Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities
Abstract
1. Introduction
2. Chemical Content of Seeds
3. Bioavailability and Dosage
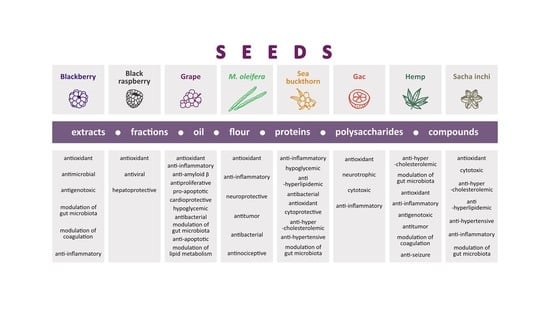
4. Chemical Content of Seeds Eaten with Whole Fruit and Biological Activity of Seed Extracts, Fractions, Oil, Proteins, Lipids, Polysaccharides, and Isolated Compounds
4.1. Blackberry (Rubus fruticosus L.)
4.1.1. Chemical Content of Blackberry Seeds
4.1.2. Biological Activities of Extracts from Blackberry Seeds
Antioxidant Activity (In Vitro)
Antimicrobial Activity (In Vitro)
Antigenotoxic Activity (In Vitro)
4.1.3. Biological Activities of Blackberry Seed Flour, Polysaccharides, and Compounds
Modulation of Gut Microbiota by Blackberry Seed Flour (In Vitro)
Modulation of Coagulation by Blackberry Seed Polysaccharides (In Vitro and In Vivo)
Anti-Inflammatory Activity of Ellagic Acid and Ellagitannins (In Vitro and In Vivo)
Activity of Sanguiin H-6 (In Vitro and In Vivo)
4.2. Black Raspberry (Rubus coreanus Miq.)
4.2.1. Chemical Content of Black Raspberry Seeds
4.2.2. Biological Activities of Extracts from Black Raspberry Seeds
Antioxidant Activity (In Vitro)
4.2.3. Hepatoprotective Activity of Black Raspberry Seed Oil (In Vitro)
4.3. Grape (Vitis vinifera L.)
4.3.1. Chemical Content of Grape Seeds
4.3.2. Biological Activity of Extracts from Grape Seeds
Antioxidant Activity (In Vitro and In Vivo)
Anti-Inflammatory Activity (In Vivo)
Inhibition of Amyloid β Oligomerization (In Vitro)
Anti-Proliferative and Pro-Apoptosis Activity Cancer Cells (In Vitro)
Cardioprotective Activity (In Vitro and In Vivo)
Hypoglycemic Activity (In Vitro and In Vivo)
Antibacterial Activity (In Vitro)
Modulation of Gut Microbiota (In Vivo)
4.3.3. Biological Activity of Grape Seed Oil and Compounds
Antioxidant Activity of Grape Seed Proanthocyanidins, Oligomeric Procyanidins, and Oil (In Vitro)
Anti-Inflammatory Activity of Unsaponifiable Fraction from Grape Seed Oil (In Vitro)
Anti-Apoptotic Activity of Grape Seed Proanthocyanidins and Procyanidins (In Vitro and In Vivo)
Anti-Proliferative and Pro-Apoptosis Activity of Grape Seed Proanthocyanidins toward Cancer Cells (In Vitro)
Cardioprotective Activity of Grape Seed Procyanidins, Proanthocyanidins, and Polyphenols (In Vitro and In Vivo)
Modulation of Lipid Metabolism by Grape Seed Procyanidins (In Vitro)
Hypoglycemic Activity of Grape Seed Oil (In Vitro)
Modulation of Gut Microbiota by Grape Seed Proanthocyanidins (In Vivo)
4.4. Moringa oleifera Lam.
4.4.1. Chemical Content of M. oleifera Seeds
4.4.2. Biological Activities of M. oleifera Seed Extracts
Antioxidant Activity (In Vivo)
Anti-Inflammatory Activity (In Vivo)
Neuroprotective Activity (In Vivo)
Cytotoxic Activity toward Cancer Cells (In Vitro)
Antibacterial Activity (In Vitro)
4.4.3. Biological Activities of M. oleifera Seed Oil, Meal, Proteins, and Compounds
Antioxidant Activity of M. oleifera Seed Proteins (In Vitro)
Anti-Inflammatory Activity of M. oleifera Seed Oil (In Vivo)
Neuroprotective Activity of M. oleifera Seed Oil (In Vivo)
Cytotoxic Activity of M. oleifera Seed Oil and Lectin toward Cancer Cells (In Vitro and In Vivo)
Antibacterial Activity of M. oleifera Seed Meal Extract, and Lectin (In Vitro)
Activity of MIC-1 (Moringin) (In Vitro and In Vivo)
Activity of Niazirin (In Vitro and In Vivo)
4.5. Sea Buckthorn (Hippophae rhamnoides L.)
4.5.1. Chemical Content of Sea Buckthorn Seeds
4.5.2. Biological Activities of Sea Buckthorn Seed Extracts
Anti-Inflammatory and Hypoglycemic (In Vivo)
Anti-Hyperlipidemic Activity (In Vivo)
Antibacterial Activity (In Vitro)
4.5.3. Biological Activity of Sea Buckthorn Seed Oil, Proteins, and Compounds
Antioxidant Activity of Sea Buckthorn Seed Oil, and Flavonoids (In Vitro and In Vivo)
Anti-Inflammatory and Hypoglycemic Activity of Sea Buckthorn Seed Flavonoids and Proteins (In Vitro and In Vivo)
Cytoprotective Activity of Sea Buckthorn Seed Alkaloids (In Vitro)
Anti-Hyperlipidemic and Anti-Hypercholesterolemic Activity of Sea Buckthorn Seed Oil and Proteins (In Vivo)
Anti-Hypertensive Activity of Sea Buckthorn Seed Oil and Total Flavones (In Vivo)
Modulation of Gut Microbiota by Sea Buckthorn Seed Oil and Protein (In Vivo)
5. Chemical Content of Seeds Eaten Separately and Biological Activity of Seed Extracts, Fractions, Oil, Proteins, Lipids, Polysaccharides, and Isolated Compounds
5.1. Gac (Momordica cochinchinensis Sprenger)
5.1.1. Chemical Content of Gac Seeds
5.1.2. Biological Activities of Gac Seed Extracts
Antioxidant Activity (In Vitro)
Neurotrophic Activity (In Vitro)
Cytotoxic Activity toward Healthy and Cancer Cells (In Vitro and In Vivo)
5.1.3. Biological Activity of Gac Seed Compounds
Antioxidant Activity of MCoCI (In Vitro)
Anti-Inflammatory Activity of Gac Seed Lignans and Saponins (In Vitro)
5.2. Hemp (Cannabis sativa L.)
5.2.1. Chemical Content of Hemp Seeds
5.2.2. Biological Activities of Hemp Seeds
Anti-Hypercholesterolemic Activity (In Vivo)
Modulation of Gut Microbiota by Hemp Seeds (In Vivo)
5.2.3. Biological Activities Hemp Seed Proteins, Polysaccharides, Lipids, and Compounds
Antioxidant Activity of Hemp Seed Proteins and Polysaccharides (In Vitro and In Vivo)
Anti-Inflammatory Activity of Hemp Seed Lipids and Lignanamides (In Vitro and In Vivo)
Cytotoxic Activity of Hemp Seed Lignanamides toward Cancer Cells (In Vitro)
Anti-Hypercholesterolemic and Anti-Hyperlipidemic Activity of Hempseed Lipid Fractions and Oil (In Vivo)
Activity of Cannabidiol (In Vitro and In Vivo)
