Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid
Abstract
1. Introduction
2. Oleic Acid and Immune Cells
2.1. Oleic Acid and Signal Transduction Mechanisms
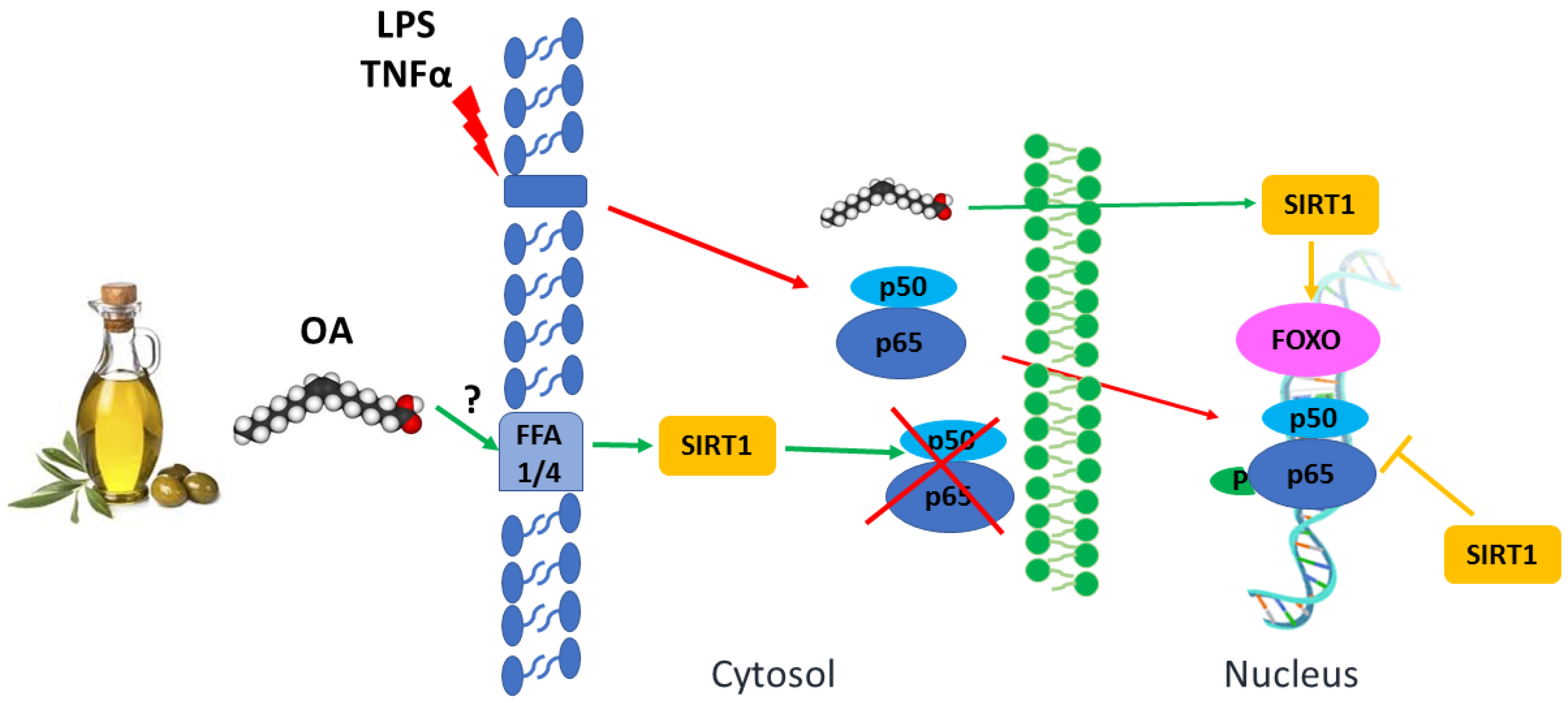
2.1.1. Oleic Acid and Cellular Membranes
2.1.2. Oleic Acid and Cytoplasmatic Signaling Pathways
2.1.3. Oleic Acid and Nuclear Receptors
3. Oleoylethanolamide
4. Oleic Acid and Epigenetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Daëron, M. The Immune System as a System of Relations. Front. Immunol. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, M. A Wellness Approach. Psychiatr. Rehabil. J. 2006, 29, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Canudas, S.; Becerra-Tomas, N.; Hernandez-Alonso, P.; Galie, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, Y.; Meinila, J.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Lăcătușu, C.M.; Grigorescu, E.D.; Floria, M.; Onofriescu, A.; Mihai, B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef]
- Marcelino, G.; Aiko Hiane, P.; de Cássia Freitas, K.; Figueiredo Santana, L.; Pott, A.; Rodrigues Donadon, J.; de Cássia Avellaneda Guimarães, R. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Tomic, I.J.; Krnic, M.; Kuriir, T.T.; Bozic, J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients 2022, 14, 757. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Truong, T.; Ly, G.; Yun, J.; Lee, D.-H.; Chung, J.-S.; Kwon, S.-M.; García, M. Protective Effects and Benefits of Olive Oil and Its Extracts on Women’s Health. Nutrients 2021, 13, 4279. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Naranjo, M.C.; Millan-Linares, M.C.; Lopez, S.; Abia, R.; Biessen, E.A.L.; Muriana, F.J.G.; Bermudez, B. Monounsaturated Fatty Acids in a High-Fat Diet and Niacin Protect from White Fat Dysfunction in the Metabolic Syndrome. Mol. Nutr. Food Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Millan-Linares, M.C.; Naranjo, M.C.; Toscano, R.; Abia, R.; Muriana, F.J.G.; Bermudez, B.; Montserrat-De La Paz, S. Minor Compounds from Virgin Olive Oil Attenuate LPS-Induced Inflammation via Visfatin-Related Gene Modulation on Primary Human Monocytes. J. Food Biochem. 2019, 43, 12941–12955. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J.G. Membrane Composition and Dynamics: A Target of Bioactive Virgin Olive Oil Constituents. Biochim. Biophys. Acta 2014, 1838, 1638–1656. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Almanza-Aguilera, E.; Hernáez, Á.; Agustí, N.; Julve, J.; Fitó, M.; Castañer, O. Beneficial Effects of Olive Oil and Mediterranean Diet on Cancer Physio-Pathology and Incidence. Semin. Cancer Biol. 2021, 73, 178–195. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Mougan, I. Fatty Acid Composition of Human Brain Phospholipids During Normal Development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Hamazaki, K.; Hamazaki, T.; Inadera, H. Fatty Acid Composition in the Postmortem Amygdala of Patients with Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. J. Psychiatr. Res. 2012, 46, 1024–1028. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic Acid Protects against Cadmium Induced Cardiac and Hepatic Tissue Injury in Male Wistar Rats: A Mechanistic Study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Zhang, Y.; Yang, P.; Zong, Y.; Qu, S.; Liu, Z. Oleic Acid Decreases the Expression of a Cholesterol Transport-Related Protein (NPC1L1) by the Induction of Endoplasmic Reticulum Stress in CaCo-2 Cells. J. Physiol. Biochem. 2011, 67, 153–163. [Google Scholar] [CrossRef]
