Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Euonymus Alatus Twig Extract
2.2. HPLC, ESI MS and NMR
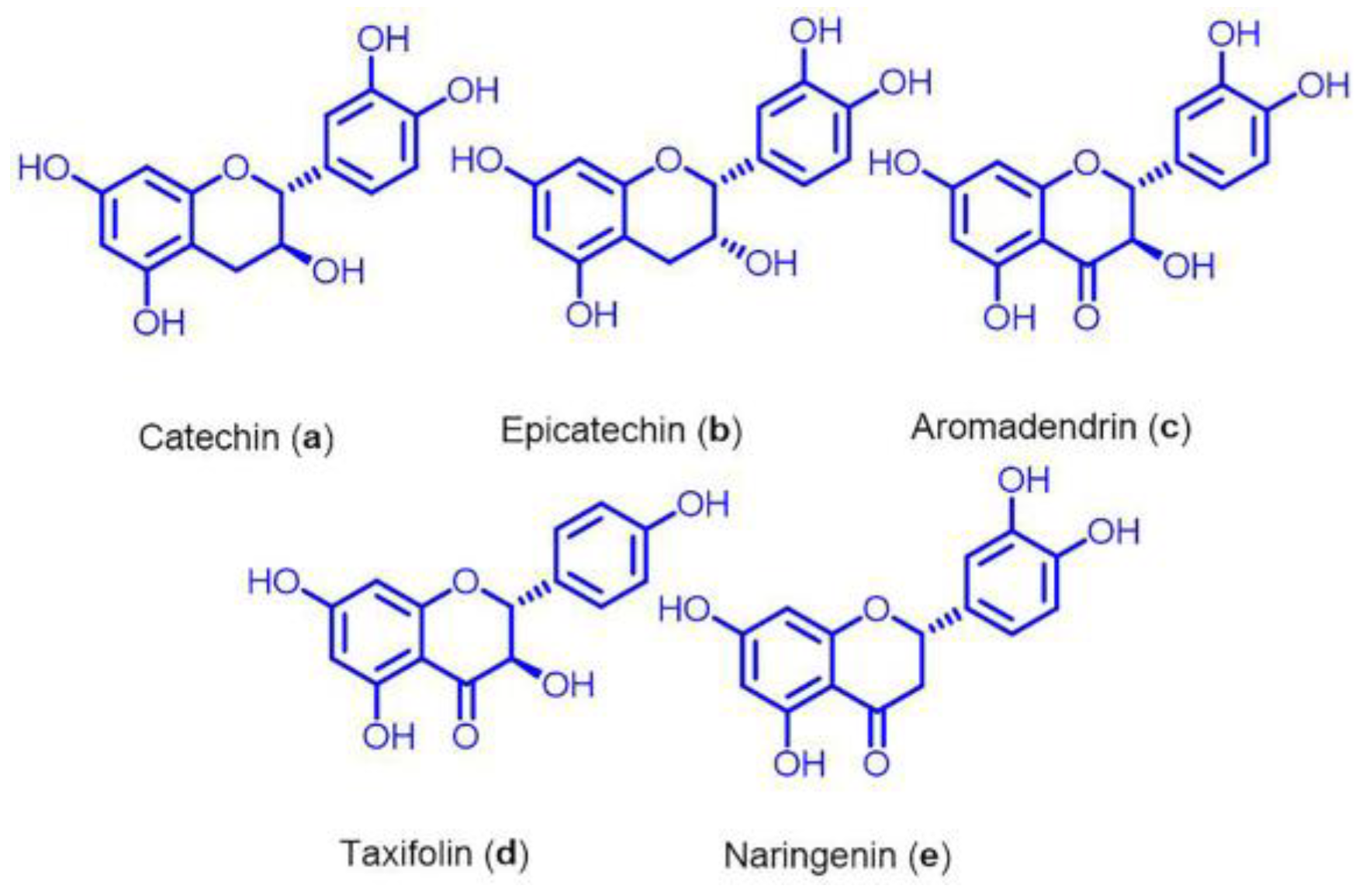
2.3. Isolation of Flavonoids and Fragmentation of Compounds
2.4. Materials
2.5. Cell
2.6. Drug Preparation
2.7. Animal
2.8. Animal Model for Cognitive Impairment
2.9. Passive Avoidance Test (PAT)
2.10. Western Blot Analysis
2.11. Cell Viability Test
2.12. Acetyl Choline Esterase Activity Assay
2.13. Statistical Analysis
3. Results
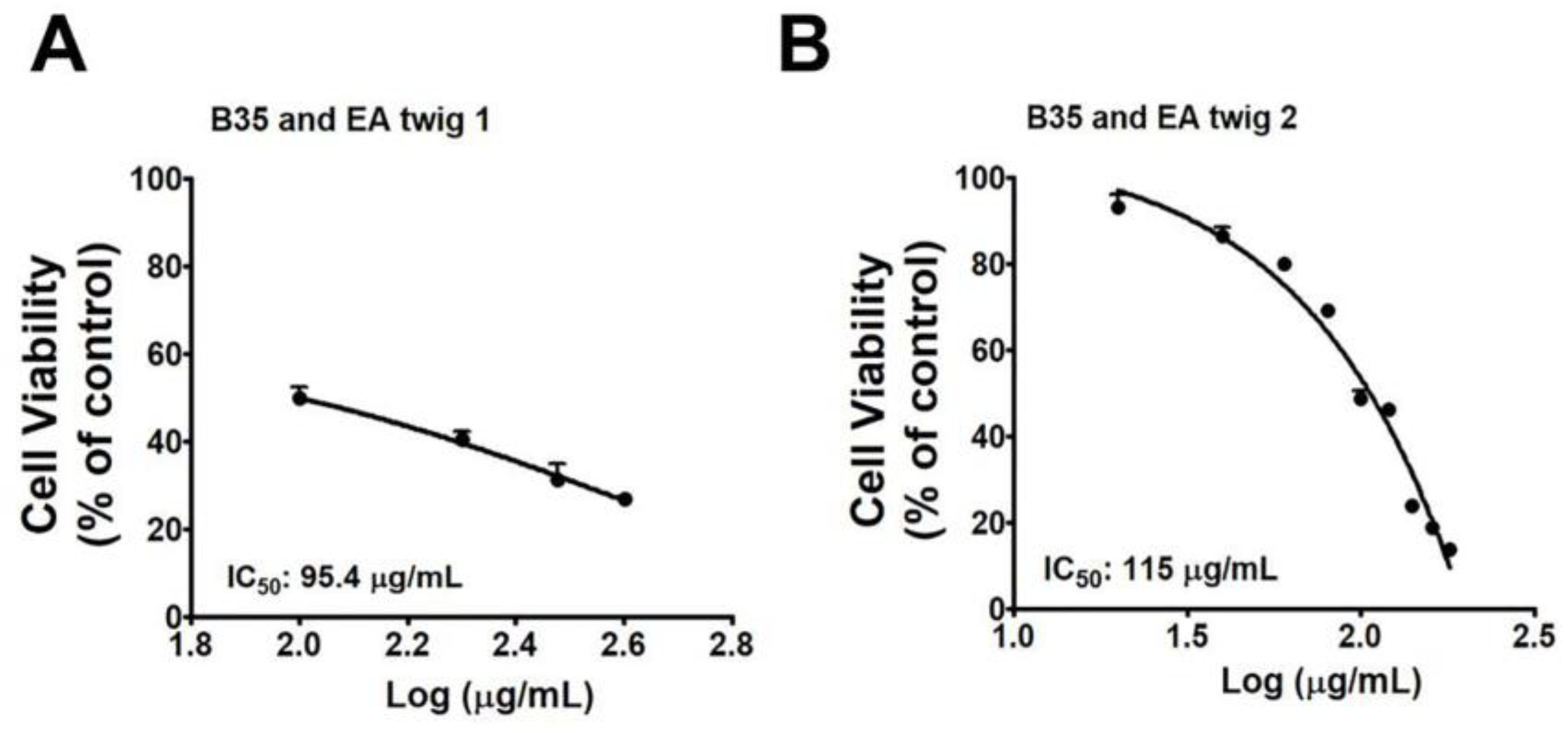
3.1. Effect of EA Twig Extract on Viability of B35 Neuroblastoma Cell Line
3.2. Effect of EA Twig Extract on the Scopolamine-Induced B35, Rat Neuroblastoma Cells
3.3. In-Vitro AChE Inhibitory Activity of EA Twig Extract
3.4. Effects of Different Flavonoids Isolated from EA Twig Extract on Scopolamine-Induced B35 Cells
3.5. Mixture Effect of EA Twig Extract 2 and Catechin (a) on BDNF and Its Downstream Protein Expression in B35 Cells
3.6. EA Twig Extract 2 Improves the Scopolamine-Induced Memory Impairments in ICR Mice
3.7. EA Twig Extract 2 Rescues ICR Mice from Scopolamine-Induced Deficits by Restoring BDNF Signalling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S. Metabolic syndrome and the cellular phase of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2017, 146, 243–258. [Google Scholar] [PubMed]
- Tillement, L.; Lecanu, L.; Papadopoulos, V. Alzheimer’s disease: Effects of β-amyloid on mitochondria. Mitochondrion 2011, 11, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Jang, M.J.; Choi, Y.-H.; Hwang, H.; Rhim, H.; Lee, B.; Choi, C.W.; Kim, M.S. Central administration of afzelin extracted from Ribes fasciculatum improves cognitive and memory function in a mouse model of dementia. Sci. Rep. 2021, 11, 9182. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural. Regen. Res. 2017, 12, 549. [Google Scholar]
- Woo, N.; Lu, B. BDNF in synaptic plasticity and memory. In Intracellular Communication in the Nervous System; NIH: Bethesda, MD, USA, 2009; pp. 135–143. [Google Scholar]
- Nieto, R.; Kukuljan, M.; Silva, H. BDNF and schizophrenia: From neurodevelopment to neuronal plasticity, learning, and memory. Front. Psychiatry 2013, 4, 45. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Arancibia, S. Physiology of BDNF: Focus on hypothalamic function. Front. Neuroendocrinol. 2004, 25, 77–107. [Google Scholar] [CrossRef]