5.3. Sacha inchi (Plukenetia volubilis L.)
5.3.1. Chemical Content of Sacha Inchi Seeds
5.3.2. Biological Activities of Sacha Inchi Seed Extracts
Antioxidant Activity (In Vitro)
Cytotoxic Activity (In Vitro)
5.3.3. Biological Activities of Sacha Inchi Seed Polysaccharide, Proteins, and Oil
Antioxidant Activity of Sacha Inchi Seed Polysaccharide and Oil (In Vitro and In Vivo)
Immunomodulatory Activity of Sacha Inchi Seed Polysaccharide and Protein (In Vitro)
Anti-Hypercholesterolemic, Antihyperlipidemic, and Anti-Hypertensive Activity of Sacha Inchi Seed Oil (In Vivo)
Anti-Inflammatory Activity of Sacha Inchi Proteins and Oil (In Vitro and In Vivo)
Modulation of Gut Microbiota by Sacha Inchi Oil and Press-Cake Protein (In Vivo)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins—Promising Molecules with Broad Biological Potential. Int. J. Mol. Sci. 2021, 22, 12972. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Zhao, C.-N.; Liu, Q.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Potential of Grape Wastes as a Natural Source of Bioactive Compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoidesL.) seeds. Int. J. Food Sci. Nutr. 2012, 63, 730–738. [Google Scholar] [CrossRef]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.Y.; Yamin, G.; Beroukhim, S.; Chen, B.; Kibalchenko, M.; Jiang, L.; Ho, L.; Wang, J.; Pasinetti, G.M.; Teplow, D.B. Inhibiting amyloid β-protein assembly: Size-activity relationships among grape seed-derived polyphenols. J. Neurochem. 2015, 135, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, M.; De Moura, C.; Junior, T.K.; Pap, N.; Mattila, P.H.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed]
- Ghanghas, N.; Mukilan, M.T.; Sharma, S.; Prabhakar, P.K. Classification, Composition, Extraction, Functional Modification and Application of Rice (Oryza sativa) Seed Protein: A Comprehensive Review. Food Rev. Int. 2022, 38, 354–383. [Google Scholar] [CrossRef]
- Garza, N.G.G.; Koyoc, J.A.C.; Castillo, J.A.T.; Zambrano, E.A.G.; Ancona, D.B.; Guerrero, L.C.; García, S.R.S. Biofunctional properties of bioactive peptide fractions from protein isolates of moringa seed (Moringa oleifera). J. Food Sci. Technol. 2017, 54, 4268–4276. [Google Scholar] [CrossRef]
- Tian, W.; Xiao, N.; Yang, Y.; Xiao, J.; Zeng, R.; Xie, L.; Qiu, Z.; Li, P.; Du, B. Structure, antioxidant and immunomodulatory activity of a polysaccharide extracted from Sacha inchi seeds. Int. J. Biol. Macromol. 2020, 162, 116–126. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Zhang, K.; Lu, Y.; Wu, Q.; Chen, J.; Li, Y.; Wu, Q.; Chen, Y. Extraction, purification, structural characterization, and gut microbiota relationship of polysaccharides: A review. Int. J. Biol. Macromol. 2022, 213, 967–986. [Google Scholar] [CrossRef]
- George, E.S.; Daly, R.M.; Tey, S.L.; Brown, R.; Wong, T.H.T.; Tan, S.-Y. Perspective: Is it Time to Expand Research on “Nuts” to Include “Seeds”? Justifications and Key Considerations. Adv. Nutr. 2022, 13, 1016–1027. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Sahu, P.K.; Chakradhari, S.; Sahu, Y.K.; Patel, K.S.; Singh, S.; Towett, E.K.; Martín-Ramos, P.; Quesada-Granados, J.J.; Rufián-Henares, J.A. Plant seeds as source of nutrients and phytochemicals for the Indian population. Int. J. Food Sci. Technol. 2022, 57, 525–532. [Google Scholar] [CrossRef]
- Bueno-Borges, L.B.; Sartim, M.A.; Gil, C.C.; Sampaio, S.V.; Rodrigues, P.H.V.; Regitano-D’Arce, M.A.B. Sacha inchi seeds from sub-tropical cultivation: Effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018, 55, 4159–4166. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of Byproducts Originating from Hemp Oil Processing. J. Agric. Food Chem. 2014, 62, 12346–12442. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Labbé, D.P.; Bairati, I.; Fradet, V.; Bazinet, L.; Têtu, B. Green tea for ovarian cancer prevention and treatment: A systematic review of the in vitro, in vivo and epidemiological studies. Gynecol. Oncol. 2012, 126, 491–498. [Google Scholar] [CrossRef]
- Gouveia, H.J.C.B.; Urquiza-Martínez, M.V.; Manhães-De-Castro, R.; Costa-De-Santana, B.J.R.; Villarreal, J.P.; Mercado-Camargo, R.; Torner, L.; Aquino, J.D.S.; Toscano, A.E.; Guzmán-Quevedo, O. Effects of the Treatment with Flavonoids on Metabolic Syndrome Components in Humans: A Systematic Review Focusing on Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 8344. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Junior, T.; de Moura, C.; Carmo, M.D.; Azevedo, L.; Esmerino, L.; Tardivo, R.; Kilpeläinen, P.; Granato, D. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic/Cytoprotective Activity of Non-Polar Extracts of Grape (Vitis labrusca cv. Bordeaux) and Blackberry (Rubus fruticosus) Seeds. Molecules 2021, 26, 4057. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.-M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.; Harada, H.; Yin, X.-M. Cyanidin-3-rutinoside, a Natural Polyphenol Antioxidant, Selectively Kills Leukemic Cells by Induction of Oxidative Stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ouyang, J.; Hu, N.; Li, G.; Wang, H. Protective Effect of Two Alkaloids from Hippophae rhamnoides Linn. against Doxorubicin-Induced Toxicity in H9c2 Cardiomyoblasts. Molecules 2021, 26, 1946. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Koche, D.; Shirsat, R.; Kawale, M. An Overview of Major Classes of Phytochemicals: Their Types and Role in Disease Prevention. Hislopia J. 2018, 9, 1–11. [Google Scholar]
- Ożarowski, M.; Karpiński, T.; Zielińska, A.; Souto, E.; Wielgus, K. Cannabidiol in Neurological and Neoplastic Diseases: Latest Developments on the Molecular Mechanism of Action. Int. J. Mol. Sci. 2021, 22, 4294. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hao, J.; He, J.; Zhang, J.; Li, Y.; Liu, R.; Li, L. Cannabisin B induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cells. Food Chem. 2013, 138, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Shen, X.; Zengin, G.; Lyu, Y.; Xiao, J.; Weng, Z. Niazirin from Moringa oleifera Lam. attenuates high glucose-induced oxidative stress through PKCζ/Nox4 pathway. Phytomedicine 2021, 86, 153066. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Rathinapriya, P.; Balaji, S.; Balan, D.J.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in Seaweeds: An Overview on Biosynthesis to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.; Georges, B.; McTier, O.; Soliman, K.F.A. Neurotrophic Effects of Mu Bie Zi (Momordica cochinchinensis) Seed Elucidated by High-Throughput Screening of Natural Products for NGF Mimetic Effects in PC-12 Cells. Neurochem. Res. 2015, 40, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Puah, B.-P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, H.-K. Sanguiin H-6 blocks endothelial cell growth through inhibition of VEGF binding to VEGF receptor. Arch. Pharmacal Res. 2005, 28, 1270–1274. [Google Scholar] [CrossRef]
- Sakai, E.; Aoki, Y.; Yoshimatsu, M.; Nishishita, K.; Iwatake, M.; Fukuma, Y.; Okamoto, K.; Tanaka, T.; Tsukuba, T. Sanguiin H-6, a constituent of Rubus parvifolius L., inhibits receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis and bone resorption in vitro and prevents tumor necrosis factor-α-induced osteoclast formation in vivo. Phytomedicine 2016, 23, 828–837. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, D.; Baek, S.-E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.-E. Cytotoxic effect of sanguiin H-6 on MCF-7 and MDA-MB-231 human breast carcinoma cells. Bioorganic Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic Activity of Sanguiin H-6 through Activation of Estrogen Receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Aguilera-Correa, J.J.; Fernández-López, S.; Cuñas-Figueroa, I.D.; Pérez-Rial, S.; Alakomi, H.-L.; Nohynek, L.; Oksman-Caldentey, K.-M.; Salminen, J.-P.; Esteban, J.; Cuadros, J.; et al. Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection. Antibiotics 2021, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Zhang, Z.; Li, Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase–silent mating type information regulation 2 homologue 1–PPARγ co-activator-1α axis in rat mesangial cells under high-dose glucosamine. Br. J. Nutr. 2015, 113, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Sun, F.J.; Lu, J.X.; Cui, L.; Chen, H.H.; Wang, C.F.; Lin, H.B. The Study of Relation of Toxicity and Pharmacodynamic Action With Cochinchina Momordica Seed Crem With Different Oil Content. Liao-Ning J. Tradit. Chin. Med. 2010, 37, 946–948. [Google Scholar]
- Yu, J.S.; Sahar, N.E.; Bi, Y.-R.; Jung, K.; Pang, C.; Huh, J.Y.; Kim, K.H. The Effects of Triterpenoid Saponins from the Seeds of Momordica cochinchinensis on Adipocyte Differentiation and Mature Adipocyte Inflammation. Plants 2020, 9, 984. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Lu, R.-R.; Qian, P.; Sun, Z.; Zhou, X.-H.; Chen, T.-P.; He, J.-F.; Zhang, H.; Wu, J. Hempseed protein derived antioxidative peptides: Purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010, 123, 1210–1218. [Google Scholar] [CrossRef]
- Liang, L.; Wang, C.; Li, S.; Chu, X.; Sun, K. Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, X.; Wang, W.; Meng, L.; Chen, D.; Zhang, C. Hypoglycemic and anti-inflammatory effects of seabuckthorn seed protein in diabetic ICR mice. Food Funct. 2016, 7, 1610–1615. [Google Scholar] [CrossRef]