- Yang, Z.H.; Nill, K.; Takechi-Haraya, Y.; Playford, M.P.; Nguyen, D.; Yu, Z.X.; Pryor, M.; Tang, J.; Rojulpote, K.V.; Mehta, N.N.; et al. Differential Effect of Dietary Supplementation with a Soybean Oil Enriched in Oleic Acid versus Linoleic Acid on Plasma Lipids and Atherosclerosis in LDLR-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 8385. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia, M.D.M.; Alonso-Torre, S.R. Antitumor Effect of Oleic Acid; Mechanisms of Action: A Review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Kim, H.; Yoon, K.S.; Choe, W.; Kang, I. Oleic Acid Reduces Lipopolysaccharide-Induced Expression of INOS and COX-2 in BV2 Murine Microglial Cells: Possible Involvement of Reactive Oxygen Species, P38 MAPK, and IKK/NF-KappaB Signaling Pathways. Neurosci. Lett. 2009, 464, 93–97. [Google Scholar] [CrossRef]
- Harvey, K.A.; Walker, C.L.; Xu, Z.; Whitley, P.; Pavlina, T.M.; Hise, M.; Zaloga, G.P.; Siddiqui, R.A. Oleic Acid Inhibits Stearic Acid-Induced Inhibition of Cell Growth and pro-Inflammatory Responses in Human Aortic Endothelial Cells. J. Lipid Res. 2010, 51, 3470–3480. [Google Scholar] [CrossRef]
- Lamers, D.; Schlich, R.; Greulich, S.; Sasson, S.; Sell, H.; Eckel, J. Oleic Acid and Adipokines Synergize in Inducing Proliferation and Inflammatory Signalling in Human Vascular Smooth Muscle Cells. J. Cell. Mol. Med. 2011, 15, 1177–1188. [Google Scholar] [CrossRef]
- Greene, E.L.; Lu, G.; Zhang, D.; Egan, B.M. Signaling Events Mediating the Additive Effects of Oleic Acid and Angiotensin II on Vascular Smooth Muscle Cell Migration. Hypertension 2001, 37, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Nahuelpan, C.; Manosalva, C.; Jara, E.; Carretta, M.D.; Conejeros, I.; Loaiza, A.; Chihuailaf, R.; Burgos, R.A. Oleic Acid Induces Intracellular Calcium Mobilization, MAPK Phosphorylation, Superoxide Production and Granule Release in Bovine Neutrophils. Biochem. Biophys. Res. Commun. 2011, 409, 280–286. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Reyes-Quiroz, M.E.; Alba, G.; Saenz, J.; Santa-María, C.; Geniz, I.; Jiménez, J.; Ramírez, R.; Martín-Nieto, J.; Pintado, E.; Sobrino, F. Oleic Acid Modulates MRNA Expression of Liver X Receptor (LXR) and Its Target Genes ABCA1 and SREBP1c in Human Neutrophils. Eur. J. Nutr. 2014, 53, 1707–1717. [Google Scholar] [CrossRef]
- Alvarez, E.; Santa Maria, C. Influence of the Age and Sex on Respiratory Burst of Human Monocytes. Mech. Ageing Dev. 1996, 90, 157–161. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P. Monounsaturated Fatty Acids and Immune Function. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Speizer, L.A.; Watson, M.J.; Brunton, L.L. Differential Effects of Omega-3 Fish Oils on Protein Kinase Activities in Vitro. Am. J. Physiol. 1991, 261, 109–114. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The Role of Virgin Olive Oil Components in the Modulation of Endothelial Function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Cho, H.J.; Wu, Y.; Wright, N.T.; Sum, A.K.; Chan, C. The Role of Fatty Acid Unsaturation in Minimizing Biophysical Changes on the Structure and Local Effects of Bilayer Membranes. Biochim. Biophys. Acta 2009, 1788, 1508–1516. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P.; Harvey, D.J.; Watts, A.; Newsholme, E.A. Incorporation of Fatty Acids by Concanavalin A-Stimulated Lymphocytes and the Effect on Fatty Acid Composition and Membrane Fluidity. Biochem. J. 1994, 300, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J. The Protective Effect of the Mediterranean Diet: Focus on Cancer and Cardiovascular Risk. Med. Princ. Pract. 2011, 20, 103–111. [Google Scholar] [CrossRef]