- González-Gutiérrez, A.; Lazo, O.M.; Bronfman, F.C. The Rab5–Rab11 Endosomal Pathway is Required for BDNF-Induced CREB Transcriptional Regulation in Hippocampal Neurons. J. Neurosci. 2020, 40, 8042–8054. [Google Scholar] [CrossRef]
- Xue, W.; Wang, W.; Gong, T.; Zhang, H.; Tao, W.; Xue, L.; Sun, Y.; Wang, F.; Chen, G. PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of Yueju but not ketamine. Sci. Rep. 2016, 6, 26331. [Google Scholar] [CrossRef]
- Shi, M.; Ding, J.; Li, L.; Bai, H.; Li, X.; Lan, L.; Fan, H.; Gao, L. Effects of Ketamine on Learning and Memory in the Hippocampus of Rats through ERK, CREB, and Arc. Brain Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Rosa, E.; Mahendram, S.; Ke, Y.D.; Ittner, L.M.; Ginsberg, S.D.; Fahnestock, M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging 2016, 48, 135–142. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, S.I.; Cho, E.J. Effect of Vigna angularis on High-Fat Diet-Induced Memory and Cognitive Impairments. J. Med. Food 2020, 23, 1155–1162. [Google Scholar] [CrossRef]
- Ayabe, T.; Ohya, R.; Taniguchi, Y.; Shindo, K.; Kondo, K.; Ano, Y. Matured hop-derived bitter components in beer improve hippocampus-dependent memory through activation of the vagus nerve. Sci. Rep. 2018, 8, 15372. [Google Scholar] [CrossRef]
- Barak, S.; Weiner, I. Towards an animal model of an antipsychotic drug-resistant cognitive impairment in schizophrenia: Scopolamine induces abnormally persistent latent inhibition, which can be reversed by cognitive enhancers but not by antipsychotic drugs. Int. J. Neuropsychopharmacol. 2009, 12, 227–241. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Gualtieri, F.; Manetti, D.; Romanelli, M.; Ghelardini, C. Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs. Curr. Pharm. Des. 2002, 8, 125–138. [Google Scholar] [CrossRef]
- Dwivedi, P.; Singh, R.; Malik, M.T.; Jawaid, T. A traditional approach to herbal nootropic agents: An overview. Int. J. Pharm. Sci. Res. 2012, 3, 630. [Google Scholar]
