Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Strain, and Media
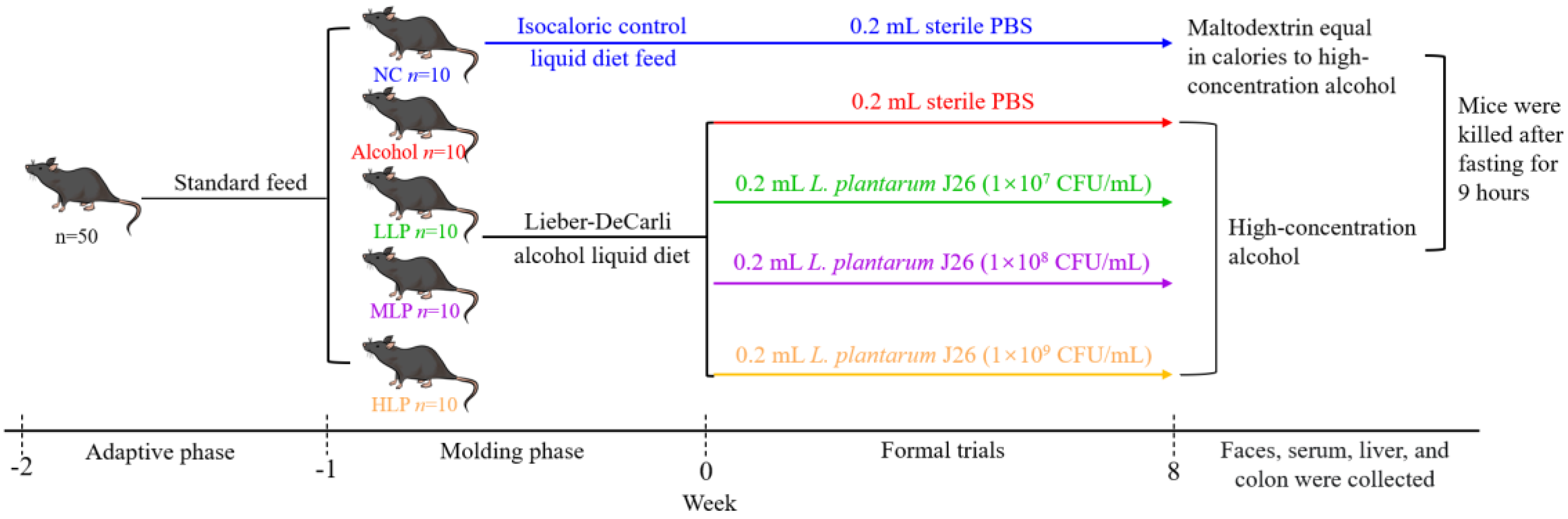
2.2. Animal Experiment and Its Parameter Analysis
2.2.1. Animal and Experimental Design
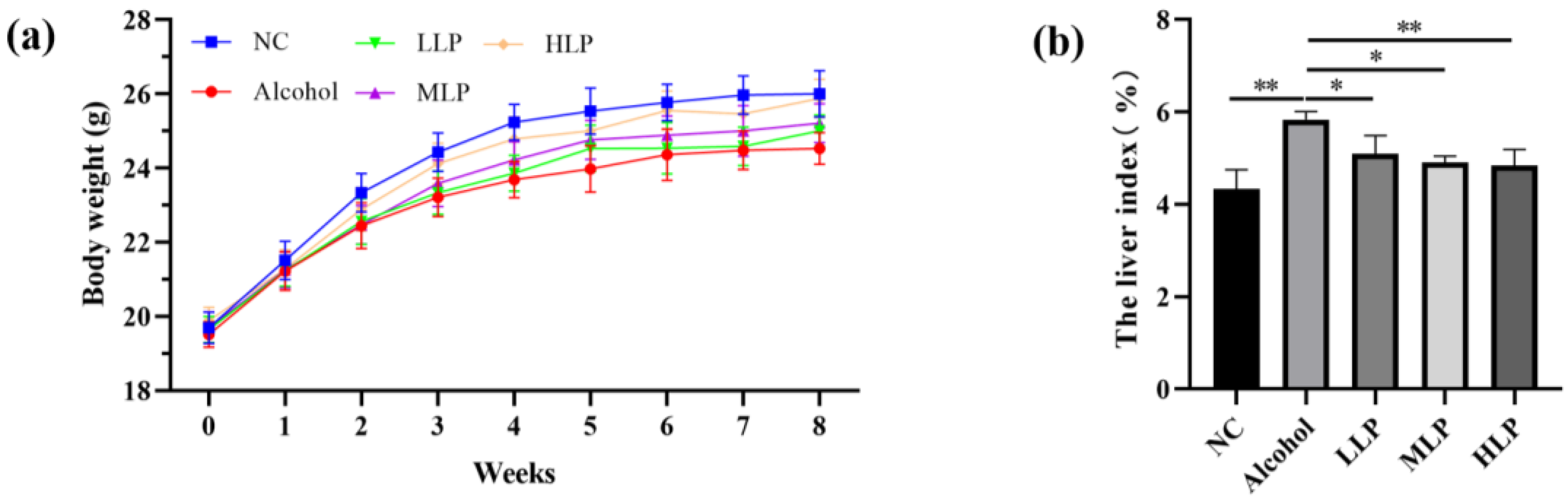
2.2.2. Body Weight and Liver Index
2.2.3. Histomorphological Observation
Anatomical Observation
Histopathological Examination
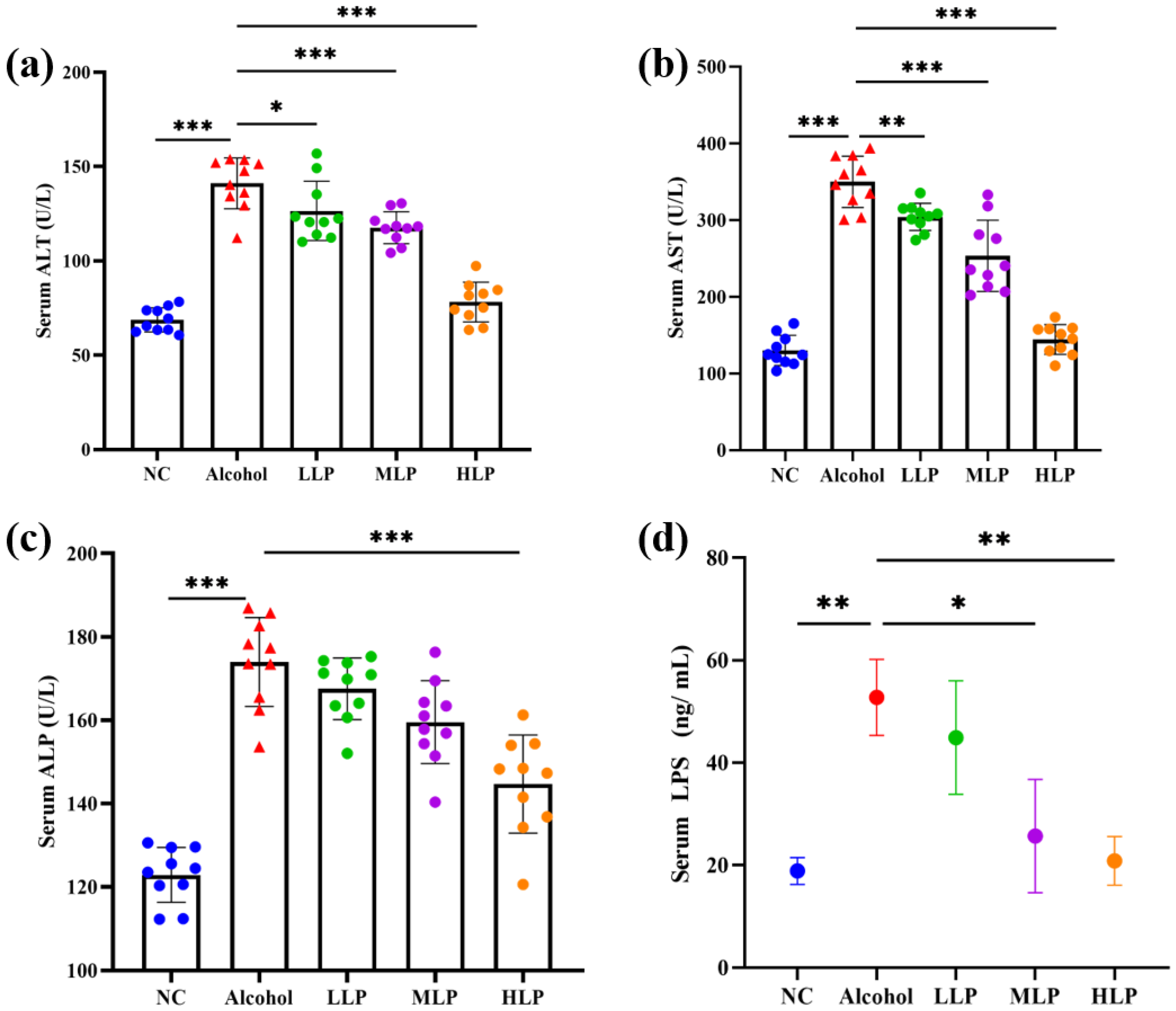
2.2.4. Biochemical Assays
2.2.5. Immunohistochemical
- (1)
- The antigen was repaired with citrate antigen repair solution by microwave method and then cooled to room temperature;
- (2)
- A total of 3% H2O2 was added and incubated for 15 min at room temperature and then washed three times;
- (3)
- A total of 3% goat serum was added and blocked for 1 h at room temperature;
- (4)
- Diluted MPO antibody was added and incubated at 4 °C overnight;
- (5)
- Washed with PBS three times, 5 min/time. The biotinylated secondary antibody was added and incubated for 1 h at room temperature and then washed with PBS again three times;
- (6)
- After adding peroxidase-labeled streptavidin, all sections were incubated at 37 °C for 30 min and then washed with PBS three times;
- (7)
- 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogenic solution was added and incubated for 1–5 min for hematoxylin counterstaining;
- (8)
- After dehydration with different concentrations of ethanol solution and diaphanization with xylene, the sections were sealed with neutral gum, and air-dried;
- (9)
- Placed under a microscope, observed, and recorded.