- Alvarez, E.; Ruiz-Gutiérrez, V.; Sobrino, F.; Santa-María, C. Age-Related Changes in Membrane Lipid Composition, Fluidity and Respiratory Burst in Rat Peritoneal Neutrophils. Clin. Exp. Immunol. 2001, 124, 95–102. [Google Scholar] [CrossRef]
- Alvarez, E.; Ruiz-Gutiérrez, V.; Santa María, C.; Machado, A. Age-Dependent Modification of Lipid Composition and Lipid Structural Order Parameter of Rat Peritoneal Macrophage Membranes. Mech. Ageing Dev. 1993, 71, 1–12. [Google Scholar] [CrossRef]
- Alvarez, E.; Conde, M.; Machado, A.; Sobrino, F.; Santa Maria, C. Decrease in Free-Radical Production with Age in Rat Peritoneal Macrophages. Biochem. J. 1995, 312, 555–560. [Google Scholar] [CrossRef]
- Carrillo, C.; Del Mar Cavia, M.; Roelofs, H.; Wanten, G.; Alonso-Torre, S.R. Activation of Human Neutrophils by Oleic Acid Involves the Production of Reactive Oxygen Species and a Rise in Cytosolic Calcium Concentration: A Comparison with N-6 Polyunsaturated Fatty Acids. Cell. Physiol. Biochem. 2011, 28, 329–338. [Google Scholar] [CrossRef]
- Manosalva, C.; Mena, J.; Velasquez, Z.; Colenso, C.K.; Brauchi, S.; Burgos, R.A.; Hidalgo, M.A. Cloning, Identification and Functional Characterization of Bovine Free Fatty Acid Receptor-1 (FFAR1/GPR40) in Neutrophils. PLoS ONE 2015, 10, e0119715. [Google Scholar] [CrossRef]
- Mena, S.J.; Manosalva, C.; Carretta, M.D.; Teuber, S.; Olmo, I.; Burgos, R.A.; Hidalgo, M.A. Differential Free Fatty Acid Receptor-1 (FFAR1/GPR40) Signalling Is Associated with Gene Expression or Gelatinase Granule Release in Bovine Neutrophils. Innate Immun. 2016, 22, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zeng, M.; Wang, Y.; Li, M.; Wu, Y.; Xu, R.; Zhang, Q.; Jia, J.; Huang, Y.; Zheng, X.; et al. Oleic Acid Alleviates LPS-Induced Acute Kidney Injury by Restraining Inflammation and Oxidative Stress via the Ras/MAPKs/PPAR-γ Signaling Pathway. Phytomedicine 2022, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gorjão, R.; Cury-Boaventura, M.F.; De Lima, T.M.; Curi, R. Regulation of Human Lymphocyte Proliferation by Fatty Acids. Cell Biochem. Funct. 2007, 25, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Verlengia, R.; Gorjão, R.; Kanunfre, C.C.; Bordin, S.; De Lima, T.M.; Curi, R. Effect of Arachidonic Acid on Proliferation, Cytokines Production and Pleiotropic Genes Expression in Jurkat Cells—A Comparison with Oleic Acid. Life Sci. 2003, 73, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, Y.M.; Lee, K.H.; Jeong, S.W.; Kwon, O.J. Oleate Protects Macrophages from Palmitate-Induced Apoptosis through the Downregulation of CD36 Expression. Biochem. Biophys. Res. Commun. 2017, 488, 477–482. [Google Scholar] [CrossRef]
- Huang, Z.H.; Gu, D.S.; Mazzone, T. Oleic Acid Modulates the Post-Translational Glycosylation of Macrophage ApoE to Increase Its Secretion. J. Biol. Chem. 2004, 279, 29195–29201. [Google Scholar] [CrossRef] [PubMed]
- Charlet, R.; Le Danvic, C.; Sendid, B.; Nagnan-Le Meillour, P.; Jawhara, S. Oleic Acid and Palmitic Acid from Bacteroides Thetaiotaomicron and Lactobacillus Johnsonii Exhibit Anti-Inflammatory and Antifungal Properties. Microorganisms 2022, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, D.; Bossila, E.A.; Zhang, Z.; Li, S.; Bao, J.; Xu, H.; Zhang, L.; Zhao, Y. FABP5 Deficiency Impaired Macrophage Inflammation by Regulating AMPK/NF-ΚB Signaling Pathway. J. Immunol. 2022, 209, 1–14. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, Y.C. Anti-Inflammatory Effects of Cicadidae Periostracum Extract and Oleic Acid through Inhibiting Inflammatory Chemokines Using PCR Arrays in LPS-Induced Lung Inflammation In Vitro. Life 2022, 12, 857. [Google Scholar] [CrossRef]