- Takai, K.; Enomoto, T. Discovery and development of muscarinic acetylcholine M4 activators as promising therapeutic agents for CNS diseases. Chem. Pharm. Bull. 2018, 66, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Mun, J.; Seo, H.W.; Ryu, S.Y.; Kim, Y.S.; Lee, B.H.; Oh, K.-S. Euonymus alatus extract attenuates LPS-induced NF-κB activation via IKKβ inhibition in RAW 264.7 cells. J. Ethnopharmacol. 2011, 134, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lenon, G.B.; Xue, C.C.; Li, C.-G. Euonymus alatus: A review on its phytochemistry and antidiabetic activity. Evid.-Based Complement. Altern. Med. 2016, 2016, 9425714. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Woo, Y.; Lim, J.S.; Oh, J.; Lee, J.S.; Kim, J.-S. Neuroprotective effects of euonymus alatus extract on scopolamine-induced memory deficits in mice. Antioxidants 2020, 9, 449. [Google Scholar] [CrossRef]
- Jeong, E.J.; Yang, H.; Kim, S.H.; Kang, S.Y.; Sung, S.H.; Kim, Y.C. Inhibitory constituents of Euonymus alatus leaves and twigs on nitric oxide production in BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1394–1398. [Google Scholar] [CrossRef]
- Astur, R.S.; Ortiz, M.L.; Sutherland, R.J. A characterization of performance by men and women in a virtual Morris water task:: A large and reliable sex difference. Behav. Brain Res. 1998, 93, 185–190. [Google Scholar] [CrossRef]
- Newhouse, P.; Newhouse, C.; Astur, R.S. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav. Brain Res. 2007, 183, 1–7. [Google Scholar] [CrossRef]
- Sen, S.; Satagopan, J.M.; Broman, K.W.; Churchill, G.A. R/qtlDesign: Inbred line cross experimental design. Mamm. Genome 2007, 18, 87–93. [Google Scholar] [CrossRef]
- Suthprasertporn, N.; Mingchinda, N.; Fukunaga, K.; Thangnipon, W. Neuroprotection of SAK3 on scopolamine-induced cholinergic dysfunction in human neuroblastoma SH-SY5Y cells. Cytotechnology 2020, 72, 155–164. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Epicatechin and catechin in cocoa inhibit amyloid β protein induced apoptosis. J. Agric. Food Chem. 2005, 53, 1445–1448. [Google Scholar] [CrossRef]
- Bhat, I.U.H.; Bhat, R. Quercetin: A bioactive compound imparting cardiovascular and neuroprotective benefits: Scope for exploring fresh produce, their wastes, and by-products. Biology 2021, 10, 586. [Google Scholar] [CrossRef]
