; Rodríguez-Flores, M.; López-Contreras, B.; Villamil-Ramírez, H.; Morán-Ramos, S.; Molina-Cruz, S.; Rivera-Paredez, B.; Antuna-Puente, B.; Velázquez-Cruz, R.; et al. Association of Gut Microbiota with Atherogenic Dyslipidemia, and Its Impact on Serum Lipid Levels after Bariatric Surgery. Nutrients 2022, 14, 3545. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, J.; Sun, L.; Lyu, X.; Chang, X.Y.; Mi, X.; Hu, M.G.; Wu, C.; Chen, X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021, 133, 111014. [Google Scholar] [CrossRef]
- Zhou, Q.; Pang, G.; Zhang, Z.; Yuan, H.; Chen, C.; Zhang, N.; Yang, Z.; Sun, L. Association Between Gut Akkermansia and Metabolic Syndrome is Dose-Dependent and Affected by Microbial Interactions: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2021, 14, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.J.; Yoon, J.Y.; Kemmitt, J.; Wright, C.; Schneider, K.; Sphabmixay, P.; Hernandez-Gordillo, V.; Holcomb, S.J.; Bhushan, B.; et al. Primary human colonic mucosal barrier crosstalk with super oxygen-sensitive Faecalibacterium prausnitzii in continuous culture. Med 2021, 2, 74–98.e79. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef]
- Corfield, A.P. The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human. Microorganisms 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, L.; De Siena, B.; Marasco, R. Lactobacillus Cell Surface Proteins Involved in Interaction with Mucus and Extracellular Matrix Components. Curr. Microbiol. 2020, 77, 3831–3841. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Chan, C.W.; Chan, P.H.; Lin, B.F. Folate Deficiency Increased Lipid Accumulation and Leptin Production of Adipocytes. Front. Nutr. 2022, 9, 852451. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, Y.; Lima, R.P.A.; Luna, R.C.P.; Monteiro, M.; da Silva, C.S.O.; do Nascimento, R.A.F.; de Farias Lima, K.Q.; Andrade, E.S.A.H.; de Lima Ferreira, F.E.L.; de Toledo Vianna, R.P.; et al. Decrease of the DNA methylation levels of the ADRB3 gene in leukocytes is related with serum folate in eutrophic adults. J. Transl. Med. 2018, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Gheorghita, R.; Lobiuc, A.; Sirbu, I.O.; Covasa, M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front. Med. 2022, 9, 1060581. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S23–S31. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; de Vos, W.M.; Nieuwdorp, M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. 2021, 33, 1098–1110. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Flores-López, A.G.; Aguilar-López, M.; Sánchez-Tapia, M.; Medina-Vera, I.; Díaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Brodmann, T.; Endo, A.; Gueimonde, M.; Vinderola, G.; Kneifel, W.; de Vos, W.M.; Salminen, S.; Gómez-Gallego, C. Safety of Novel Microbes for Human Consumption: Practical Examples of Assessment in the European Union. Front. Microbiol. 2017, 8, 1725. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Abdeen, S.K.; Kolodziejczyk, A.A.; Elinav, E. Commensal inter-bacterial interactions shaping the microbiota. Curr. Opin. Microbiol. 2021, 63, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Zhao, J.; Meng, F.; Yao, C.; Bao, E.; Sun, N.; Chen, X.; Cheng, W.; Hua, H.; et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics 2022, 12, 976–998. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flaig, B.; Garza, R.; Singh, B.; Hamamah, S.; Covasa, M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients 2023, 15, 228. https://doi.org/10.3390/nu15010228
Flaig B, Garza R, Singh B, Hamamah S, Covasa M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients. 2023; 15(1):228. https://doi.org/10.3390/nu15010228
Chicago/Turabian StyleFlaig, Brandon, Rachel Garza, Bhavdeep Singh, Sevag Hamamah, and Mihai Covasa. 2023. "Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota" Nutrients 15, no. 1: 228. https://doi.org/10.3390/nu15010228
APA StyleFlaig, B., Garza, R., Singh, B., Hamamah, S., & Covasa, M. (2023). Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients, 15(1), 228. https://doi.org/10.3390/nu15010228