- Müller, A.K.; Albrecht, F.; Rohrer, C.; Koeberle, A.; Werz, O.; Schlörmann, W.; Glei, M.; Lorkowski, S.; Wallert, M. Olive Oil Extracts and Oleic Acid Attenuate the LPS-Induced Inflammatory Response in Murine RAW264.7 Macrophages but Induce the Release of Prostaglandin E2. Nutrients 2021, 13, 4437. [Google Scholar] [CrossRef]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Parks, D.J.; Blanchard, S.G.; Brown, P.J.; Sternbach, D.D.; Lehmann, J.M.; Wisely, G.B.; Willson, T.M.; et al. Molecular Recognition of Fatty Acids by Peroxisome Proliferator-Activated Receptors. Mol. Cell 1999, 3, 397–403. [Google Scholar] [CrossRef]
- Carrillo, C.; Giraldo, M.; Cavia, M.M.; Alonso-Torre, S.R. Effect of Oleic Acid on Store-Operated Calcium Entry in Immune-Competent Cells. Eur. J. Nutr. 2017, 56, 1077–1084. [Google Scholar] [CrossRef]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic Acid Stimulates Complete Oxidation of Fatty Acids through Protein Kinase A-Dependent Activation of SIRT1-PGC1 Complex*. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dangat, K.; Kale, A.; Sable, P.; Chavan-Gautam, P.; Joshi, S. Effects of Altered Maternal Folic Acid, Vitamin B12 and Docosahexaenoic Acid on Placental Global DNA Methylation Patterns in Wistar Rats. PLoS ONE 2011, 6, e17706. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, X.; Jia, L.; Mondal, A.K.; Diallo, A.; Hawkins, G.A.; Das, S.K.; Parks, J.S.; Yu, L.; Shi, H.; et al. Inhibiting DNA Methylation by 5-Aza-2’-Deoxycytidine Ameliorates Atherosclerosis through Suppressing Macrophage Inflammation. Endocrinology 2014, 155, 4925–4938. [Google Scholar] [CrossRef]
- Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; Koyama, Y.; Sato-Tomita, A.; Matsuzaka, T.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 744–756. [Google Scholar] [CrossRef]
- Yang, Q.B.; He, Y.L.; Zhong, X.W.; Xie, W.G.; Zhou, J.G. Resveratrol Ameliorates Gouty Inflammation via Upregulation of Sirtuin 1 to Promote Autophagy in Gout Patients. Inflammopharmacology 2019, 27, 47–56. [Google Scholar] [CrossRef]
- Layrolle, P.; Payoux, P.; Chavanas, S. PPAR Gamma and Viral Infections of the Brain. Int. J. Mol. Sci. 2021, 22, 8876. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Correction to: Oleic Acid Ameliorates Palmitic Acid Induced Hepatocellular Lipotoxicity by Inhibition of ER Stress and Pyroptosis. Nutr. Metab. 2020, 17, 1–18. [Google Scholar] [CrossRef]
- Gao, R.; Ma, Z.; Hu, Y.; Chen, J.; Shetty, S.; Fu, J. Sirt1 Restrains Lung Inflammasome Activation in a Murine Model of Sepsis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L847–L853. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, S.; Pandey, S.; Akhtar, M.S.; Rumman, M.; Singh, B.; Mahdi, A.A. SIRT1 Mediates Neuroprotective and Neurorescue Effects of Camel α-Lactalbumin and Oleic Acid Complex on Rotenone-Induced Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 1263–1272. [Google Scholar] [CrossRef]
- Camell, C.; Smith, C.W. Dietary Oleic Acid Increases M2 Macrophages in the Mesenteric Adipose Tissue. PLoS ONE 2013, 8, e75147. [Google Scholar] [CrossRef]
- Kaneshiro, K.R.; Egelhofer, T.A.; Rechtsteiner, A.; Cockrum, C.; Strome, S. Sperm-Inherited H3K27me3 Epialleles Are Transmitted Transgenerationally in Cis. Proc. Natl. Acad. Sci. USA 2022, 119, 1–14. [Google Scholar] [CrossRef] [PubMed]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 1–18. [Google Scholar] [CrossRef]