- Xue, R.; Du, M.; Zhou, T.-Y.; Ai, W.-Z.; Zhang, Z.-S.; Xiang, X.-W.; Zhou, Y.-F.; Wen, Z.-S. Polysaccharides from Hemp Seed Protect against Cyclophosphamide-Induced Intestinal Oxidative Damage via Nrf2-Keap1 Signaling Pathway in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 1813798. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kehinde, B.A.; Sharma, P.; Sharma, D.; Kaur, S. Recently isolated food-derived antihypertensive hydrolysates and peptides: A review. Food Chem. 2021, 346, 128719. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Du, Z.-M.; Wang, Y.-W.; Feng, Y.-X.; Zhang, R.; Yan, X.-B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Le, A.V.; Huynh, T.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines 2018, 5, 104. [Google Scholar] [CrossRef]
- Capecchi, A.; Probst, D.; Reymond, J.-L. One molecular fingerprint to rule them all: Drugs, biomolecules, and the metabolome. J. Cheminform. 2020, 12, 43. [Google Scholar] [CrossRef]
- Cui, C.; Shi, A.; Bai, S.; Yan, P.; Li, Q.; Bi, K. Novel Antihypertensive Prodrug from Grape Seed Proanthocyanidin Extract via Acid-Mediated Depolymerization in the Presence of Captopril: Synthesis, Process Optimization, and Metabolism in Rats. J. Agric. Food Chem. 2018, 66, 3700–3707. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxidative Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The fate of flavonoids after oral administration: A comprehensive overview of its bioavailability. Crit. Rev. Food Sci. Nutr. 2021, 62, 6169–6186. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. J. Pharm. Sci. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From waste to health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M.; Saadat, Y.R.; Khezri, K.; Jafari, S.; Ahmadian, E.; Jahandizi, N.G.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytotherapy Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose Conversion Between Animals and Humans: A Practical Solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from blackberry (Rubus fruticosus ) growing in Poland. J. Sci. Food Agric. 2017, 97, 3576–3583. [Google Scholar] [CrossRef]
- Wang, J.; Lian, P.; Yu, Q.; Wei, J.; Kang, W. Antithrombotic mechanism of polysaccharides in Blackberry (Rubus spp.) seeds. Food Nutr. Res. 2017, 61, 1379862. [Google Scholar] [CrossRef]
- Lechowicz, K.; Wrońska-Pilarek, D.; Bocianowski, J.; Maliński, T. Pollen morphology of Polish species from the genus Rubus L. (Rosaceae) and its systematic importance. PLoS ONE 2020, 15, e0221607. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Vajs, V.; Milosavljević, S.; Stanković, M. Blackberry Seed Extracts and Isolated Polyphenolic Compounds Showing Protective Effect on Human Lymphocytes DNA. J. Food Sci. 2011, 76, C1039–C1043. [Google Scholar] [CrossRef]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.T.; Qin, X.; Kaul, S.; Barrientos, G.; Zou, Z.; Mathias, C.B.; Thomas, D.; Bose, D.D. The polyphenol ellagic acid exerts anti-inflammatory actions via disruption of store-operated calcium entry (SOCE) pathway activators and coupling mediators. Eur. J. Pharmacol. 2020, 875, 173036. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus Fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Rhyu, D.Y.; Tanaka, T.; Park, J.C.; Kitani, K. Potential of Sanguiin H-6 against Oxidative Damage in Renal Mitochondria and Apoptosis Mediated by Peroxynitrite in vivo. Nephron 2002, 92, 133–141. [Google Scholar] [CrossRef]
- Lee, D.; Ko, H.; Kim, Y.-J.; Kim, S.-N.; Choi, K.-C.; Yamabe, N.; Kim, K.H.; Kang, K.S.; Kim, H.Y.; Shibamoto, T. Inhibition of A2780 Human Ovarian Carcinoma Cell Proliferation by a Rubus Component, Sanguiin H-6. J. Agric. Food Chem. 2016, 64, 801–805. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Antioxidant activities of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour. Technol. 2008, 99, 4503–4509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.-I.; Bae, S.Y.; Hong, Y.-M.; Kwon, S.-O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses 2016, 8, 157. [Google Scholar] [CrossRef]
- Choi, M.-H.; Shim, S.-M.; Kim, G.-H. Protective effect of black raspberry seed containing anthocyanins against oxidative damage to DNA, protein, and lipid. J. Food Sci. Technol. 2016, 53, 1214–1221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teng, H.; Lin, Q.; Li, K.; Yuan, B.; Song, H.; Peng, H.; Yi, L.; Wei, M.-C.; Yang, Y.-C.; Battino, M.; et al. Hepatoprotective effects of raspberry (Rubus coreanus Miq.) seed oil and its major constituents. Food Chem. Toxicol. 2017, 110, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Kang, H.; Lee, S.-G. Antioxidant and anti-inflammatory effects of seed ethanol extracts of Rubus coreanus miquel. J. Plant Biotechnol. 2022, 49, 155–161. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, S.Y.; Oh, M.; Seok, J.H.; Kim, S.; Chung, Y.B.; Gowda, K.G.; Mun, J.Y.; Chung, M.S.; Kim, K.H. Antiviral effects of black raspberry (Rubus coreanus) seed extract and its polyphenolic compounds on norovirus surrogates. Biosci. Biotechnol. Biochem. 2016, 80, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Urciuoli, S.; Arlorio, M.; Paoli, P.; Mulinacci, N. In depth study of phenolic profile and PTP-1B inhibitory power of cold-pressed grape seed oils of different varieties. Food Chem. 2019, 271, 380–387. [Google Scholar] [CrossRef]
- Kadri, S.; El Ayed, M.; Mabrouk, M.; Limam, F.; Elkahoui, S.; Aouani, E.; Mokni, M. Characterization, anti-oxidative effect of grape seed powder and in silico affinity profiling of polyphenolic and extra-phenolic compounds for calpain inhibition. J. Pharm. Biomed. Anal. 2019, 164, 365–372. [Google Scholar] [CrossRef]
- He, X.; Guo, X.; Ma, Z.; Li, Y.; Kang, J.; Zhang, G.; Gao, Y.; Liu, M.; Chen, H.; Kang, X. Grape seed proanthocyanidins protect PC12 cells from hydrogen peroxide-induced damage via the PI3K/AKT signaling pathway. Neurosci. Lett. 2021, 750, 135793. [Google Scholar] [CrossRef]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Di, Y.; Lian, Z.; Zhu, B.; Xu, X.; Guo, D.; Huang, Q.; Jiang, C.; Kong, J.; Shi, J. Grape seed proanthocyanidins suppressed macrophage foam cell formation by miRNA-9 via targeting ACAT1 in THP-1 cells. Food Funct. 2020, 11, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Bosso, A.; Cassino, C.; Motta, S.; Panero, L.; Tsolakis, C.; Guaita, M. Polyphenolic Composition and In Vitro Antioxidant Activity of Red Grape Seeds as Byproducts of Short and Medium-Long Fermentative Macerations. Foods 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef]
- Foshati, S.; Rouhani, M.H.; Amani, R. The effect of grape seed extract supplementation on oxidative stress and inflammation: A systematic review and meta-analysis of controlled trials. Int. J. Clin. Pract. 2021, 75, e14469. [Google Scholar] [CrossRef]
- Liu, H.-H.; Cao, Y.-X.; Sun, D.; Jin, J.-L.; Zhang, H.-W.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Gao, Y.; Dong, Q.-T.; et al. High-sensitivity C-reactive protein and hypertension: Combined effects on coronary severity and cardiovascular outcomes. Hypertens. Res. 2019, 42, 1783–1793. [Google Scholar] [CrossRef]
- Feringa, H.H.; Laskey, D.; Dickson, J.E.; Coleman, C. The Effect of Grape Seed Extract on Cardiovascular Risk Markers: A Meta-Analysis of Randomized Controlled Trials. J. Am. Diet. Assoc. 2011, 111, 1173–1181. [Google Scholar] [CrossRef]
- Soleimani, M.; Homayoun, M.; Targhi, R.G. Anti-proliferative and anti-apoptotic effects of grape seed extract on chemo-resistant OVCAR-3 ovarian cancer cells. Res. Pharm. Sci. 2020, 15, 390–400. [Google Scholar] [CrossRef]
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-Lowering Activity of the Major Polyphenols in Grape Seed. Molecules 2011, 16, 5054–5061. [Google Scholar] [CrossRef]
- Bijak, M.; Sut, A.; Kosiorek, A.; Saluk-Bijak, J.; Golanski, J. Dual Anticoagulant/Antiplatelet Activity of Polyphenolic Grape Seeds Extract. Nutrients 2019, 11, 93. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, J.; Zhao, S.; You, B.; Ji, X.; Wang, Y.; Cui, X.; Wang, Q.; Gao, H. Grape seed proanthocyanidin extract alleviates ouabain-induced vascular remodeling through regulation of endothelial function. Mol. Med. Rep. 2012, 6, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Nishioka, S.; Kiuchi, M.; Imada, Y.; Makino, K.; Nakagawa, K.; Tanaka, R.; Matsumura, Y.; Ohkita, M. Grape Extract from Chardonnay Seeds Restores Deoxycorticosterone Acetate–Salt-Induced Endothelial Dysfunction and Hypertension in Rats. Biol. Pharm. Bull. 2020, 43, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Shahnaz, T.; Sorayya, K. Effect of 8 weeks’ supplementation grape seed extract on insulin resistance in iranian adolescents with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 197–203. [Google Scholar] [CrossRef]