2.2.6. RNA Extraction and RT-Quantitative PCR (RT-qPCR)
2.2.7. Western Blotting Analysis
2.2.8. Metagenomic Analysis of Gut Microbiome
2.3. Statistical Analysis
3. Results
3.1. Effects of L. plantarum J26 on Body Weight and Liver Index in the Mice
3.2. Effects of L. plantarum J26 on Liver Histopathology in Liver of Mice
3.3. Effects of L. plantarum J26 on the Levels of Liver Function Enzymes, LPS and Lipid Content in the Serum of Mice
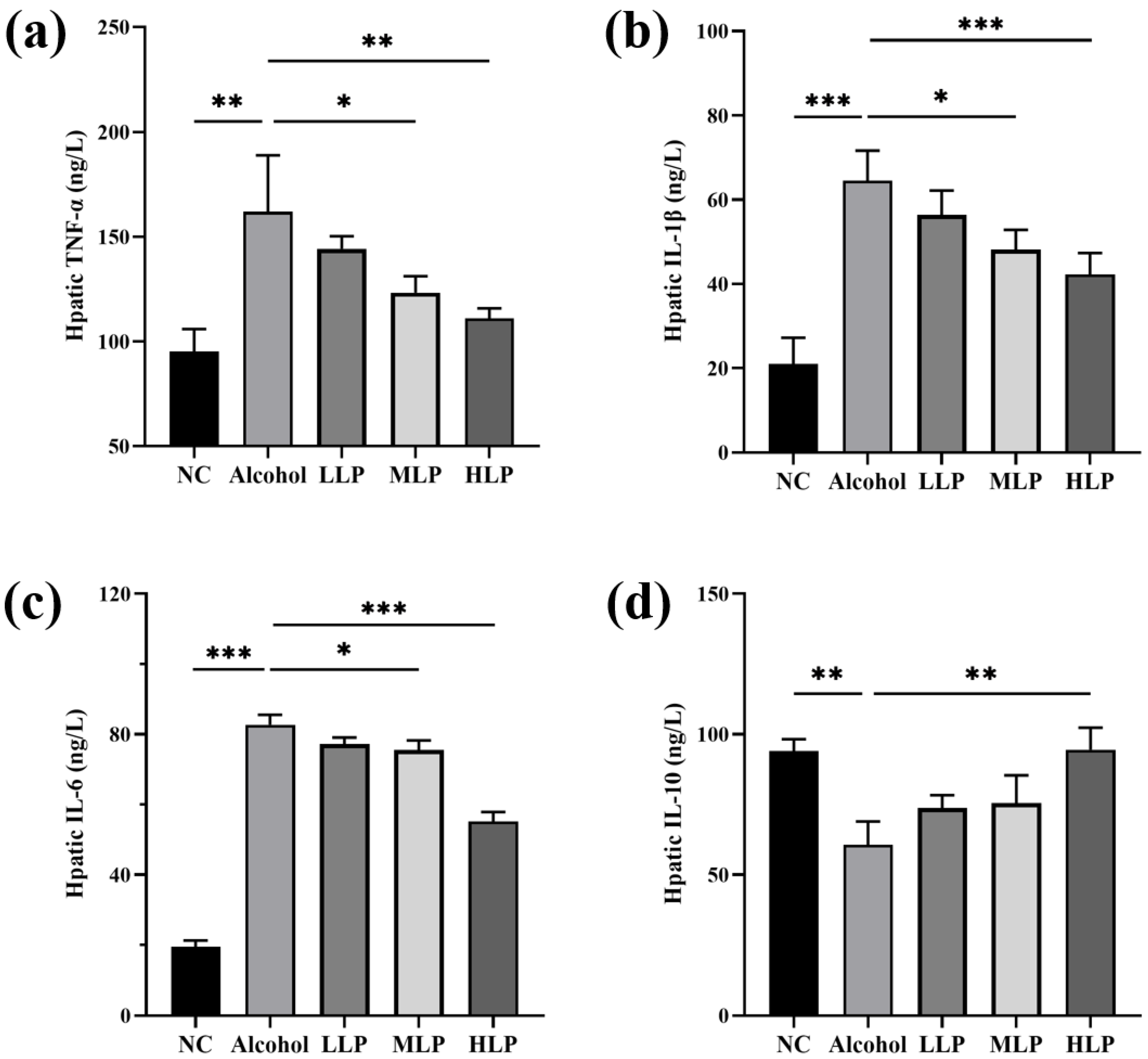
3.4. Effect of L. plantarum J26 on the Levels of MPO and Inflammatory Factors in the Liver of Mice
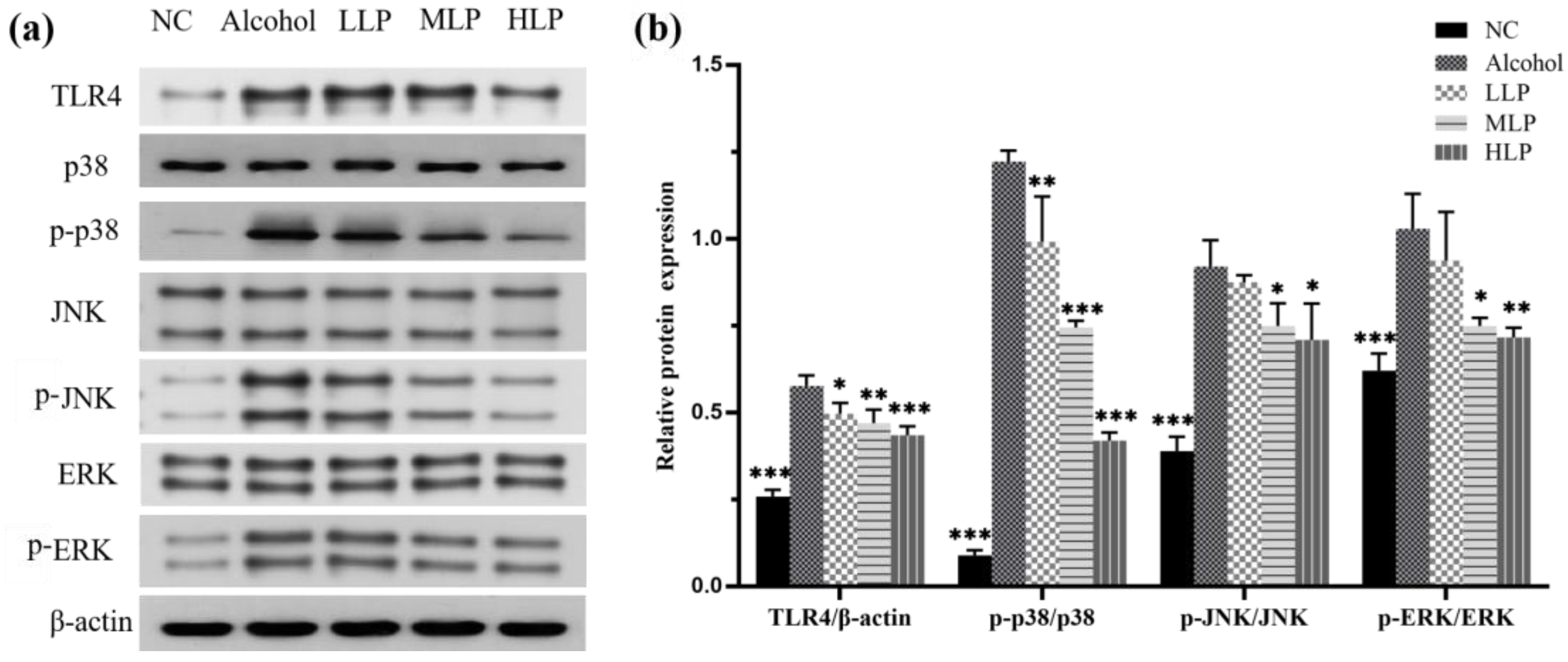
3.5. Effects of L. plantarum J26 on Protein Expression Related to MAPK Signaling Pathway in Mice Liver
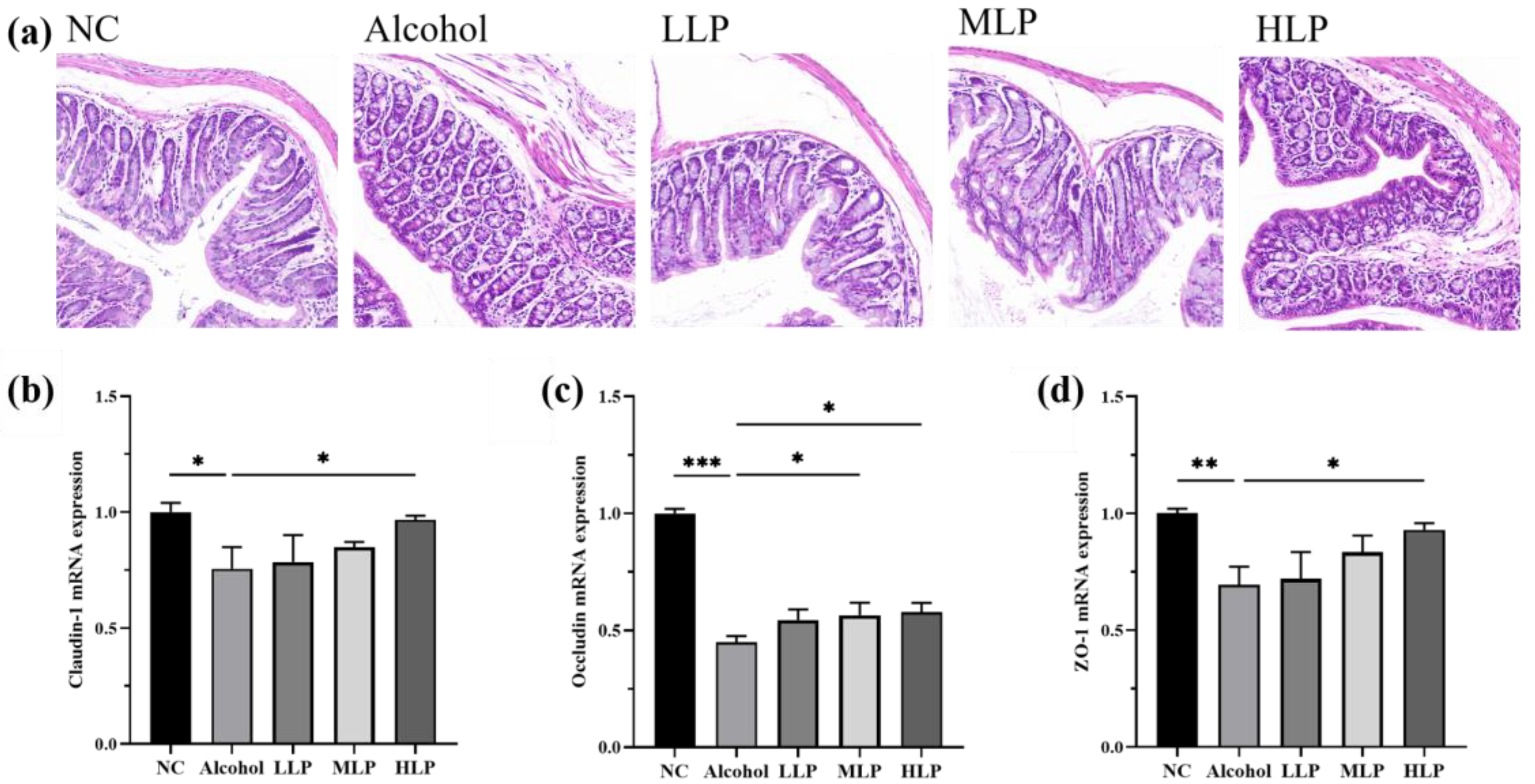
3.6. Protective Effects of L. plantarum J26 on Alcohol-Induced Intestinal Injury in Mice
3.7. Effects of L. plantarum J26 on the Gut Microbiota in Mice
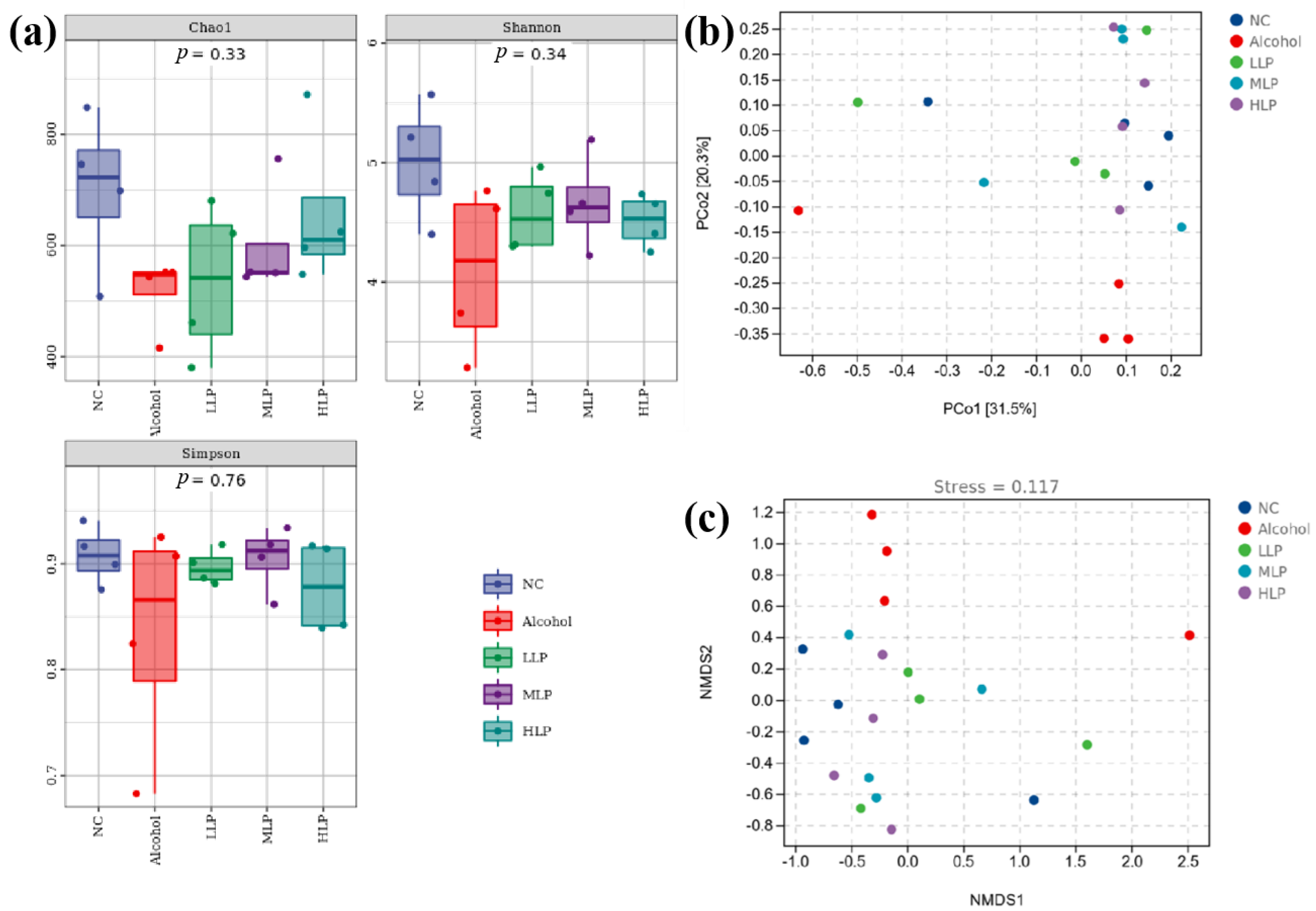
3.7.1. Gut Microbiota Diversity in Mice
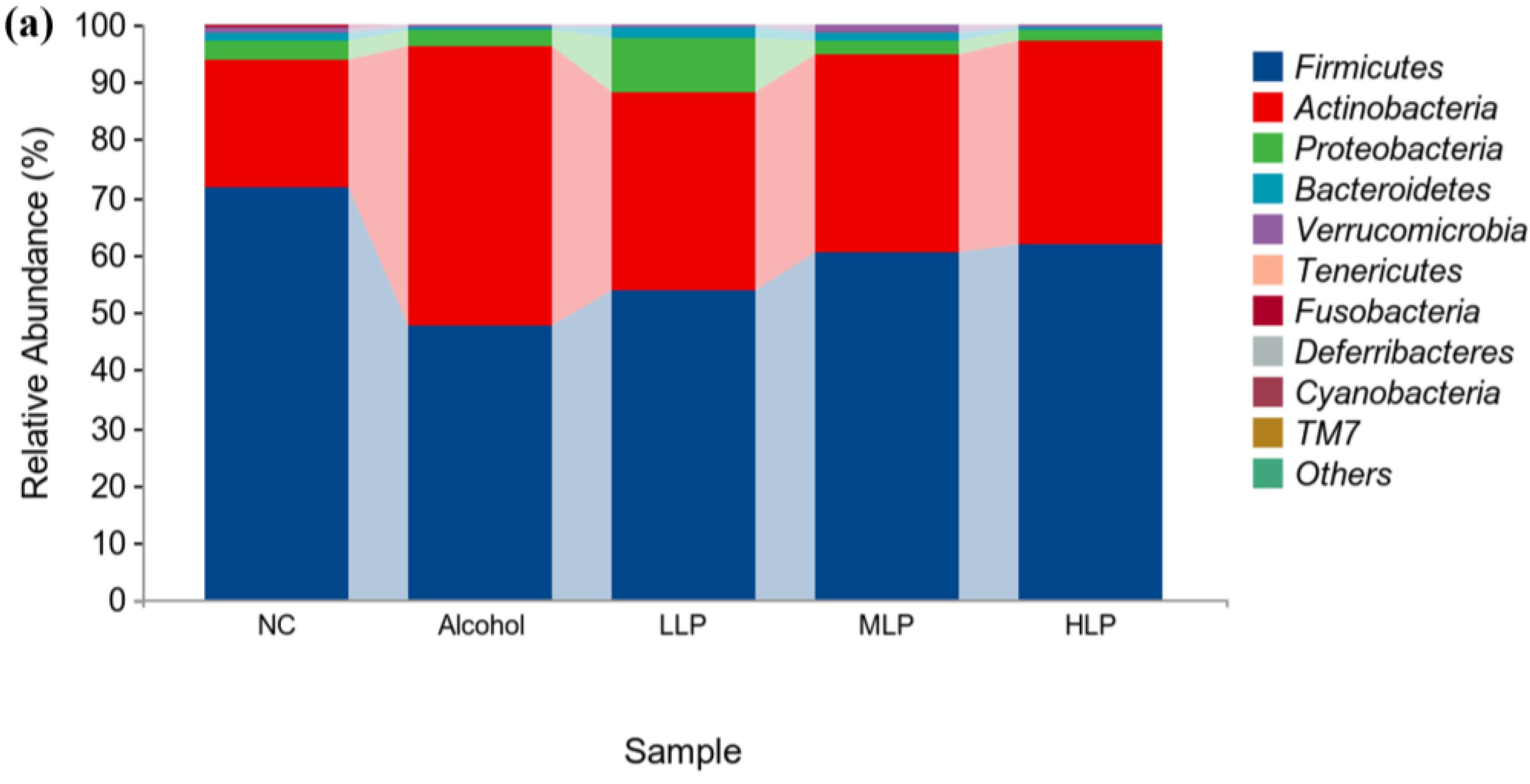
3.7.2. Analysis of Microbial Colony Composition at the Phylum Level
3.7.3. Analysis of Microbial Colony Composition at the Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Grewal, A.S.; Kanojia, N.; Rani, L.; Sharma, N.; Singh, S. Alcoholic and non-alcoholic liver diseases: Promising molecular drug targets and their clinical development. Curr. Drug Discov. Technol. 2021, 18, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kawai, M.; Kakugawa, Y.; Nishino, Y.; Ohuchi, N.; Minami, Y. Alcohol consumption and breast cancer risk according to hormone receptor status in japanese women: A case-control study. Tohoku J. Exp. Med. 2018, 244, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Cassard, A.-M.; Ciocan, D. Microbiota, a key player in alcoholic liver disease. Clin. Mol. Hepatol. 2018, 24, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Kong, L.; Shen, Q.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Recent insights into the hepatoprotective effects of lactic acid bacteria in alcoholic liver disease. Trends Food Sci. Technol. 