- Hong, C.; Bradley, M.N.; Rong, X.; Wang, X.; Wagner, A.; Grijalva, V.; Castellani, L.W.; Salazar, J.; Realegeno, S.; Boyadjian, R.; et al. LXRα Is Uniquely Required for Maximal Reverse Cholesterol Transport and Atheroprotection in ApoE-Deficient Mice. J. Lipid Res. 2012, 53, 1126–1133. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kwekkeboom, J.C.; Westhoff, S.; Giera, M.; Rombouts, Y.; van Harmelen, V.; Huizinga, T.W.J.; Deelder, A.; Kloppenburg, M.; Toes, R.E.M. Adipocyte-Derived Lipids Modulate CD4+ T-Cell Function. Eur. J. Immunol. 2013, 43, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Pompura, S.L.; Wagner, A.; Kitz, A.; LaPerche, J.; Yosef, N.; Dominguez-Villar, M.; Hafler, D.A. Oleic Acid Restores Suppressive Defects in Tissue-Resident FOXP3 Tregs from Patients with Multiple Sclerosis. J. Clin. Investig. 2021, 131, 1–15. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental Defects and P53 Hyperacetylation in Sir2 Homolog (SIRT1)-Deficient Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef]
- Yang, H.; Bi, Y.J.; Xue, L.X.; Wang, J.; Lu, Y.; Zhang, Z.G.; Chen, X.; Chu, Y.; Yang, R.; Wang, R.; et al. Multifaceted Modulation of SIRT1 in Cancer and Inflammation. Crit. Rev. Oncog. 2015, 20, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Chen, X.; Luo, R.; Li, L.; Wang, C.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic Acid Protects Insulin-Secreting INS-1E Cells against Palmitic Acid-Induced Lipotoxicity along with an Amelioration of ER Stress. Endocrine 2019, 64, 512–524. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-Regulation of the Inflammatory Response by Peroxisome Proliferator-Activated Receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Medeiros-De-Moraes, I.M.; Gonçalves-De-Albuquerque, C.F.; Kurz, A.R.M.; De Jesus Oliveira, F.M.; Pereira de Abreu, V.H.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, Y.-S.; Lee, D.H.; Lee, H.; Jin Park, H.; Lee, D.; Kim, H. Neuroprotective Effects of Oleic Acid in Rodent Models of Cerebral Ischaemia. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, L.; Socks, E.; Balasubramanian, P.; McGowan, R.; Herdt, T.M.; Kianian, R.; MohanKumar, S.M.J.; MohanKumar, P.S. Oleic Acid Stimulates Monoamine Efflux through PPAR-α: Differential Effects in Diet-Induced Obesity. Life Sci. 2020, 255, 1–7. [Google Scholar] [CrossRef]
- Bideyan, L.; Fan, W.; Kaczor-Urbanowicz, K.E.; Priest, C.; Casero, D.; Tontonoz, P. Integrative Analysis Reveals Multiple Modes of LXR Transcriptional Regulation in Liver. Proc. Natl. Acad. Sci. USA 2022, 119, 1–11. [Google Scholar] [CrossRef]
- Strickland, B.A.; Ansari, S.A.; Dantoft, W.; Uhlenhaut, N.H. How to Tame Your Genes: Mechanisms of Inflammatory Gene Repression by Glucocorticoids. FEBS Lett. 2022, 596, 2596–2616. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, N.S.; Camponogara, C.; Gehrcke, M.; Giuliani, L.M.; da Silva, D.T.; Maurer, L.H.; Dias, P.; Emanuelli, T.; Cruz, L.; Oliveira, S.M. Oleic Acid-Containing Semisolid Dosage Forms Exhibit in vivo Anti-Inflammatory Effect via Glucocorticoid Receptor in a UVB Radiation-Induced Skin Inflammation Model. Inflammopharmacology 2020, 28, 773–786. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int. J. Mol. Sci. 2021, 22, 1034. [Google Scholar] [CrossRef]
- Romano, A.; Tempesta, B.; Provensi, G.; Passani, M.B.; Gaetani, S. Central Mechanisms Mediating the Hypophagic Effects of Oleoylethanolamide and N-Acylphosphatidylethanolamines: Different Lipid Signals? Front. Pharmacol. 2015, 6, 137. [Google Scholar] [CrossRef]
- Geurts, L.; Everard, A.; Van Hul, M.; Essaghir, A.; Duparc, T.; Matamoros, S.; Plovier, H.; Castel, J.; Denis, R.G.P.; Bergiers, M.; et al. Adipose Tissue NAPE-PLD Controls Fat Mass Development by Altering the Browning Process and Gut Microbiota. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Tutunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M.; Roshanravan, N.; Shakeri-Bavil, A.; Asghari-Jafarabadi, M.; Farrin, N.; Mobasseri, M. Expression of NF-ΚB, IL-6, and IL-10 Genes, Body Composition, and Hepatic Fibrosis in Obese Patients with NAFLD-Combined Effects of Oleoylethanolamide Supplementation and Calorie Restriction: A Triple-Blind Randomized Controlled Clinical Trial. J. Cell. Physiol. 2021, 236, 417–426. [Google Scholar] [CrossRef]