- Asbaghi, O.; Nazarian, B.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Chamani, M.; Asemi, Z. The effects of grape seed extract on glycemic control, serum lipoproteins, inflammation, and body weight: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 239–253. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Selvi, T.; Sakariah, K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Brown, J.C.; Huang, G.; Haley-Zitlin, V.; Jiang, X. Antibacterial Effects of Grape Extracts on Helicobacter Pylori. Appl. Environ. Microbiol. 2009, 75, 848–852. [Google Scholar] [CrossRef]
- Lu, F.; Liu, F.; Zhou, Q.; Hu, X.; Zhang, Y. Effects of grape pomace and seed polyphenol extracts on the recovery of gut microbiota after antibiotic treatment in high-fat diet-fed mice. Food Sci. Nutr. 2019, 7, 2897–2906. [Google Scholar] [CrossRef]
- Han, H.; Wang, H.; Du, Y.; Gao, L. Grape Seed Procyanidins Attenuates Cisplatin-induced Human Embryonic Renal Cell Cytotoxicity by Modulating Heme Oxygenase-1 in Vitro. Cell Biochem. Biophys. 2019, 77, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Iida, K.; Kang, M.-I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Millan-Linares, M.C.; Bermudez, B.; Martin, M.E.; Muñoz, E.; Abia, R.; Millan, F.; Muriana, F.J.G.; la Paz, S.M.-D. Unsaponifiable fraction isolated from grape (Vitis vinifera L.) seed oil attenuates oxidative and inflammatory responses in human primary monocytes. Food Funct. 2018, 9, 2517–2523. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.; Chen, Y.; Sheng, S.; Huang, Z. Grape seed procyanidins suppress the apoptosis and senescence of chondrocytes and ameliorates osteoarthritis via the DPP4-Sirt1 pathway. Food Funct. 2020, 11, 10493–10505. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhan, L.; Guo, D.; Xiang, Y.; Tian, M.; Zhang, Y.; Wu, H.; Wei, Y.; Ma, G.; Han, Z. Grape seed proanthocyanidins inhibit proliferation of pancreatic cancer cells by modulating microRNA expression. Oncol. Lett. 2019, 17, 2777–2787. [Google Scholar] [CrossRef]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef]
- Wei, S.; Zheng, Y.; Zhang, M.; Zheng, H.; Yan, P. Grape seed procyanidin extract inhibits adipogenesis and stimulates lipolysis of porcine adipocytes in vitro1. J. Anim. Sci. 2018, 96, 2753–2762. [Google Scholar] [CrossRef]
- Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa Oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Huang, Q.; Liu, R.; Liu, J.; Zhang, F.; Liu, S.; Jiang, Y. Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3β activity and activating the Nrf2/HO-1 pathway. Phytomedicine 2022, 95, 153856. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, Y.; Yang, W.; Ge, Y.; Xu, L.; Ren, T.; Zhang, H.; Zhuo, R.; Peng, L.; Chen, C.; et al. Moringa oleifera seed extract protects against brain damage in both the acute and delayed stages of ischemic stroke. Exp. Gerontol. 2019, 122, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, W.-S.; Suo, D.-Q.; Li, Y.; Peng, L.; Xu, L.-X.; Zeng, K.-Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef]
- Aldakheel, R.K.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Gondal, M.A.; Mostafa, A.; Baykal, A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals 2020, 13, 193. [Google Scholar] [CrossRef]
- Adebayo, I.; Arsad, H.; Samian, M.R. Antiproliferative effect on breast cancer (MCF7) of Moringa oleifera seed extracts. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Enan, G.; Al-Mohammadi, A.-R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.; Taha, M.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC554891 by Moringa oleifera Seed Extract either Singly or in Combination with Antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. In vitro antihypertensive and antioxidative properties of trypsin-derived Moringa oleifera seed globulin hydrolyzate and its membrane fractions. Food Sci. Nutr. 2019, 7, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Cretella, A.B.M.; Soley, B.D.S.; Pawloski, P.L.; Ruziska, R.M.; Scharf, D.R.; Ascari, J.; Cabrini, D.A.; Otuki, M.F. Expanding the anti-inflammatory potential of Moringa oleifera: Topical effect of seed oil on skin inflammation and hyperproliferation. J. Ethnopharmacol. 2020, 254, 112708. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Aja, P.M.; Nwankwo, O.E.; Awoke, J.N.; Maduagwuna, E.K.; Aloke, C. Moringa oleiferaseed oil or virgin coconut oil supplementation abrogates cerebral neurotoxicity induced by antineoplastic agent methotrexate by suppression of oxidative stress and neuro-inflammation in rats. J. Food Biochem. 2019, 43, e12748. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Zoheir, K.M.; Kishta, M.S.; Shalby, A.B.; Ezzo, M.I. Nano-Micelle of Moringa Oleifera Seed Oil Triggers Mitochondrial Cancer Cell Apoptosis. Asian Pac. J. Cancer Prev. 2016, 17, 4929–4933. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, A.; Hasan, I.; Chakrabortty, A.; Zaman, S.; Islam, S.S.; Ahmed, F.R.S.; Kabir, K.A.; Nurujjaman; Uddin, B.; Alam, M.T.; et al. Moringa oleifera seed lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of Bak and NF-κB gene expression. Int. J. Biol. Macromol. 2018, 107, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wichaphon, J.; Klangpetch, W. Antimicrobial and antioxidant activities of defatted Moringa oleifera seed meal extract obtained by ultrasound-assisted extraction and application as a natural antimicrobial coating for raw chicken sausages. Int. J. Food Microbiol. 2020, 332, 108770. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.; Trentin, D.; Napoleão, T.; Primon-Barros, M.; Xavier, A.; Carneiro, N.; Paiva, P.; Macedo, A.; Coelho, L. Multi-effect of the water-soluble Moringa oleifera lectin against Serratia marcescens and Bacillus sp.: Antibacterial, antibiofilm and anti-adhesive properties. J. Appl. Microbiol. 2017, 123, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A. Moringin, A Stable Isothiocyanate from Moringa oleifera, Activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/α-CD Pretreatment Induces Neuroprotection in an In Vitro Model of Alzheimer’s Disease: A Transcriptomic Study. Curr. Issues Mol. Biol. 2021, 43, 197–214. [Google Scholar] [CrossRef]
- Sailaja, B.S.; Aita, R.; Maledatu, S.; Ribnicky, D.; Verzi, M.P.; Raskin, I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE 2021, 16, e0248691. [Google Scholar] [CrossRef]
- Giacoppo, S.; Iori, R.; Bramanti, P.; Mazzon, E. Topical moringin cream relieves neuropathic pain by suppression of inflammatory pathway and voltage-gated ion channels in murine model of multiple sclerosis. Mol. Pain 2017, 13, 4318. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. Moringin activates Wnt canonical pathway by inhibiting GSK3β in a mouse model of experimental autoimmune encephalomyelitis. Drug Des. Dev. Ther. 2016, 10, 3291–3304. [Google Scholar] [CrossRef]
- Bao, Y.; Xiao, J.; Weng, Z.; Lu, X.; Shen, X.; Wang, F. A phenolic glycoside from Moringa oleifera Lam. improves the carbohydrate and lipid metabolisms through AMPK in db/db mice. Food Chem. 2020, 311, 125948. [Google Scholar] [CrossRef]
- Ma, N.; Tang, Q.; Wu, W.-T.; Huang, X.-A.; Xu, Q.; Rong, G.-L.; Chen, S.; Song, J.-P. Three Constituents of Moringa oleifera Seeds Regulate Expression of Th17-Relevant Cytokines and Ameliorate TPA-Induced Psoriasis-Like Skin Lesions in Mice. Molecules 2018, 23, 3256. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Lis, B.; Olas, B.; Grabarczyk, Ł.; Stochmal, A.; Żuchowski, J. Biological properties of Elaeagnus rhamnoides (L.) A. Nelson twig and leaf extracts. BMC Complement. Altern. Med. 2019, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, K.; Alirezalu, A.; Akbari, Z. Evaluation of chemical constitute, fatty acids and antioxidant activity of the fruit and seed of sea buckthorn (Hippophae rhamnoides L.) grown wild in Iran. Nat. Prod. Res. 2016, 30, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gao, W.; Ou-Yang, D.-W.; Zhang, J.; Kong, D.-Y. Three new flavonoids, hippophins K–M, from the seed residue of Hippophae rhamnoides subsp. sinensis. Nat. Prod. Res. 2014, 28, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zheng, L.; Zhao, C.; Jin, Q.; Wang, X. Chemical composition and antioxidant capacity of extracts from the whole berry, pulp and seed of Hippophae¨ rhamnoides ssp. yunnanensis. Nat. Prod. Res. 2019, 33, 3596–3600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Sun, B.; Qi, C. Understanding the role of extracts from sea buckthorn seed residues in anti-melanogenesis properties on B16F10 melanoma cells. Food Funct. 2018, 9, 5402–5416. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Pang, Z.-R.; Pan, M.-R.; Zhang, W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm. Biol. 2017, 55, 1207–1214. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef]
- Negi, P.; Chauhan, A.; Sadia, G.; Rohinishree, Y.; Ramteke, R. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005, 92, 119–124. [Google Scholar] [CrossRef]
- Arora, R.; Mundra, S.; Yadav, A.; Srivastava, R.B.; Stobdan, T. Antimicrobial activity of seed, pomace and leaf extracts of sea buckthorn (Hippophae rhamnoides L.) against foodborne and food spoilage pathogens. Afr. J. Biotechnol. 2012, 11, 10424–10430. [Google Scholar] [CrossRef]