- Peruche, B.; Krienglstein, J. Neuroblastoma cells for testing neuroprotective drug effects. J. Pharmacol. Methods 1991, 26, 139–148. [Google Scholar] [CrossRef]
- Phaisan, S.; Yusakul, G.; Sakdamas, A.; Taluengjit, N.; Sakamoto, S.; Putalun, W. A green and effective method using oils to remove chlorophyll from Chromolaena odorata (L.) RM King & H. Rob. Songklanakarin J. Sci. Technol. 2020, 42, 1084–1090. [Google Scholar]
- Wenk, G.L. Amnesia and Alzheimer’s disease: Which neurotransmitter system is responsible? Neurobiol. Aging 1988, 9, 640–641. [Google Scholar] [CrossRef]
- Van Beek, A.H.; Claassen, J.A. The cerebrovascular role of the cholinergic neural system in Alzheimer’s disease. Behav. Brain Res. 2011, 221, 537–542. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Khan, H.; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331. [Google Scholar]
- Zhou, J.; Yang, W.-S.; Suo, D.-Q.; Li, Y.; Peng, L.; Xu, L.-X.; Zeng, K.-Y.; Ren, T.; Wang, Y.; Zhou, Y. Moringa oleifera seed extract alleviates scopolamine-induced learning and memory impairment in mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimers Dis. 2020, 2020, 6372059. [Google Scholar] [CrossRef] [PubMed]
- Berkeley, J.L.; Gomeza, J.; Wess, J.; Hamilton, S.E.; Nathanson, N.M.; Levey, A.I. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell. Neurosci. 2001, 18, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Song, H.-S.; Park, M.N.; Kim, S.-H.; Shim, B.-S.; Kim, B. Ethanol Extract of Oldenlandia diffusa Herba Attenuates Scopolamine-Induced Cognitive Impairments in Mice via Activation of BDNF, P-CREB and Inhibition of Acetylcholinesterase. Int. J. Mol. Sci. 2018, 19, 996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, Y.H.; Kim, J.; Goo, N.; Jeong, Y.; Bae, H.J.; Jung, S.Y.; Lee, J.; Ryu, J.H. Casticin ameliorates scopolamine-induced cognitive dysfunction in mice. J. Ethnopharmacol. 2020, 259, 112843. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in learning and memory: A review of recent research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Bae, H.J.; Kim, J.; Jeon, S.J.; Kim, J.; Goo, N.; Jeong, Y.; Cho, K.; Cai, M.; Jung, S.Y.; Kwon, K.J. Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice. J. Ethnopharmacol. 2020, 258, 112923. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant role of catechin in health and disease. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–271. [Google Scholar]