- Grabacka, M.; Pierzchalska, M.; Płonka, P.M.; Pierzchalski, P. The Role of PPAR Alpha in the Modulation of Innate Immunity. Int. J. Mol. Sci. 2021, 22, 10545. [Google Scholar] [CrossRef]
- Antón, M.; Alén, F.; Gómez de Heras, R.; Serrano, A.; Pavón, F.J.; Leza, J.C.; García-Bueno, B.; Rodríguez de Fonseca, F.; Orio, L. Oleoylethanolamide Prevents Neuroimmune HMGB1/TLR4/NF-KB Danger Signaling in Rat Frontal Cortex and Depressive-like Behavior Induced by Ethanol Binge Administration. Addict. Biol. 2017, 22, 724–741. [Google Scholar] [CrossRef]
- Payahoo, L.; Khajebishak, Y.; Jafarabadi, M.A.; Ostadrahimi, A. Oleoylethanolamide Supplementation Reduces Inflammation and Oxidative Stress in Obese People: A Clinical Trial. Adv. Pharm. Bull. 2018, 8, 479–487. [Google Scholar] [CrossRef]
- Sayd, A.; Antón, M.; Alén, F.; Caso, J.R.; Pavón, J.; Leza, J.C.; De Fonseca, F.R.; García-Bueno, B.; Orio, L. Systemic Administration of Oleoylethanolamide Protects from Neuroinflammation and Anhedonia Induced by LPS in Rats. Int. J. Neuropsychopharmacol. 2015, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, X.; Chen, W. Inhibitory Effects of Oleoylethanolamide (OEA) on H₂O₂-Induced Human Umbilical Vein Endothelial Cell (HUVEC) Injury and Apolipoprotein E Knockout (ApoE-/-) Atherosclerotic Mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6301–6311. [Google Scholar] [PubMed]
- Hu, J.; Zhu, Z.; Ying, H.; Yao, J.; Ma, H.; Li, L.; Zhao, Y. Oleoylethanolamide Protects Against Acute Liver Injury by Regulating Nrf-2/HO-1 and NLRP3 Pathways in Mice. Front. Pharmacol. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Zolese, G.; Bacchetti, T.; Masciangelo, S.; Ragni, L.; Ambrosi, S.; Ambrosini, A.; Marini, M.; Ferretti, G. Effect of Acylethanolamides on Lipid Peroxidation and Paraoxonase Activity. Biofactors 2008, 33, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Roshanravan, N.; Tutunchi, H.; Ostadrahimi, A.; Pouraghaei, M.; Kafil, B. Oleoylethanolamide, A Bioactive Lipid Amide, as A Promising Treatment Strategy for Coronavirus/COVID-19. Arch. Med. Res. 2020, 51, 464–467. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, R.; Blanco, E.; Serrano, A.; Pavon, F.J.; Parsons, L.H.; Maldonado, R.; Robledo, P.; Fernandez-Espejo, E.; De Fonseca, F.R. The Systemic Administration of Oleoylethanolamide Exerts Neuroprotection of the Nigrostriatal System in Experimental Parkinsonism. Int. J. Neuropsychopharmacol. 2014, 17, 455–468. [Google Scholar] [CrossRef]
- Sabahi, M.; Ahmadi, S.A.; Kazemi, A.; Mehrpooya, M.; Khazaei, M.; Ranjbar, A.; Mowla, A. The Effect of Oleoylethanolamide (OEA) Add-On Treatment on Inflammatory, Oxidative Stress, Lipid, and Biochemical Parameters in the Acute Ischemic Stroke Patients: Randomized Double-Blind Placebo-Controlled Study. Oxid. Med. Cell. Longev. 2022, 2022, 5721167. [Google Scholar] [CrossRef]
