Habitual Fish Oil Supplementation and Incident Chronic Kidney Disease in the UK Biobank
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Ascertainment of Exposure
2.3. Ascertainment of Covariates
2.4. Study Outcome
2.5. Statistical Analysis
3. Results
3.1. Study Participants and Characteristics
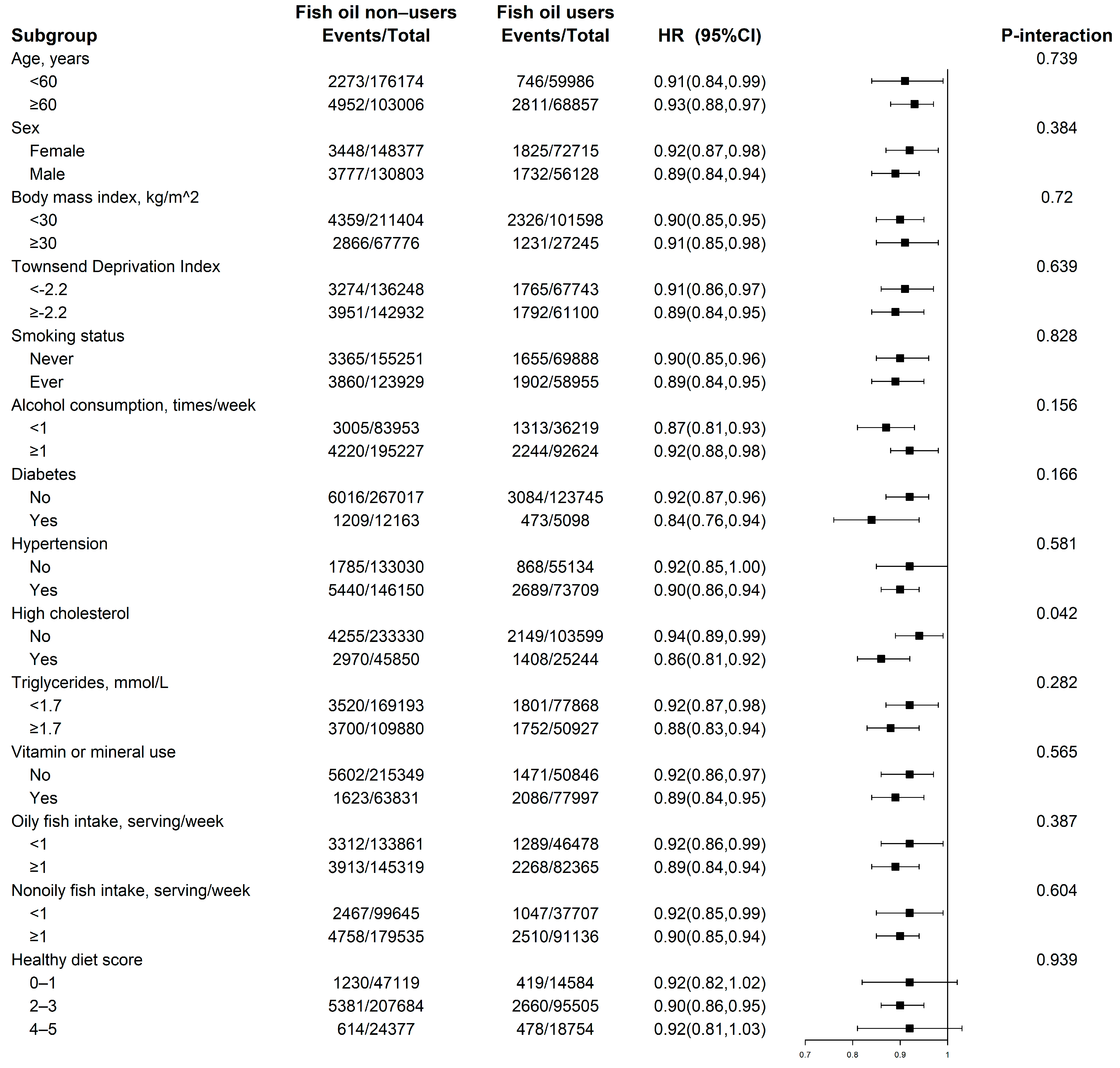
3.2. Fish Oil Supplements and Incident CKD
3.3. Oily and Nonoily Fish Consumption with Incident CKD
3.4. Plasma Omega-3 PUFA and DHA with Incident CKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Welsh, C.E.; Celis-Morales, C.A.; Mackay, D.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Cleland, J.G.; Gill, J.M.R.; Jhund, P.S.; et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat. Med. 2019, 25, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Adler, A.I. Recent findings on the effects of marine-derived n-3 polyunsaturated fatty acids on urinary albumin excretion and renal function. Curr. Atheroscler. Rep. 2012, 14, 535–541. [Google Scholar] [CrossRef]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: Molecular and metabolic alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef]
- Shapiro, H.; Theilla, M.; Attal-Singer, J.; Singer, P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 110–121. [Google Scholar] [CrossRef]
- Park, I.; Xun, P.; Tsinovoi, C.L.; Klemmer, P.; Liu, K.; He, K. Intakes of long-chain omega-3 polyunsaturated fatty acids and non-fried fish in relation to incidence of chronic kidney disease in young adults: A 25-year follow-up. Eur. J. Nutr. 2020, 59, 399–407. [Google Scholar] [CrossRef]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Miller, E.R., 3rd; Ruggiero, C.; Cherubini, A.; Guralnik, J.M.; Ferrucci, L. Plasma polyunsaturated fatty acids and the decline of renal function. Clin. Chem. 2008, 54, 475–481. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef]
- Hoogeveen, E.K.; Geleijnse, J.M.; Kromhout, D.; Stijnen, T.; Gemen, E.F.; Kusters, R.; Giltay, E.J. Effect of omega-3 fatty acids on kidney function after myocardial infarction: The Alpha Omega Trial. Clin. J. Am. Soc. Nephrol. 2014, 9, 1676–1683. [Google Scholar] [CrossRef]