- Vashishtha, V.; Barhwal, K.; Kumar, A.; Hota, S.K.; Chaurasia, O.P.; Kumar, B. Effect of seabuckthorn seed oil in reducing cardiovascular risk factors: A longitudinal controlled trial on hypertensive subjects. Clin. Nutr. 2017, 36, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Q.; Zhao, M.; Yang, P.; Hu, X.; Ouyang, D. Flavonoid glycosides from seeds of Hippophae rhamnoides subsp. Sinensis with α-glucosidase inhibition activity. Fitoterapia 2019, 137, 104248. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, J.; Zhang, W.; Zhuang, X.; Wang, J.; Xu, R.; Xu, Z.; Qu, W. Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 2008, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Prasad, R.; Jayamurthy, P.; Pal, K.; Arumughan, C.; Sawhney, R. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007, 14, 770–777. [Google Scholar] [CrossRef]
- Hao, W.; He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Zhao, Y.; Ma, K.Y.; He, W.-S.; Chen, Z.-Y. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. 2019, 10, 5669–5681. [Google Scholar] [CrossRef]
- Yuan, H.; Shi, F.; Meng, L.; Wang, W. Effect of sea buckthorn protein on the intestinal microbial community in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018, 107, 1168–1174. [Google Scholar] [CrossRef]
- Xu, X.-R.; Luo, C.-H.; Cao, B.; Xu, R.-C.; Wang, F.; Wei, X.-C.; Zhang, T.; Han, L.; Zhang, D.-K. A Potential Anti-Tumor Herb Bred in a Tropical Fruit: Insight into the Chemical Components and Pharmacological Effects of Momordicae Semen. Molecules 2019, 24, 3949. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, Z.; Xiong, Y.; Zhang, Y.; Li, X. Cytotoxic and anti-inflammatory constituents from Momordica cochinchinensis seeds. Fitoterapia 2019, 139, 104360. [Google Scholar] [CrossRef]
- Du, Z.; Xia, Z.; Huang, Y.; Peng, Y.; Cao, B.; Li, C.; Liang, Y.; Zhao, F.; Zhang, M.; Chen, Z.; et al. Cardiotoxicity induced by Cochinchina momordica seed extract in zebrafish. J. Appl. Toxicol. 2021, 41, 1222–1231. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef]
- Mazzio, E.; Badisa, R.; Eyunni, S.; Ablordeppey, S.; George, B.; Soliman, K.F.A. Bioactivity-Guided Isolation of Neuritogenic Factor from the Seeds of the Gac Plant (Momordica cochinchinensis). Evid. Based Complement. Altern. Med. 2018, 2018, 8953958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Y.-M.; Zhan, Y.-Z.; Liu, C.-X. Momordica cochinchinensis Seed Extracts Suppress Migration and Invasion of Human Breast Cancer ZR-75-30 Cells Via Down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, A.Y.-K.; Ng, T.-B.; Fong, W.-P. Antioxidative effect of a chymotrypsin inhibitor from Momordica cochinchinensis (Cucurbitaceae) seeds in a primary rat hepatocyte culture. J. Pept. Sci. 2005, 11, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical Analysis of Minor Bioactive Components and Cannabidiolic Acid in Commercial Hemp Seed Oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Rashid, A.; Ali, V.; Khajuria, M.; Faiz, S.; Gairola, S.; Vyas, D. GC–MS based metabolomic approach to understand nutraceutical potential of Cannabis seeds from two different environments. Food Chem. 2021, 339, 128076. [Google Scholar] [CrossRef]
- Luo, Q.; Yan, X.; Bobrovskaya, L.; Ji, M.; Yuan, H.; Lou, H.; Fan, P. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol. Cell. Biochem. 2017, 428, 129–137. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef]
- Ben Necib, R.; Manca, C.; Lacroix, S.; Martin, C.; Flamand, N.; Di Marzo, V.; Silvestri, C. Hemp seed significantly modulates the endocannabinoidome and produces beneficial metabolic effects with improved intestinal barrier function and decreased inflammation in mice under a high-fat, high-sucrose diet as compared with linseed. Front. Immunol. 2022, 13, 5080. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Gupta, M.; Kulshreshtha, E. Hempseed (Cannabis sativa) lipid fractions alleviate high-fat diet-induced fatty liver disease through regulation of inflammation and oxidative stress. Heliyon 2020, 6, e04422. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Q.; Fan, P. Cannabisin F from Hemp (Cannabis sativa) Seed Suppresses Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglia as SIRT1 Modulator. Int. J. Mol. Sci. 2019, 20, 507. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Garg, A.; Kaushal, N. Hempseed (Cannabis sativa) offers effective alternative over statins in ameliorating hypercholesterolemia associated nephropathy. Clin. Biochem. 2021, 93, 104–111. [Google Scholar] [CrossRef]
- Mokhtari, I.; Taaifi, Y.; Harnafi, M.; Belhaj, K.; Mansouri, F.; Melhaoui, R.; Addi, M.; Hano, C.; Amrani, S.; Harnafi, H.; et al. Hypolipidemic Effect of Hemp Seed Oil from the Northern Morocco Endemic Beldiya Ecotype in a Mice Model: Comparison with Fenofibrate Hypolipidemic Drugs. J. Food Qual. 2022, 2022, 9142395. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A. The Effect of Hemp (Cannabis sativa L.) Seeds and Hemp Seed Oil on Vascular Dysfunction in Obese Male Zucker Rats. Nutrients 2021, 13, 2575. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Compton, W.M.; Einstein, E.B. The Need for Evidence Regarding Cannabidiol. JAMA Netw. Open 2020, 3, e2021067. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J.; Hellerová, Š. Industrial hemp (Cannabis sativa L.) as a possible source of cannabidiol. J. Central Eur. Agric. 2021, 22, 110–118. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wen, J.; Ma, X.; Lin, F.; Jiang, Z.; Du, B. Structural, functional properties and immunomodulatory activity of isolated Inca peanut (Plukenetia volubilis L.) seed albumin fraction. Int. J. Biol. Macromol. 2018, 118, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.-F.; Quiñones-Segura, Y.; Reinoso, Z.S.; Díaz, D.L.; Abril, J. Physicochemical properties of oils extracted from γ-irradiated Sacha Inchi (Plukenetia volubilis L.) seeds. Food Chem. 2017, 237, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.M.C.; Rave, L.J.G.; Soto, J.A. Biological Activity of Sacha Inchi (Plukenetia volubilis Linneo) and Potential Uses in Human Health: A Review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef]
- Chasquibol, N.A.; Gallardo, G.; Gómez-Coca, R.B.; Trujillo, D.; Moreda, W.; Pérez-Camino, M.C. Glyceridic and Unsaponifiable Components of Microencapsulated Sacha Inchi (Plukenetia huayllabambana L. and Plukenetia volubilis L.) Edible Oils. Foods 2019, 8, 671. [Google Scholar] [CrossRef]
- Srichamnong, W.; Ting, P.; Pitchakarn, P.; Nuchuchua, O.; Temviriyanukul, P. Safety assessment of Plukenetia volubilis (Inca peanut) seeds, leaves, and their products. Food Sci. Nutr. 2018, 6, 962–969. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef]
- Alayón, A.N.; Ávila, J.G.O.; Jiménez, I.E. Metabolic status is related to the effects of adding of sacha inchi (Plukenetia volubilis L.) oil on postprandial inflammation and lipid profile: Randomized, crossover clinical trial. J. Food Biochem. 2019, 43, e12703. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C. A randomized, double-blind placebo-controlled study on acceptability, safety and efficacy of oral administration of sacha inchi oil (Plukenetia volubilis L.) in adult human subjects. Food Chem. Toxicol. 2014, 65, 168–176. [Google Scholar] [CrossRef]
- Quinteros, M.; Vilcacundo, R.; Carpio, C.; Carrillo, W. Digestibility and anti-inflammatory activity in vitro of sacha inchi (Plukenetia volubilis L.) proteins. Asian J. Pharm. Clin. Res. 2016, 9, 303–306. [Google Scholar]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Wang, K.; Wu, S.; Li, P.; Xiao, N.; Wen, J.; Lin, J.; Lu, S.; Cai, X.; Xu, Y.; Du, B. Sacha Inchi Oil Press-Cake Protein Hydrolysates Exhibit Anti-Hyperuricemic Activity via Attenuating Renal Damage and Regulating Gut Microbiota. Foods 2022, 11, 2534. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, J.; Xiao, N.; Cai, X.; Yang, Y.; Deng, J.; Zhang, L.-H.; Du, B. Sacha inchi oil alleviates gut microbiota dysbiosis and improves hepatic lipid dysmetabolism in high-fat diet-fed rats. Food Funct. 2020, 11, 5827–5841. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Godarzi, S.M.; Gorji, A.V.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrologia 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran J Basic Med. Sci 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef]
- Mihanfar, A.; Darband, S.G.; Sadighparvar, S.; Kaviani, M.; Mirza-Aghazadeh-Attari, M.; Yousefi, B.; Majidinia, M. In vitro and in vivo anticancer effects of syringic acid on colorectal cancer: Possible mechanistic view. Chem. Biol. Interact. 2021, 337, 109337. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Oyelere, A.K. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 2022, 207, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.; Arbid, M.S. Role of caftaric acid in lead-associated nephrotoxicity in rats via antidiuretic, antioxidant and anti-apoptotic activities. J. Complement. Integr. Med. 2018, 15, 20170024. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Soliman, R.E. Chlorogenic and Caftaric Acids in Liver Toxicity and Oxidative Stress Induced by Methamphetamine. J. Toxicol. 2014, 2014, 583494. [Google Scholar] [CrossRef]
- Zhang, X.; Ishida, R.; Yuhara, Y.; Kamiya, T.; Hatano, T.; Okamoto, G.; Arimoto-Kobayashi, S. Anti-genotoxic activity of Vitis coignetiae Pulliat towards heterocyclic amines and isolation and identification of caftaric acid as an antimutagenic component from the juice. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 723, 182–189. [Google Scholar] [CrossRef]