- Pouryousefi, E.; Javadi, M.; Hashemipour, S.; Nooshabadi, M.R.; Haghighian, H.K. Improved Glycemic Status, Insulin Resistance and Inflammation after Receiving Oral Oleoylethanolamide Supplement in People with Prediabetes: A Randomized Controlled Trial. Diabetol. Metab. Syndr. 2022, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Lalooha, F.; Nooshabadi, M.R.; Haghighian, H.K. Decreased Dysmenorrhea Pain in Girls by Reducing Oxidative Stress and Inflammatory Biomarkers Following Supplementation with Oleoylethanolamide: A Randomized Controlled Trial. J. Obstet. Gynaecol. Res. 2022, 48, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Bonechi, E.; Provensi, G.; Costa, A.; Clarke, G.; Ballerini, C.; De Filippo, C.; Passani, M.B. Oleoylethanolamide Treatment Affects Gut Microbiota Composition and the Expression of Intestinal Cytokines in Peyer’s Patches of Mice. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Occurrence and Biological Activity of Palmitoleic Acid Isomers in Phagocytic Cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef]
- Guijas, C.; Meana, C.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Foamy Monocytes Are Enriched in Cis-7-Hexadecenoic Fatty Acid (16:1n-9), a Possible Biomarker for Early Detection of Cardiovascular Disease. Cell Chem. Biol. 2016, 23, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Ghai, M.; Kader, F. A Review on Epigenetic Inheritance of Experiences in Humans. Biochem. Genet. 2022, 60, 1107–1140. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Gabbianelli, R. Primers on Nutrigenetics and Nutri(Epi)Genomics: Origins and Development of Precision Nutrition. Biochimie 2019, 160, 156–171. [Google Scholar] [CrossRef]
- Kiec-Wilk, B.; Sliwa, A.; Mikolajczyk, M.; Malecki, M.T.; Mathers, J.C. The CpG Island Methylation Regulated Expression of Endothelial Proangiogenic Genes in Response to β-Carotene and Arachidonic Acid. Nutr. Cancer 2011, 63, 1053–1063. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium Butyrate Inhibits the NF-Kappa B Signaling Pathway and Histone Deacetylation, and Attenuates Experimental Colitis in an IL-10 Independent Manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Silva-Martínez, G.A.; Rodríguez-Ríos, D.; Alvarado-Caudillo, Y.; Vaquero, A.; Esteller, M.; Carmona, F.J.; Moran, S.; Nielsen, F.C.; Wickström-Lindholm, M.; Wrobel, K.; et al. Arachidonic and Oleic Acid Exert Distinct Effects on the DNA Methylome. Epigenetics 2016, 11, 321–334. [Google Scholar] [CrossRef]
- Del Pilar Valencia-Morales, M.; Zaina, S.; Heyn, H.; Carmona, F.J.; Varol, N.; Sayols, S.; Condom, E.; Ramírez-Ruz, J.; Gomez, A.; Moran, S.; et al. The DNA Methylation Drift of the Atherosclerotic Aorta Increases with Lesion Progression. BMC Med. Genom. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Schuldt, L.; Von Brandenstein, K.; Jacobs, C.; Symmank, J. Oleic Acid-Related Anti-Inflammatory Effects in Force-Stressed PdL Fibroblasts Are Mediated by H3 Lysine Acetylation Associated with Altered IL10 Expression. Epigenetics 2022, 17, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Hwang, J.-T.; Park, J.H.; Choi, H.-K. Free Fatty Acid-Induced Histone Acetyltransferase Activity Accelerates Lipid Accumulation in HepG2 Cells INTRODUCTION 2). Nutr. Res. Pract. 2019, 13, 196–204. [Google Scholar] [CrossRef]
- García-Segura, L.; Pérez-Andrade, M.; Miranda-Ríos, J. The Emerging Role of MicroRNAs in the Regulation of Gene Expression by Nutrients. J. Nutr. Nutr. 2013, 6, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.-H.; Feng, X.; Zhang, Y.-W.; Lou, X.-Y.; Cheng, Y.; Zhou, H.-H. Let-7 in Cardiovascular Diseases, Heart Development and Cardiovascular Differentiation from Stem Cells. Int. J. Mol. Sci. 2013, 14, 23086–23102. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas, M.; Bressan, J.; Martínez, J.A.; Milagro, F.I. Regulatory Roles of MiR-155 and Let-7b on the Expression of Inflammation-Related Genes in THP-1 Cells: Effects of Fatty Acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Gil-Zamorano, J.; Martin, R.; Daimiel, L.; Richardson, K.; Giordano, E.; Nicod, N.; García-Carrasco, B.; Soares, S.M.A.; Iglesias-Gutiérrez, E.; Lasunción, M.A.; et al. Docosahexaenoic Acid Modulates the Enterocyte Caco-2 Cell Expression of MicroRNAs Involved in Lipid Metabolism. J. Nutr. 2014, 144, 575–585. [Google Scholar] [CrossRef]