- de Boer, I.H.; Zelnick, L.R.; Ruzinski, J.; Friedenberg, G.; Duszlak, J.; Bubes, V.Y.; Hoofnagle, A.N.; Thadhani, R.; Glynn, R.J.; Buring, J.E.; et al. Effect of Vitamin D and Omega-3 Fatty Acid Supplementation on Kidney Function in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2019, 322, 1899–1909. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Juraschek, S.P.; Appel, L.J.; Madala, M.; Anderson, C.A.; Bleys, J.; Guallar, E. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2009, 89, 1937–1945. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Collins, R. What makes UK Biobank special? Lancet 2012, 379, 1173–1174. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, e6. [Google Scholar] [CrossRef]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; de Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the Capture and Adjudication of Prevalent and Incident Diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Song, Y.; Ma, H.; Zhou, T.; Liang, Z.; Qi, L. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: A cohort study in UK Biobank. PLoS Med. 2021, 18, e1003767. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Chen, C.; Sun, Y.; Chen, J.; Tan, X.; Xia, F.; Zhang, J.; Lu, Y.; Wang, N. Sleep Patterns, Genetic Susceptibility, and Incident Chronic Kidney Disease: A Prospective Study of 370 671 Participants. Front. Neurosci. 2022, 16, 725478. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Su, G.; Zhang, L.; Qin, X.; Marshall, S.; González-Ortiz, A.; Clase, C.M.; Campbell, K.L.; Xu, H.; Carrero, J.J. Modifiable Lifestyle Factors for Primary Prevention of CKD: A Systematic Review and Meta-Analysis. J. Am. Soc. Nephrol. 2021, 32, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Chang, C.H.; Tseng, P.T.; Chen, N.Y.; Lin, P.C.; Lin, P.Y.; Chang, J.P.; Kuo, F.Y.; Lin, J.; Wu, M.C.; Su, K.P. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 2018, 129, 1–12. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
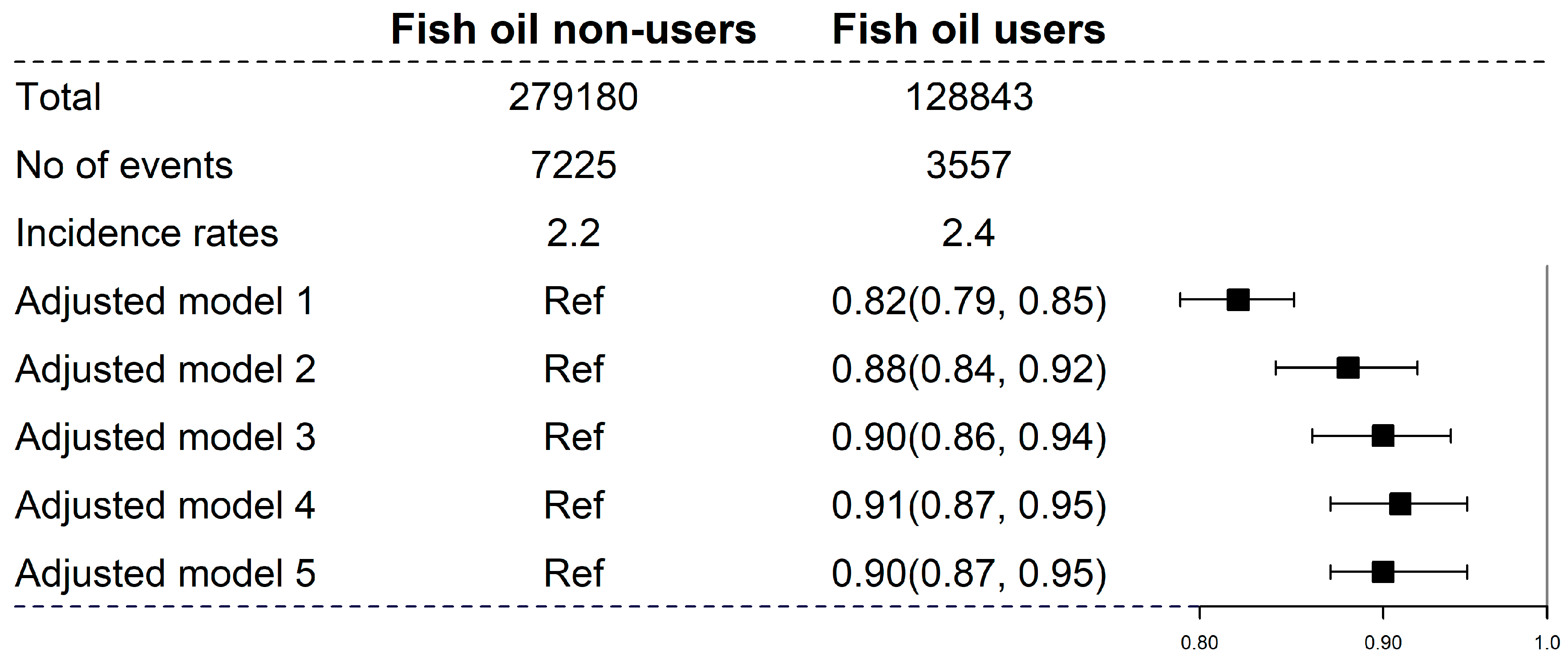
| Total | Fish Oil Non-Users | Fish Oil Users | p-Value | |
|---|---|---|---|---|
| N | 408,023 | 279,180 | 128,843 | |
| Age, years | 56.3 ± 8.1 | 55.3 ± 8.1 | 58.5 ± 7.4 | <0.001 |