- Bai, F.; Fang, L.; Hu, H.; Yang, Y.; Feng, X.; Sun, D. Vanillic acid mitigates the ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 531–537. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef]
- Sreelekshmi, M.; Raghu, K.G. Vanillic acid mitigates the impairments in glucose metabolism in HepG2 cells through BAD–GK interaction during hyperinsulinemia. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef]
- Brimson, J.M.; Onlamoon, N.; Tencomnao, T.; Thitilertdecha, P. Clerodendrum petasites S. Moore: The therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomed. Pharmacother. 2019, 118, 109319. [Google Scholar] [CrossRef]
- Yao, X.; Jiao, S.; Qin, M.; Hu, W.; Yi, B.; Liu, D. Vanillic Acid Alleviates Acute Myocardial Hypoxia/Reoxygenation Injury by Inhibiting Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 8348035. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kamboj, A.; Wanjari, M.; Sharma, A.K. Nephroprotective effect of Vanillic acid in STZ-induced diabetic rats. J. Diabetes Metab. Disord. 2021, 20, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Safari, S.; Mirazi, N.; Karimi, S.A.; Komaki, A. Effects of vanillic acid on Aβ1-40-induced oxidative stress and learning and memory deficit in male rats. Brain Res. Bull. 2021, 170, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-W.; Wei, I.-H.; Lin, F.-Y.; Li, C.-T.; Chen, K.-T.; Tsai, M.-H. Roles of Akt and ERK in mTOR-Dependent Antidepressant Effects of Vanillic Acid. ACS Omega 2020, 5, 3709–3716. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Modun, D.; Music, I.; Katalinic, V.; Salamunic, I.; Boban, M. Comparison of protective effects of catechin applied in vitro and in vivo on ischemia-reperfusion injury in the isolated rat hearts. Croat. Med. J. 2003, 44, 690–696. [Google Scholar]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.-Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Estrella, N.; Gil Lorenzo, A.; Cruzado, M.; Castro, C. Quercetin and catechin synergistically inhibit angiotensin II-induced redox-dependent signalling pathways in vascular smooth muscle cells from hypertensive rats. Free Radic. Res. 2012, 46, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-W.; Cai, S.; Zhao, T.-S.; Li, M.; Tian, Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free. Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A Multifunctional Flavonol in Biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, M.; Xing, J.; Liu, Y.; Li, H. Myricetin ameliorates atherosclerosis in the low-density-lipoprotein receptor knockout mice by suppression of cholesterol accumulation in macrophage foam cells. Nutr. Metab. 2019, 16, 25. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Akefe, I.O.; Ayo, J.O.; Sinkalu, V.O. Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS ONE 2020, 15, e0236251. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Osteoprotective Effects of Kaempferol: The Evidence from In Vivo And In Vitro Studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Park, S.-E.; Kim, S.-J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef]
- Ochiai, A.; Ben Othman, M.; Sakamoto, K. Kaempferol ameliorates symptoms of metabolic syndrome by improving blood lipid profile and glucose tolerance. Biosci. Biotechnol. Biochem. 2021, 85, 2169–2176. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Lin, J.-P.; Yang, J.-S.; Lin, J.-J.; Lai, K.-C.; Lu, H.-F.; Ma, C.-Y.; Wu, R.S.-C.; Wu, K.-C.; Chueh, F.-S.; Wood, W.G.; et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2011, 27, 480–484. [Google Scholar] [CrossRef]
- Skalski, B.; Lis, B.; Pecio, Ł.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Isorhamnetin and its new derivatives isolated from sea buckthorn berries prevent H2O2/Fe—Induced oxidative stress and changes in hemostasis. Food Chem. Toxicol. 2019, 125, 614–620. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Ho, M.-L.; Chen, P.-N.; Chu, S.-C.; Kuo, D.-Y.; Kuo, W.-H.; Chen, J.-Y.; Hsieh, Y.-S. Peonidin 3-Glucoside Inhibits Lung Cancer Metastasis by Downregulation of Proteinases Activities and MAPK Pathway. Nutr. Cancer 2010, 62, 505–516. [Google Scholar] [CrossRef]
- Ren, Z.; Raut, N.A.; Lawal, T.O.; Patel, S.R.; Lee, S.M.; Mahady, G.B. Peonidin-3-O-glucoside and cyanidin increase osteoblast differentiation and reduce RANKL -induced bone resorption in transgenic medaka. Phytotherapy Res. 2021, 35, 6255–6269. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Kongthitilerd, P.; Thilavech, T.; Marnpae, M.; Rong, W.; Yao, S.; Adisakwattana, S.; Cheng, H.; Suantawee, T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother. 2022, 146, 112494. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Yibchokkanun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidinn33rutinoside Alleviates Postprandial Hyperglycemia and Its Synergism with Acarbose by Inhibition of Intestinal Aglucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kodama, E.N.; Inoue, Y.; Tani, H.; Matsuura, Y.; Zhang, J.; Tanaka, T.; Hattori, T. Procyanidin B1 Purified from Cinnamomi Cortex Suppresses Hepatitis C Virus Replication. Antivir. Chem. Chemother. 2010, 20, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Ishihara, S.; Okamoto, T.; Doi, S.; Harui, K.; Higashino, Y.; Kawasaki, T.; Nakajima, N.; Saito, A. Inhibitory Activity of Synthesized Acetylated Procyanidin B1 Analogs against HeLa S3 Cells Proliferation. Molecules 2014, 19, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Palozza, P.; Fernandez-Larrea, J.; Ardévol, A.; Bladé, C.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M.T. Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free. Radic. Res. 2011, 45, 611–619. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Liu, T.; Li, J.; Wu, L.; Yu, Q.; Li, S.; Zhou, Y.; Zhang, J.; Chen, J.; et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J. Cell. Mol. Med. 2019, 23, 6479–6493. [Google Scholar] [CrossRef]
- Nie, X.; Tang, W.; Zhang, Z.; Yang, C.; Qian, L.; Xie, X.; Qiang, E.; Zhao, J.; Zhao, W.; Xiao, L.; et al. Procyanidin B2 mitigates endothelial endoplasmic reticulum stress through a PPARδ-Dependent mechanism. Redox Biol. 2020, 37, 101728. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, G.; Zhang, F.; Yang, X.; Chen, Y.; Duan, Y.; Yu, M.; Zhang, S.; Han, J. Procyanidin B2 Reduces Vascular Calcification through Inactivation of ERK1/2-RUNX2 Pathway. Antioxidants 2021, 10, 916. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhuan, Q.; Li, J.; Du, X.; Huang, Z.; Hou, Y.; Fu, X. Procyanidin B2 Improves Oocyte Maturation and Subsequent Development in Type 1 Diabetic Mice by Promoting Mitochondrial Function. Reprod. Sci. 2020, 27, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, M.; Tian, S. Procyanidin B2 prevents lupus nephritis development in mice by inhibiting NLRP3 inflammasome activation. Innate Immun. 2018, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, Q.; Lu, W.; Shen, J.; Zhou, D.; Wang, Y.; Gao, S.; Wang, Z. Grape seed procyanidin B2 promotes the autophagy and apoptosis in colorectal cancer cells via regulating PI3K/Akt signaling pathway. OncoTargets Ther. 2019, 12, 4109–4118. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef]
- Chandramohan, R.; Pari, L. Antihyperlipidemic effect of tyrosol, a phenolic compound in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2021, 31, 507–516. [Google Scholar] [CrossRef]
- Borovskaya, T.G.; Vychuzhanina, A.V.; Grigor’Eva, V.A.; Kollantay, O.V.; Goldberg, V.E.; Dygai, A.M. Evaluation of the Effect of p-Tyrosol on the Level of DNA Damage in the DNA Comet Assay In Vivo. Bull. Exp. Biol. Med. 2020, 169, 233–236. [Google Scholar] [CrossRef]
- Chandramohan, R.; Saravanan, S.; Pari, L. Beneficial effects of tyrosol on altered glycoprotein components in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1631–1637. [Google Scholar] [CrossRef]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Paul, D.; Maulik, N. Akt/FOXO3a/SIRT1-Mediated Cardioprotection by n-Tyrosol against Ischemic Stress in Rat in Vivo Model of Myocardial Infarction: Switching Gears toward Survival and Longevity. J. Agric. Food Chem. 2008, 56, 9692–9698. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential Metabolic Modulators in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Park, S.; Yang, C.; Song, G.; Lim, W. Disruption of Endoplasmic Reticulum and ROS Production in Human Ovarian Cancer by Campesterol. Antioxidants 2021, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.D.; Yu, H.C.; Xiong, Y.; Feng, J. Effect of dehydroepiandrosterone on cartilage and synovium of knee joints with osteoarthritis in rabbits. Rheumatol. Int. 2006, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.I.B.; Oliveira, S.M.; Tonello, R.; Rossato, M.F.; da Silva Brum, E.; Ferreira, J.; Trevisan, G. Anti-nociceptive effect of stigmasterol in mouse models of acute and chronic pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1163–1172. [Google Scholar] [CrossRef]