| General Effects | Specific Effects | Pathways | Cells | References |
|---|---|---|---|---|
| Pro-inflammatory | ↑ ROS | PKC/Ca+2 | Neutrophils | [43,44] |
| ↑ Granule release | PKC/Ca+2 | Neutrophils | [30,45] | |
| ↑ MMP9 | MAPK | Neutrophils | [45] | |
| ↑ Phagocytosis | – | Neutrophils | [46] | |
| ↑ Proliferation | Ca2+/calcineurin/ NFAT | Lymphocytes | [48,49,50] | |
| Anti-inflammatory | ↑ M2 | – | Macrophages | [51] |
| ↓ COX2, TNFα, IL-6, IL-12, NF-κB, iNOS, PGE2 | AMPK/MAPK/PI3K | Macrophages/Caco cells/Lung epithelial cells | [52,53,54,55] | |
| ↑ HO-1, GPx, SOD, IL-10 ↓ COX2, TNFα, IL-6, IL-12, NF-κB, | MAPK/Nrf2/PPARγ | Phagocytic cells | [56,57] | |
| ↑ Treg | Oxidative phosphorilation | Lymphocytes | [58] | |
| ↓ Nf-κB | Lys 310 acetilated/SIRT1 | Macrophages | [59] | |
| ↑ Let7b | Histone acetilated | Macrophages/Caco cells | [60,61] | |
| Apoptosis | ↓ Apoptosis | CD36 expression | Macrophages | [62] |
| ↑ FOXO3, HSF-1 | SIRT1 | Neurons | [63] | |
| Neuroprotection | ↓ ROS, IL-8, IL-6, TNFα | HIF-1α deacetylate | Neurons (Parkinson’s disease) | [63] |
| ↑ Monoamino release, dendrites and axon development | PPARγ | Neurons (hypothalamus) | [64] | |
| Lipid metabolism And energy | ↓ Lipotoxicity | ----- | INS-1 cells | [65] |
| ↑ Membrane fluidity | Membrane composition | Hep G2 cells | [32] | |
| ↑ AMPK | ---- | Macrophages | [53] | |
| ↑ β oxidation | PGC1α/SIRT1 | Skeletal muscle cells | [66] | |
| ↓ Lipotoxicity | ER stress/pyroptosis/caspase1 | Hep G2 cells | [67] | |
| ↑ Apo E secretion | Glycosylation | Macrophages | [68] | |
| ↓ Atherosclerosis lesion | Hipomethylation | THP-1 cells | [69,70] | |
| ↑ LXRα, ABCA1 ↓SREBP1c | MAPK | Neutrophils | [71] | |
| Glycemic Metabolism | ↓ IR | ER stress | HFD rats β cells | [65] |
| General Effects | Specific Effects | Pathways | Cells | Reference |
|---|---|---|---|---|
| Anti-inflammatory | ↑ SOD, GPx | Nrf2/HO-1 | Hepatic cells | [92,94] |
| ↓ Macrophages activation | – | Macrophages | [94] | |
| ↓ IL-6, TNFα, MCP1, IL-1β | NRLP3/caspase 1 | Liver/plasma | [93,94] | |
| ↑ IκB, IL-10 ↓ TLR4 | PPARα | PBMCs | [89] | |
| Apoptosis | ↓ Bax, Bcl2 | Caspase 3 | Hepatic cells | [94] |
| Glycemic Metabolism | ↓ IR | PPAR | Plasma | [95] |
| ↓ Food intake | – | – | [89] | |
| Neuroprotection | ↓ Alcohol damage | TLR4 | Neurons | [96] |
| ↓ Pro-inflammatory Cytokines/oxidative/nitrosative stress | – | – | [97] | |
| ↓ Neuronal damage, NF-κB, iNOS, COX2, NO, lipid peroxidation | PPAR | Frontal cortex cells | [98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. https://doi.org/10.3390/nu15010224
Santa-María C, López-Enríquez S, Montserrat-de la Paz S, Geniz I, Reyes-Quiroz ME, Moreno M, Palomares F, Sobrino F, Alba G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients. 2023; 15(1):224. https://doi.org/10.3390/nu15010224
Chicago/Turabian StyleSanta-María, Consuelo, Soledad López-Enríquez, Sergio Montserrat-de la Paz, Isabel Geniz, María Edith Reyes-Quiroz, Manuela Moreno, Francisca Palomares, Francisco Sobrino, and Gonzalo Alba. 2023. "Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid" Nutrients 15, no. 1: 224. https://doi.org/10.3390/nu15010224
APA StyleSanta-María, C., López-Enríquez, S., Montserrat-de la Paz, S., Geniz, I., Reyes-Quiroz, M. E., Moreno, M., Palomares, F., Sobrino, F., & Alba, G. (2023). Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients, 15(1), 224. https://doi.org/10.3390/nu15010224