| Male, No. (%) | 186,931 (45.8) | 130,803 (46.9) | 56,128 (43.6) | <0.001 |
| White, No. (%) | 388,982 (95.3) | 265,559 (95.1) | 123,423 (95.8) | <0.001 |
| TDI | −1.4 ± 3.0 | −1.3 ± 3.1 | −1.6 ± 2.9 | <0.001 |
| BMI, kg/m2 | 27.3 ± 4.7 | 27.4 ± 4.8 | 27.1 ± 4.4 | <0.001 |
| Smoking status, No. (%) | <0.001 | |||
| Never | 225,139 (55.2) | 155,251 (55.6) | 69,888 (54.2) | |
| Former | 141,165 (34.6) | 92,480 (33.1) | 48,685 (37.8) | |
| Current | 41,719 (10.2) | 31,449 (11.3) | 10,270 (8.0) | |
| Alcohol consumption, No. (%) | <0.001 | |||
| Never | 30,070 (7.4) | 21,392 (7.7) | 8678 (6.7) | |
| <1 times/week | 90,102 (22.1) | 62,561 (22.4) | 27,541 (21.4) | |
| 1–2 times/week | 106,302 (26.1) | 72,635 (26.0) | 33,667 (26.1) | |
| 3–4 times/week | 96,943 (23.8) | 65,341 (23.4) | 31,602 (24.5) | |
| >4 times/week | 84,606 (20.7) | 57,251 (20.5) | 27,355 (21.2) | |
| Disease history, No. (%) | ||||
| Diabetes | 17,261 (4.2) | 12,163 (4.4) | 5098 (4.0) | <0.001 |
| Hypertension | 219,859 (53.9) | 146,150 (52.3) | 73,709 (57.2) | <0.001 |
| High cholesterol | 71,094 (17.4) | 45,850 (16.4) | 25,244 (19.6) | <0.001 |
| Healthy diet score | 2.4 ± 0.9 | 2.3 ± 0.9 | 2.6 ± 0.9 | <0.001 |
| Vitamin and mineral supplementation, No. (%) | 141,828 (34.8) | 63,831 (22.9) | 77,997 (60.5) | <0.001 |
| Oily fish intake, No. (%) | <0.001 | |||
| Never | 43,939 (10.8) | 34,584 (12.4) | 9355 (7.3) | |
| <1 serving/week | 136,400 (33.4) | 99,277 (35.6) | 37,123 (28.8) | |
| 1 serving/week | 154,621 (37.9) | 101,230 (36.3) | 53,391 (41.4) | |
| ≥2 serving/week | 73,063 (17.9) | 44,089 (15.8) | 28,974 (22.5) | |
| Nonoily fish intake, No. (%) | <0.001 | |||
| Never | 18,560 (4.5) | 15,049 (5.4) | 3511 (2.7) | |
| <1 serving/week | 118,792 (29.1) | 84,596 (30.3) | 34,196 (26.5) | |
| 1 serving/week | 203,803 (49.9) | 136,067 (48.7) | 67,736 (52.6) | |
| ≥2 serving/week | 66,868 (16.4) | 43,468 (15.6) | 23,400 (18.2) | |
| Plasma Omega-3 PUFA, mmol/L | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | <0.001 |
| Plasma DHA, mmol/L | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | <0.001 |
| eGFR, mL/min/1.73 m2 | 91.7 ± 11.9 | 92.2 ± 12.1 | 90.5 ± 11.5 | <0.001 |