- Poulose, N.; Sajayan, A.; Ravindran, A.; Chandran, A.; Priyadharshini, G.B.; Selvin, J.; Kiran, G.S. Anti-diabetic Potential of a Stigmasterol from the Seaweed Gelidium spinosum and Its Application in the Formulation of Nanoemulsion Conjugate for the Development of Functional Biscuits. Front. Nutr. 2021, 8, 694362. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Essel, L.B.; Forkuo, A.D.; Atobiga, C. Stigmasterol Alleviates Cutaneous Allergic Responses in Rodents. BioMed Res. Int. 2018, 2018, 3984068. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N. Stigmasterol Modulates Allergic Airway Inflammation in Guinea Pig Model of Ovalbumin-Induced Asthma. Mediat. Inflamm. 2017, 2017, 2953930. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Alqahtani, S.; Ali, D.; Alkahtani, S.; Alarifi, S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Dev. Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. et Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Liu, J.; Pan, X.; Zhao, Q. Stigmasterol Exerts Neuro-Protective Effect Against Ischemic/Reperfusion Injury Through Reduction of Oxidative Stress And Inactivation Of Autophagy. Neuropsychiatr. Dis. Treat. 2019, 15, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Wang, J.; Dong, Y.; Meng, S.; Li, R.; Niu, X.; Deng, X. β-sitosterol interacts with pneumolysin to prevent Streptococcus pneumoniae infection. Sci. Rep. 2015, 5, 17668. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, Y.; Huang, W.; Liu, Y.; Lu, Y.; Zhao, J. β-Sitosterol Ameliorates Endometrium Receptivity in PCOS-Like Mice: The Mediation of Gut Microbiota. Front. Nutr. 2021, 8, 667130. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-Y.; Li, L.; Hao, Q.-M.; Qin, Y.; Ma, C.-S. β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacol. 2020, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Xu, L.; Huang, M.; Deng, B.; Zhang, W.; Zeng, Z.; Yinzhi, S. β-Sitosterol Protects against Myocardial Ischemia/Reperfusion Injury via Targeting PPARγ/NF-κB Signalling. Evid. Based Complement. Altern. Med. 2020, 2020, 267940. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Schwenke, D.C.; Rudel, L.L.; Sorci-Thomas, M.G.; Thomas, M.J. α-Tocopherol protects against diet induced atherosclerosis in New Zealand white rabbits. J. Lipid Res. 2002, 43, 1927–1938. [Google Scholar] [CrossRef]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free. Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Kiyose, C.; Takeuchi, H.; Yabe, Y.; Nojima, T.; Nagase, M.; Takahashi-Muto, C.; Tanaka-Yachi, R. Effect of δ-Tocopherol on Mice Adipose Tissues and Mice Adipocytes Induced Inflammation. J. Oleo Sci. 2021, 70, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Liu, A.; Wang, G.; Bosland, M.C.; Yang, C.S. δ-Tocopherol inhibits the development of prostate adenocarcinoma in prostate specific Pten−/− mice. Carcinogenesis 2018, 39, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Xu, Y.-M.; Lau, A.T.Y. Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate. Molecules 2021, 26, 7512. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.P.M.; Brusco, I.; Brum, E.S.; Fialho, M.F.P.; Camponogara, C.; Scussel, R.; Machado-De-Ávila, R.A.; Trevisan, G.; Oliveira, S.M. Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem. Int. 2020, 134, 104673. [Google Scholar] [CrossRef]
- Borges, F.R.; Silva, M.D.; Córdova, M.M.; Schambach, T.R.; Pizzolatti, M.G.; Santos, A.R. Anti-inflammatory action of hydroalcoholic extract, dichloromethane fraction and steroid α-spinasterol from Polygala sabulosa in LPS-induced peritonitis in mice. J. Ethnopharmacol. 2014, 151, 144–150. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, R.; Pieróg, M.; Wlaź, P. α-Spinasterol, a TRPV1 receptor antagonist, elevates the seizure threshold in three acute seizure tests in mice. J. Neural Transm. 2015, 122, 1239–1247. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers with Social Anxiety Disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive Phenolic Amides from Celtis africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Schoene, N. N-Caffeoyltyramine arrests growth of U937 and Jurkat cells by inhibiting protein tyrosine phosphorylation and inducing caspase-3. Cancer Lett. 2003, 202, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
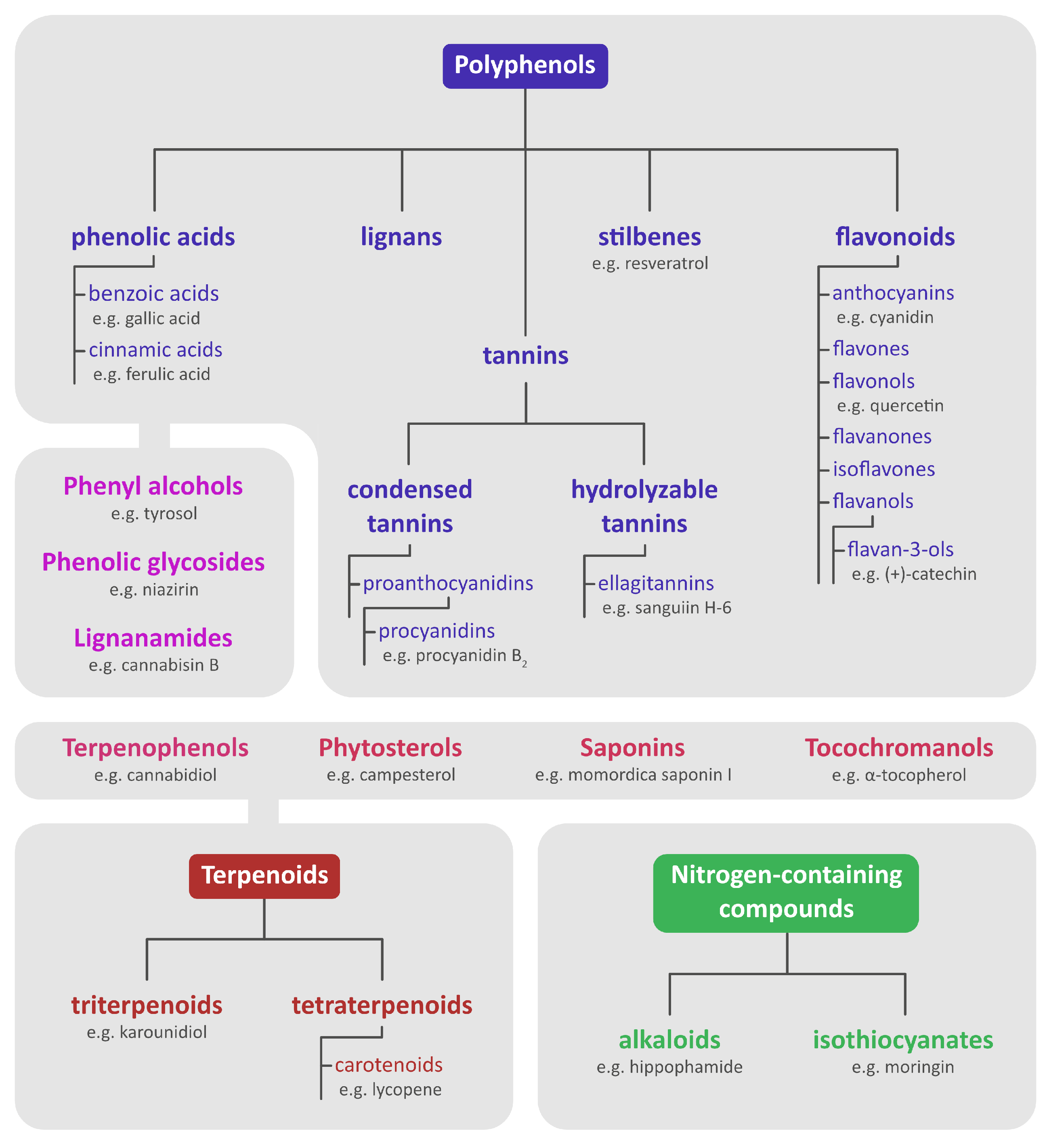
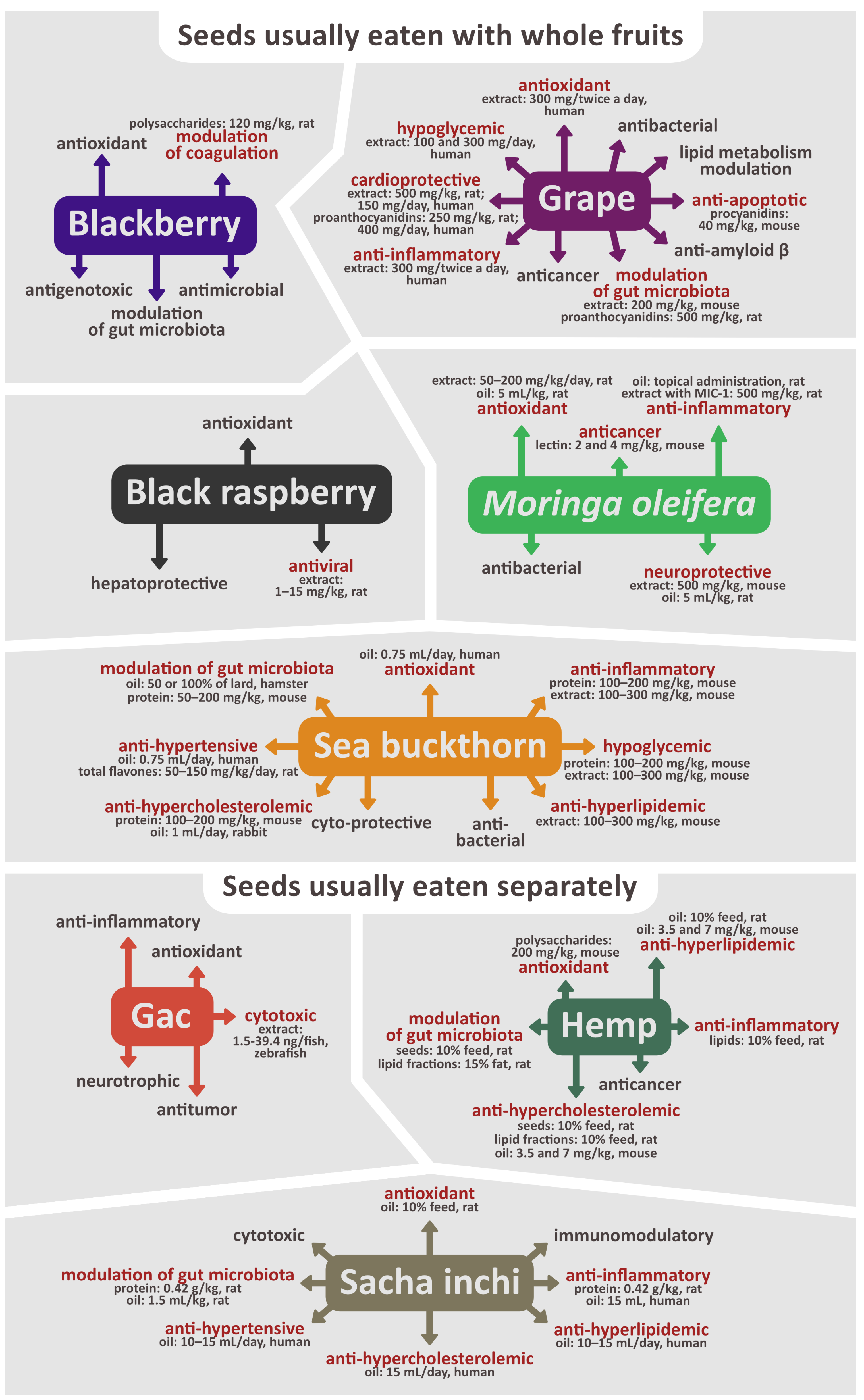
| Type of Compound | Compound | Plant Species | Biological Activity | Citations |
|---|---|---|---|---|
| Phenolic acids | Protocatechuic acid | Blackberry Black raspberry Grape Sea buckthorn | Antioxidant **, anti-inflammatory **, antibacterial, antiviral **, antifibrotic, anti-atherosclerotic, anti-amyloid β **, anti-hypertensive **, decreasing insulin resistance **, hypolipidemic **, hypoglycemic ** Anti-aging, antidiabetic **, anticancer **, antiosteoporosis **; cardioprotective **, neuroprotective ** | [6,28,99,156,205,206] |
| p-Coumaric acid | Blackberry Black raspberry Grape | Antioxidant **, anti-inflammatory **, antimicrobial, antiviral, antiplatelet **, antiproliferative **, decreasing insulin resistance ** Anticancer **, antidiabetic ** | [6,99,207,208] | |
| Gallic acid * | Blackberry Black raspberry Grape M. oleifera | Antioxidant **, prooxidant, anti-inflammatory **, antimicrobial, antiviral, hypoglycemic **, hypolipidemic ** Anticancer **, anti-obesity **, cardioprotective **, gastroprotective **, hepatoprotective **, neuroprotective ** | [6,7,67,92,109,209] | |
| Syringic acid | Blackberry Black raspberry Grape | Antioxidant **, anti-inflammatory **, antimicrobial, anti-angiogenic, antiendotoxic ** Anticancer **, antidiabetic **, cardioprotective **, hepatoprotective **, neuroprotective ** | [6,210,211,212] | |
| Caffeic acid * | Blackberry Black raspberry Grape M. oleifera | Antioxidant **, prooxidant, anti-inflammatory **, modulation of gut microbiota **, suppression of metalloproteinases expression Anticancer **, hepatoprotective ** | [6,7,92,98,213,214] | |
| Caftaric acid | Grape | Antioxidant **, anti-apoptotic, antimutagenic, antidiuretic **, anti-hypercholesterolemic ** Anticancer, hepatoprotective **, nephroprotective ** | [1,215,216,217] | |
| Vanillic acid | Grape | Antioxidant **, anti-inflammatory **, antibacterial, antidepressant **, antihypertensive ** Anti-asthma **, antidiabetic, cardioprotective **, nephroprotective **, neuroprotective ** | [99,218,219,220,221,222,223,224,225] | |
| Chlorogenic acid | Grape Sea buckthorn | Antioxidant **, anti-inflammatory **, immunomodulatory **, antibacterial, antiviral, anti-hypercholesterolemic **, anti-hyperlipidemic **, hypoglycemic **, antimutagenic Anti-cancer, anti-obesity **, cardioprotective **, hepatoprotective ** | [1,156,216,226] | |
| Ferulic acid | Black raspberry Grape | Antioxidant **, anti-inflammatory **, antiplatelet **, anti-apoptotic **, antifibrosis ** | [92,99,170,227] | |
| Cardioprotective ** | ||||
| Ellagic acid * | Blackberry Black raspberry M. oleifera | Antioxidant **, anti-inflammatory **, antibacterial, anti-viral **, anti-apoptotic, antidepressant, anti-atherosclerotic **, antihyperglycemic **, antihyperlipidemic **, anti-angiogenic ** Antidiabetic **, anticancer **, cardioprotective **, hepatoprotective, nephroprotective **, neuroprotective ** | [3,7,82,87,92] | |
| Flavonoids | Catechins * | Blackberry Black raspberry Grape M. oleifera Sea buckthorn | Antioxidant **, anti-inflammatory **, antibacterial **, antiviral, anti-apoptosis ** antihypertensive **, protecting from UV radiation ** Anticancer **, anti-obesity **, cardioprotective **, hepatoprotective ** | [6,31,109,156,228,229,230,231,232,233,234,235] |
| Quercetin * | Blackberry Black raspberry Grape M. oleifera Sea buckthorn | Antioxidant **, prooxidant, anti-inflammatory **, antimicrobial, anti-hypertensive **, hypoglycemic **, anti-hyperlipidemic **, anti-hypercholesterolemic ** Anticancer **, anti-obesity **, anti-diabetes **, cardioprotective ** | [5,6,7,8,92,170,180,233,236,237] | |
| Myricetin | Blackberry Sea buckthorn | Antioxidant **, prooxidant **, anti-inflammatory **, antiviral, anti-atherosclerotic **, antiplatelet ** Anticancer **, cardioprotective **, neuroprotective | [6,156,238,239,240] | |
| Kaempferol * | Blackberry Grape M. oleifera Sea buckthorn | Antioxidant **, anti-inflammatory **, antithrombotic **, antihyperglycemic **, antihyperlipidemic **, anti-atherosclerotic ** Anticancer **, antidiabetic **, anti-obesity **, cardioprotective **, gastroprotective **, hepatoprotective **, neuroprotective **, osteoprotective ** | [6,7,11,98,154,180,241,242,243,244,245,246] | |
| Rutin | Black raspberry Grape Sea buckthorn | Antioxidant **, anti-inflammatory **, antibacterial, antihyperglycemic **, anti-hypercholesterolemic **, antihypertensive **, antiplatelet, antiulcer ** Anti-asthmatic **, anti-arthritic **, anticancer **, anticataract **, antidiabetic **, anti-osteoporotic **, cardioprotective **, hepatoprotective **, nephroprotective **, neuroprotective **, wound healing ** | [5,76,92,156,170,247,248] | |
| Isorhamnetin * | Sea buckthorn | Antioxidant **, anti-inflammatory **, anti-apoptotic **, antibacterial, antiviral **, anti-atherogenic **, anticholinesterase, anticoagulant, antihypertensive, antifibrosis **, hypoglycemic ** Anticancer **, anti-osteoporosis **, anti-obesity **, cardioprotective **, hepatoprotective **, nephroprotective **, neuroprotective ** | [8,249,250] | |