2022, 125, 91–99. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent insights into the role of immune cells in alcoholic liver disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Zhong, X.; Cui, P.; Jiang, J.; Ning, C.; Liang, B.; Zhou, J.; Tian, L.; Zhang, Y.; Lei, T.; Zuo, T.; et al. Streptococcus, the predominant bacterium to predict the severity of liver injury in alcoholic liver disease. Front. Cell. Infect. Microbiol. 2021, 11, 649060. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Song, Z.; Han, H.; Ge, X.; Desert, R.; Athavale, D.; Komakula, S.S.B.; Magdaleno, F.; Chen, W.; Lantvit, D.; et al. Intestinal osteopontin protects from alcohol-induced liver injury by preserving the gut microbiome and the intestinal barrier function. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 813–839. [Google Scholar] [CrossRef]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kong, D. The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed. Pharmacother. 2021, 135, 111235. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, C.; Li, Y.; Dai, W.; Piao, C.; Liu, J.; Yu, H.; Li, X.; et al. Probiotics in treating with alcoholic liver disease and nonalcoholic fatty liver disease. Food Rev. Int. 2021, 1–19. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, Y. Protective effects of Lactobacillus plantarum NDC 75017 against lipopolysaccharide-induced liver injury in mice. Inflammation 2014, 37, 1599–1607. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Sun, H.; Shan, Y.; Liu, Y.; Li, L.; Qu, B.; Man, C. Induction of cytokines via NF-kappa B and p38 MAP kinase signalling pathways associated with the immunomodulation by Lactobacillus plantarum NDC 75017 in vitro and in vivo. J. Funct. Foods 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT-Food Sci. Technol. 2021, 139, 110590. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, S.; Dang, F.; Wang, Z.; Man, C.; Jiang, Y. Screening and study on activity in the simulated gastrointestinal conditions of a cholesterol-lowering Lactic acid bacteria. China Dairy Ind. 2018, 46, 4–7. [Google Scholar]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989, 24, 197–211. [Google Scholar]