| UACR, mg/g | 9.0 ± 5.7 | 8.8 ± 5.6 | 9.4 ± 5.8 | <0.001 |
| Fish Consumption | Never | <1 Serving/Week | 1 Serving/Week | ≥2 Serving/Week | p for Trend | Per 1 Serving/Week |
|---|---|---|---|---|---|---|
| Oily fish | ||||||
| No. of events | 1227 | 3374 | 4122 | 2059 | ||
| Incidence rates * | 2.4 | 2.1 | 2.3 | 2.4 | ||
| Adjusted model 1 † | Ref | 0.77 (0.72, 0.82) | 0.71 (0.67, 0.76) | 0.68 (0.64, 0.73) | <0.001 | 0.94 (0.92, 0.96) |
| Adjusted model 2 † | Ref | 0.90 (0.84, 0.96) | 0.91 (0.85, 0.97) | 0.86 (0.80, 0.93) | 0.003 | 0.98 (0.96, 1.00) |
| Adjusted model 3 † | Ref | 0.88 (0.82, 0.94) | 0.87 (0.82, 0.94) | 0.83 (0.77, 0.90) | <0.001 | 0.97 (0.95, 0.99) |
| Adjusted model 4 † | Ref | 0.91 (0.84, 0.98) | 0.90 (0.84, 0.97) | 0.86 (0.79, 0.93) | 0.002 | 0.97 (0.95, 0.99) |
| Adjusted model 5 † | Ref | 0.91 (0.85, 0.98) | 0.90 (0.84, 0.97) | 0.86 (0.79, 0.94) | 0.002 | 0.97 (0.95, 0.99) |
| Nonoily fish | ||||||
| No. of events | 478 | 3036 | 5517 | 1751 | ||
| Incidence rates * | 2.2 | 2.2 | 2.3 | 2.2 | ||
| Adjusted model 1 † | Ref | 0.84 (0.76, 0.93) | 0.79 (0.72, 0.87) | 0.78 (0.71, 0.87) | <0.001 | 0.97 (0.95, 0.99) |
| Adjusted model 2 † | Ref | 0.94 (0.85, 1.03) | 0.94 (0.85, 1.03) | 0.92 (0.83, 1.02) | 0.281 | 0.99 (0.97, 1.01) |
| Adjusted model 3 † | Ref | 0.84 (0.76, 0.92) | 0.83 (0.75, 0.91) | 0.81 (0.72, 0.89) | 0.004 | 0.98 (0.96, 1.00) |
| Adjusted model 4 † | Ref | 0.89 (0.80, 0.99) | 0.88 (0.79, 0.98) | 0.86 (0.77, 0.97) | 0.065 | 0.98 (0.96, 1.00) |
| Adjusted model 5 † | Ref | 0.89 (0.80, 0.99) | 0.88 (0.79, 0.98) | 0.86 (0.77, 0.97) | 0.057 | 0.98 (0.96, 1.00) |
| Quantiles of Omega-3 PUFA, mmol/L | p for Trend | Per SD Increment | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Omega-3 polyunsaturated fatty acid | ||||||
| Range | <0.37 | 0.37–<0.49 | 0.49–<0.64 | ≥0.64 | ||
| No. of events | 632 | 657 | 680 | 614 | ||
| Incidence rates † | 2.2 | 2.3 | 2.4 | 2.1 | ||
| Adjusted model 1‡ | Ref | 0.89 (0.80, 0.99) | 0.83 (0.74, 0.93) | 0.67 (0.60, 0.76) | <0.001 | 0.87 (0.83, 0.90) |
| Adjusted model 2 ‡ | Ref | 0.90 (0.81, 1.01) | 0.89 (0.80, 1.00) | 0.79 (0.71, 0.89) | <0.001 | 0.93 (0.89, 0.97) |