| Luteolin * | Grape | Antioxidant **, anti-inflammatory **, antidepressant **, hypoglycemic **, improving insulin sensitivity ** Anticancer **, anti-obesity **, cardioprotective **, hepatoprotective **, neuroprotective ** | [1,70,170] | |
| Anthocyanins | Peonidin-3-O-glucoside | Blackberry Black raspberry | Stimulating bone formation ** Anticancer | [6,251,252] |
| Cyanidin-3-glucoside | Black raspberry Grape | Antioxidant **, anti-inflammatory **, antimicrobial, antiviral, antiatherogenic **, antithrombotic, hypoglycemic **, decreasing insulin resistance ** Anticancer **, antidiabetic **, anti-obesity **, cardioprotective **, neuroprotective ** | [5,6,96,253] | |
| Cyanidin-3-rutinoside | Black raspberry | Antioxidant, prooxidant, proapoptotic (in cancer cells), hypoglycemic ** Anticancer, antidiabetic | [30,92,254,255] | |
| Procyanidins | Procyanidin B1 | Blackberry Black raspberry | Anti-inflammatory, antiviral Anticancer | [6,256,257,258] |
| Procyanidin B2 | Grape | Antioxidant **, anti-inflammatory **, anti-apoptotic, proapoptotic **, anti-atherogenic, anti-angiogenic **, pro-angiogenic **, improving endothelial function, improving mitochondrial function, anti-fibrosis ** Anticancer **, hepatoprotective **, wound healing ** | [53,259,260,261,262,263,264,265] | |
| Stilbenes | Resveratrol | Black raspberry Grape | Antioxidant **, anti-inflammatory **, antimicrobial, anti-apoptotic **, antiglycation **, antiplatelet, increased NO synthesis **, stimulating bone formation ** Anti-ageing **, anticancer **, antidiabetic **, cardioprotective **, neuroprotective ** | [5,74,92,101,266] |
| Phenyl alcohols | Tyrosol | Grape | Antioxidant **, anti-inflammatory **, antibacterial, anti-apoptotic, antiplatelet, antihyperlipidemic **, hypoglycemic **, anti-genotoxic ** Anti-obesity, cardioprotective **, anti-diabetes **, neuroprotective ** | [38,99,267,268,269,270,271] |
| Phenolic glycosides | Niazirin | M. oleifera | Antioxidant **, anti-inflammatory **, antiproliferative **, hypoglycemic **, anti-hyperlipidemic **, decreasing insulin resistance ** Antidiabetic ** | [39,150,151] |
| Phytosterols | Campesterol | Blackberry M. oleifera | Prooxidant (in cancer cells), anti-neuroinflammatory, hypocholesterolemic Anticancer | [7,79,192,272,273,274] |
| Stigmasterol | Blackberry M. oleifera | Antioxidant, prooxidant, anti-inflammatory **, anti-apoptotic **, anti-hypercholesterolemic **, anti-hyperlipidemic **, anti-nociceptive ** Antiallergic **, anti-asthmatic **, anti-arthritis **, anticancer **, antidiabetic **, neuroprotective ** | [7,79,192,272,273,275,276,277,278,279,280,281,282] | |
| β-sitosterol | Blackberry M. oleifera Sea buckthorn | Antioxidant **, anti-inflammatory **, antibacterial **, anti-acetylcholinesterase, anti-amyloid β, anti-hypercholesterolemic **, anti-hyperlipidemic **, anxiolytic **, immunomodulatory **, modulation of gut microbiota ** Anticancer **, antidiabetic **, cardioprotective **, hepatoprotective **, neuroprotective ** | [7,79,155,192,272,273,281,283,284,285,286,287] | |
| Tocopherols | α-tocopherol | M. oleifera Sea buckthorn | Antioxidant **, anti-inflammatory **, antiproliferative **, anti-hypercholesterolemic **Anticancer **, cardioprotective ** | [7,34,175,288,289,290,291] |
| γ-tocopherol | Blackberry M. oleifera Sea buckthorn | Antioxidant **, anti-inflammatory ** Anti-asthmatic **, antiallergic **, anticancer ** | [7,34,79,175,196,288,292] | |
| δ-tocopherol | Blackberry M. oleifera | Antioxidant, anti-inflammatory, antiproliferative **, proapoptotic ** Anticancer ** | [7,79,175,196,288,293,294] | |
| Ellagitannins | Sanguiin H-6 | Blackberry Black raspberry | Antioxidant **, antibacterial **, anti-apoptotic **, anti-angiogenic, anti-osteoclastogenic **, anti-genotoxic, estrogenic Anticancer, anti-osteoporosis ** | [2,48,49,50,51,52,82,89,92] |
| Isothiocyanates | Moringa isothiocyanate-1 (moringin) | M. oleifera | Antioxidant, anti-inflammatory **, pro-apoptotic (in cancer cells), antinociceptive ** Antidiabetic **, neuroprotective, pain-relieving ** | [31,39,130,145,146,147,295,296] |
| Alkaloids | N-p-coumaroyl-4-aminobutan-1-ol | Sea buckthorn | Antioxidant, cytoprotective | [33] |
| Hippophamide | Sea buckthorn | Antioxidant, cytoprotective | [33] |
| Type of Compound | Compound | Plant Species | Biological Activity | Citations |
|---|---|---|---|---|
| Phenolic acids | Gallic acid * | Gac | Antioxidant **, prooxidant, anti-inflammatory **, antimicrobial, antiviral, hypoglycemic **, hypolipidemic ** Anticancer **, anti-obesity **, cardioprotective **, gastroprotective **, hepatoprotective **, neuroprotective ** | [6,7,67,92,109,209] |
| Ferulic acid | Gac | Antioxidant **, anti-inflammatory **, antiplatelet **, anti-apoptotic **, antifibrosis ** Cardioprotective ** | [92,99,170,227] | |
| Flavonoids | Quercetin * | Gac Hemp | Antioxidant **, prooxidant, anti-inflammatory **, antimicrobial, anti-hypertensive **, hypoglycemic **, anti-hyperlipidemic **, anti-hypercholesterolemic ** Anticancer **, anti-obesity **, anti-diabetes **, cardioprotective ** | [5,6,7,8,92,170,180,233,236,237] |
| Kaempferol * | Hemp | Antioxidant **, anti-inflammatory **, antithrombotic **, antihyperglycemic **, antihyperlipidemic **, anti-atherosclerotic ** Anticancer **, antidiabetic **, anti-obesity **, cardioprotective **, gastroprotective **, hepatoprotective **, neuroprotective **, osteoprotective ** | [6,7,11,98,154,180,241,242,243,244,245,246] | |
| Rutin | Gac | Antioxidant **, anti-inflammatory **, antibacterial, antihyperglycemic **, anti-hypercholesterolemic **, antihypertensive **, antiplatelet, antiulcer ** Anti-asthmatic **, anti-arthritic **, anticancer **, anticataract **, antidiabetic **, anti-osteoporotic **, cardioprotective **, hepatoprotective **, nephroprotective **, neuroprotective **, wound healing ** | [5,76,92,156,170,247,248] | |
| Luteolin * | Gac | Antioxidant **, anti-inflammatory **, antidepressant **, hypoglycemic **, improving insulin sensitivity ** Anticancer **, anti-obesity **, cardioprotective **, hepatoprotective **, neuroprotective ** | [1,70,170] | |
| Phytosterols | α-spinasterol | Gac | Anti-inflammatory **, antinociceptive **, antiseizure **, antiulcer, antiproliferative Anticancer, pain-relieving ** | [169,297,298,299] |
| Campesterol | Sacha inchi | Prooxidant (in cancer cells), anti-neuroinflammatory, hypocholesterolemic Anticancer | [7,79,192,272,273,274] | |
| Stigmasterol | Sacha inchi | Antioxidant, prooxidant, anti-inflammatory **, anti-apoptotic **, anti-hypercholesterolemic **, anti-hyperlipidemic **, anti-nociceptive ** Antiallergic **, anti-asthmatic **, anti-arthritis **, anticancer **, antidiabetic **, neuroprotective ** | [7,79,192,272,273,275,276,277,278,279,280,281,282] | |
| β-sitosterol | Sacha inchi | Antioxidant **, anti-inflammatory **, antibacterial **, anti-acetylcholinesterase, anti-amyloid β, anti-hypercholesterolemic **, anti-hyperlipidemic **, anxiolytic **, immunomodulatory **, modulation of gut microbiota ** Anticancer **, antidiabetic **, cardioprotective **, hepatoprotective **, neuroprotective ** | [7,79,155,192,272,273,281,283,284,285,286,287] | |
| Terpenoids | Lycopene | Gac | Antioxidant **, anti-inflammatory **, anti-atherogenic **, antiplatelet **, antihypertensive **, anti-hyperlipidemic **, anti-seizure **, proapoptotic (in cancer cells) ** Anticancer **, anti-obesity **, cardioprotective **, neuroprotective ** | [42,43,44,45,46] |
| Terpenophenols | Cannabidiol | Hemp | Antioxidant **, anti-inflammatory **, anti-apoptotic **, proapoptotic **, anti-angiogenic **, antiseizure **, anti-amyloid β **, antidepressant **, antipsychotic **, anxiolytic **, antiarthritic **, immunomodulatory **, vasodilatory ** Anticancer **, cardioprotective **, neuroprotective ** | [36,188,189,300,301] |
| Tocopherols | α-tocopherol | Hemp | Antioxidant **, anti-inflammatory **, antiproliferative **, anti-hypercholesterolemic **Anticancer **, cardioprotective ** | [7,34,175,288,289,290,291] |
| γ-tocopherol | Hemp Sacha inchi | Antioxidant **, anti-inflammatory ** Anti-asthmatic **, antiallergic **, anticancer ** | [7,34,79,175,196,288,292] | |
| δ-tocopherol | Hemp Sacha inchi | Antioxidant, anti-inflammatory, antiproliferative **, proapoptotic ** Anticancer ** | [7,79,175,196,288,293,294] | |
| Lignanamides | N-caffeoyltyramine * | Hemp | Antioxidant, anti-inflammatory, antiproliferative | [179,302,303] |
| Grossamide | Hemp | Anti-neuroinflammatory Neuroprotective | [178] | |
| Cannabisin B | Hemp | Autophagic, antiproliferative | [37,180] | |
| Cannabisin F | Hemp | Anti-neuroinflammatory Neuroprotective | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. https://doi.org/10.3390/nu15010187
Sławińska N, Olas B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients. 2023; 15(1):187. https://doi.org/10.3390/nu15010187
Chicago/Turabian StyleSławińska, Natalia, and Beata Olas. 2023. "Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities" Nutrients 15, no. 1: 187. https://doi.org/10.3390/nu15010187
APA StyleSławińska, N., & Olas, B. (2023). Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients, 15(1), 187. https://doi.org/10.3390/nu15010187