- Jin, H.-R.; Lee, S.; Choi, S.-J. Pharmacokinetics and protective effects of tartary buckwheat flour extracts against ethanol-induced liver injury in rats. Antioxidants 2020, 9, 913. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Solovieva, N.V.; Leikhter, S.N.; Shidakova, N.A.; Lebedeva, O.V.; Sidorov, P.I.; Bazhukova, T.A.; Soloviev, A.G.; Barve, S.S.; McClain, C.J.; et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol 2008, 42, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Gam, D.H.; Park, J.H.; Kim, S.H.; Kang, M.H.; Kim, S.B.; Kim, J.W. Production of bioactive substances to alleviates hangover and ethanol-induced liver damage through fermentation of oenanthe javanica using Lactiplantibacillus plantarum. Molecules 2022, 27, 1175. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; McMullen, M.; Pritchard, M.T.; Nagy, L.E. Regulation of macrophage activation in alcoholic liver disease. J. Gastroenterol. Hepatol. 2008, 23, 501. [Google Scholar]
- Thurman, R.G. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Gastroenterol. 1998, 275, G605–G611. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Kono, H.; Yin, M.; Nakagami, M.; Uesugi, T.; Arteel, G.E.; Gabele, E.; Rusyn, I.; Yamashina, S.; Froh, M.; et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic. Biol. Med. 2001, 31, 1544–1549. [Google Scholar] [CrossRef]
- Dhanda, A.D.; Collins, P.L. Immune dysfunction in acute alcoholic hepatitis. World J. Gastroenterol. 2015, 21, 11904–11913. [Google Scholar] [CrossRef]
- Luo, P.P.; Wang, F.; Wong, N.K.; Lv, Y.; Li, X.X.; Li, M.H.; Tipoe, G.L.; So, K.F.; Xu, A.M.; Chen, S.Y.; et al. Divergent roles of Kupffer Cell TLR2/3 signaling in alcoholic liver disease and the protective role of EGCG. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 145–160. [Google Scholar] [CrossRef]
- Sangineto, M.; Grabherr, F.; Adolph, T.E.; Grander, C.; Reider, S.; Jaschke, N.; Mayr, L.; Schwaerzler, J.; Dallio, M.; Moschen, A.R.; et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. 2020, 40, 1610–1619. [Google Scholar] [CrossRef]