| Adjusted model 3 ‡ | Ref | 0.91 (0.82, 1.02) | 0.90 (0.81, 1.01) | 0.83 (0.74, 0.94) | 0.004 | 0.94 (0.90, 0.98) |
| Adjusted model 4 ‡ | Ref | 0.87 (0.78, 0.98) | 0.83 (0.74, 0.94) | 0.73 (0.63, 0.83) | <0.001 | 0.89 (0.84, 0.94) |
| Adjusted model 5 ‡ | Ref | 0.87 (0.78, 0.98) | 0.82 (0.73, 0.93) | 0.72 (0.63, 0.83) | <0.001 | 0.89 (0.84, 0.94) |
| Docosahexaenoic acid | ||||||
| Range | <0.18 | 0.18–<0.22 | 0.22–<0.28 | ≥0.28 | ||
| No. of events | 801 | 629 | 592 | 561 | ||
| Incidence rates † | 2.8 | 2.2 | 2.1 | 2 | ||
| Adjusted model 1 ‡ | Ref | 0.72 (0.65, 0.80) | 0.62 (0.55, 0.69) | 0.51 (0.46, 0.57) | <0.001 | 0.78 (0.74, 0.81) |
| Adjusted model 2 ‡ | Ref | 0.82 (0.74, 0.91) | 0.76 (0.68, 0.85) | 0.73 (0.64, 0.82) | <0.001 | 0.89 (0.85, 0.93) |
| Adjusted model 3 ‡ | Ref | 0.84 (0.76, 0.94) | 0.80 (0.72, 0.90) | 0.78 (0.69, 0.89) | <0.001 | 0.91 (0.87, 0.95) |
| Adjusted model 4 ‡ | Ref | 0.84 (0.76, 0.94) | 0.80 (0.71, 0.89) | 0.76 (0.67, 0.87) | <0.001 | 0.91 (0.87, 0.96) |
| Adjusted model 5 ‡ | Ref | 0.83 (0.75, 0.93) | 0.79 (0.70, 0.89) | 0.76 (0.67, 0.87) | <0.001 | 0.91 (0.87, 0.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ye, Z.; Yang, S.; Zhang, Y.; Wu, Q.; Zhou, C.; He, P.; Zhang, Y.; Hou, F.; Qin, X. Habitual Fish Oil Supplementation and Incident Chronic Kidney Disease in the UK Biobank. Nutrients 2023, 15, 22. https://doi.org/10.3390/nu15010022
Liu M, Ye Z, Yang S, Zhang Y, Wu Q, Zhou C, He P, Zhang Y, Hou F, Qin X. Habitual Fish Oil Supplementation and Incident Chronic Kidney Disease in the UK Biobank. Nutrients. 2023; 15(1):22. https://doi.org/10.3390/nu15010022
Chicago/Turabian StyleLiu, Mengyi, Ziliang Ye, Sisi Yang, Yanjun Zhang, Qimeng Wu, Chun Zhou, Panpan He, Yuanyuan Zhang, Fanfan Hou, and Xianhui Qin. 2023. "Habitual Fish Oil Supplementation and Incident Chronic Kidney Disease in the UK Biobank" Nutrients 15, no. 1: 22. https://doi.org/10.3390/nu15010022
APA StyleLiu, M., Ye, Z., Yang, S., Zhang, Y., Wu, Q., Zhou, C., He, P., Zhang, Y., Hou, F., & Qin, X. (2023). Habitual Fish Oil Supplementation and Incident Chronic Kidney Disease in the UK Biobank. Nutrients, 15(1), 22. https://doi.org/10.3390/nu15010022