- Yang, X.; He, F.; Zhang, Y.; Xue, J.; Li, K.; Zhang, X.; Zhu, L.; Wang, Z.; Wang, H.; Yang, S. Inulin ameliorates alcoholic liver disease via suppressing LPS-TLR4-M axis and modulating gut microbiota in mice. Alcohol. Clin. Exp. Res. 2019, 43, 411–424. [Google Scholar] [CrossRef]
- Loguercio, C.; De Simone, T.; Federico, A.; Terracciano, F.; Tuccillo, C.; Di Chicco, M.; Carteni, M. Gut-liver axis: A new point of attack to treat chronic liver damage? Am. J. Gastroenterol. 2002, 97, 2144–2146. [Google Scholar] [CrossRef]
- Pedrazza, L.; Schneider, T.; Bartrons, R.; Ventura, F.; Luis Rosa, J. The ubiquitin ligase HERC1 regulates cell migration via RAF-dependent regulation of MKK3/p38 signaling. Sci. Rep. 2020, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ding, H.; Fang, J.; Fang, X.; Liu, H.; Wang, J.; Chen, C.; Zhang, W. Aquaporin 9 represents a novel target of chronic liver injury that may antagonize its progression by reducing lipotoxicity. Oxid. Med. Cell. Longev. 2021, 2021, 5653700. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cao, H.; Rodrigues, R.M.; Xu, M.; Ren, T.; He, Y.; Hwang, S.; Feng, D.; Ren, R.; Yang, P.; et al. Chronic-plus-binge alcohol intake induces production of proinflammatory mtDNA-enriched extracellular vesicles and steatohepatitis via ASK1/p38MAPK alpha-dependent mechanisms. JCI Insight 2020, 5, e136496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Wang, L.; Chen, W.-D.; Duan, Y.-T.; Sun, M.-J.; Huang, J.-J.; Peng, D.-Y.; Yu, N.-J.; Wang, Y.-Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 9, 963598. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Li, Y.; Dong, S.; Liu, H.; Chen, J.; Wang, Y.; Zhao, S.; Zhang, Y.; Zhang, H. Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur. J. Nutr. 2016, 55, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Meena, A.S.; Dalal, K.; Canelas, C.; Samak, G.; Pierre, J.F.; Rao, R. Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 2021, 11, 826. [Google Scholar] [CrossRef]
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef]
- Adolph, T.E.; Grander, C.; Moschen, A.R.; Tilg, H. Liver-microbiome axis in health and disease. Trends Immunol. 2018, 39, 712–723. [Google Scholar] [CrossRef]
- Huang, H.; Lin, Z.; Zeng, Y.; Lin, X.; Zhang, Y. Probiotic and glutamine treatments attenuate alcoholic liver disease in a rat model. Exp. Ther. Med. 2019, 18, 4733–4739. [Google Scholar] [CrossRef]
- Sun, S.; Wang, K.; Sun, L.; Cheng, B.; Qiao, S.; Dai, H.; Shi, W.; Ma, J.; Liu, H. Therapeutic manipulation of gut microbiota by polysaccharides of Wolfiporia cocos reveals the contribution of the gut fungi-induced PGE(2) to alcoholic hepatic steatosis. Gut Microbes 2020, 12, 1830693. [Google Scholar] [CrossRef] [PubMed]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The gastrointestinal microbiome alcohol effects on the composition of intestinal microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar] [PubMed]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; Shields, C.E.D.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe 2018, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Surana, N.K.; Kasper, D.L. The yin yang of bacterial polysaccharides: Lessons learned from B. fragilis PSA. Immunol. Rev. 2012, 245, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, M.; Yu, Q.; Chen, Y.; Chen, X.; Yang, J.; Huang, L.; Guo, X.; Xie, J. Cyclocarya paliurus polysaccharide improves metabolic function of gut microbiota by regulating short-chain fatty acids and gut microbiota composition. Food Res. Int. 2021, 141, 110119. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Kalliomaki, M.; Arkkila, P.; Satokari, R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef]
- Koay, Y.C.; Wali, J.A.; Luk, A.W.S.; Macia, L.; Cogger, V.C.; Pulpitel, T.J.; Wahl, D.; Solon-Biet, S.M.; Holmes, A.; Simpson, S.J.; et al. Ingestion of resistant starch by mice markedly increases microbiome-derived metabolites. FASEB J. 2019, 33, 8033–8042. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Wang, X.; Luo, L.; Sun, K.; Zeng, L. Gut microbiome and metabolome response of Pu-erh tea on metabolism disorder induced by chronic alcohol consumption. J. Agric. Food Chem. 2020, 68, 6615–6627. [Google Scholar] [CrossRef]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of low-protein diet and inulin on microbiota and clinical parameters in patients with chronic kidney disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef]
- Pinero, F.; Vazquez, M.; Bare, P.; Rohr, C.; Mendizabal, M.; Sciara, M.; Alonso, C.; Fay, F.; Silva, M. A different gut microbiome linked to inflammation found in cirrhotic patients with and without hepatocellular carcinoma. Ann. Hepatol. 2019, 18, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef] [PubMed]



| Type | Gene Name | Base Sequence (5′ → 3′) |
|---|---|---|
| Tight junction protein | Claudin-1 | F: AGATACAGTGCAAAGTCTTCGA R: CAGGATGCCAATTACCATCAAG |
| Occludin | F: TGCTTCATCGCTTCCTTAGTAA R: GGGTTCACTCCCATTATGTACA | |
| ZO-1 | F: CTGGTGAAGTCTCGGAAAAATG R: CATCTCTTGCTGCCAAACTATC |
| Group | TG (mmol/L) | TC (mmol/L) |
|---|---|---|
| NC | 0.93 ± 0.07 * | 1.52 ± 0.20 ** |
| Alcohol | 1.25 ± 0.08 | 2.23 ± 0.08 |
| LLP | 1.10 ± 0.10 | 1.95 ± 0.19 |
| MLP | 1.08 ± 0.10 | 1.70 ± 0.13 * |
| HLP | 0.89 ± 0.05 * | 1.65 ± 0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cheng, S.; Huo, J.; Dong, K.; Ding, Y.; Man, C.; Zhang, Y.; Jiang, Y. Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients 2023, 15, 190. https://doi.org/10.3390/nu15010190
Li H, Cheng S, Huo J, Dong K, Ding Y, Man C, Zhang Y, Jiang Y. Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients. 2023; 15(1):190. https://doi.org/10.3390/nu15010190
Chicago/Turabian StyleLi, Hongxuan, Shasha Cheng, Jiacheng Huo, Kai Dong, Yixin Ding, Chaoxin Man, Yu Zhang, and Yujun Jiang. 2023. "Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways" Nutrients 15, no. 1: 190. https://doi.org/10.3390/nu15010190
APA StyleLi, H., Cheng, S., Huo, J., Dong, K., Ding, Y., Man, C., Zhang, Y., & Jiang, Y. (2023). Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients, 15(1), 190. https://doi.org/10.3390/nu15010190