- Chobot, V.; Huber, C.; Trettenhahn, G.; Hadacek, F. (±)-catechin: Chemical weapon, antioxidant, or stress regulator? J. Chem. Ecol. 2009, 35, 980–996. [Google Scholar] [CrossRef]
- Andrade, J.P.; Assunção, M. Green Tea Effects on Age-Related Neurodegeneration: A Focus on Neuronal Plasticity. In Diet and Nutrition in Dementia and Cognitive Decline; Elsevier: Amsterdam, The Netherlands, 2015; pp. 915–924. [Google Scholar]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Gunesch, S.; Hoffmann, M.; Kiermeier, C.; Fischer, W.; Pinto, A.F.; Maurice, T.; Maher, P.; Decker, M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox Biol. 2020, 29, 101378. [Google Scholar] [CrossRef]
- Abd El-Razek, M.H. NMR assignments of four catechin epimers. Asian J. Chem. 2007, 19, 4867. [Google Scholar] [CrossRef]
- Cecile, C.; Stephanie, D.; Stephane, L.; Bernadette, C.; Christian, R. Characterization of methylation site of monomethyl flavan-3-ols by liquid chromatography/electrospray ionization tan-dem mass spectrometry. Rapid Commun. Mass. Spectrom. 2000, 14, 2312–2319. [Google Scholar]
- Jung, W.K.; Tae, B.K.; Heejung, Y.; Fischer, W.; Sang, H.S. Phenolic compounds isolated from Opuntia ficus-indica fruits. Nat. Prod. Sci. 2016, 22, 117–121. [Google Scholar] [CrossRef]
- Guilin, C.; Xun, L.; Flora, S.; Mingquan, G. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Jing, L.; Kun, J.; Li, J.W.; Guo, Y.; Jue, W.; Yang, W.; Yi, B.J.; Qing, L.; Tie, J.W. HPLC–MS/MS determination of flavonoids in Gleditsiae Spina for its quality assessment. J. Sep. Sci. 2018, 41, 1752–1763. [Google Scholar] [CrossRef]
- Deny, S.; Hasnah, M.S.; Farediah, A.; Rasadah, M.A.; Norio, A.; Mariko, K. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 2007, 103, 710–716. [Google Scholar] [CrossRef]
- Shun, K.; Kazuho, H.; Kiyotaka, H.; Hiroaki, T.; Morifumi, H. Identification of Sternbin and Naringenin as Detoxified Metabolites from the Rice Flavanone Phytoalexin Sakuranetin by Pyricularia oryzae. Chem. Biodivers. 2017, 14, e1600240. [Google Scholar] [CrossRef]



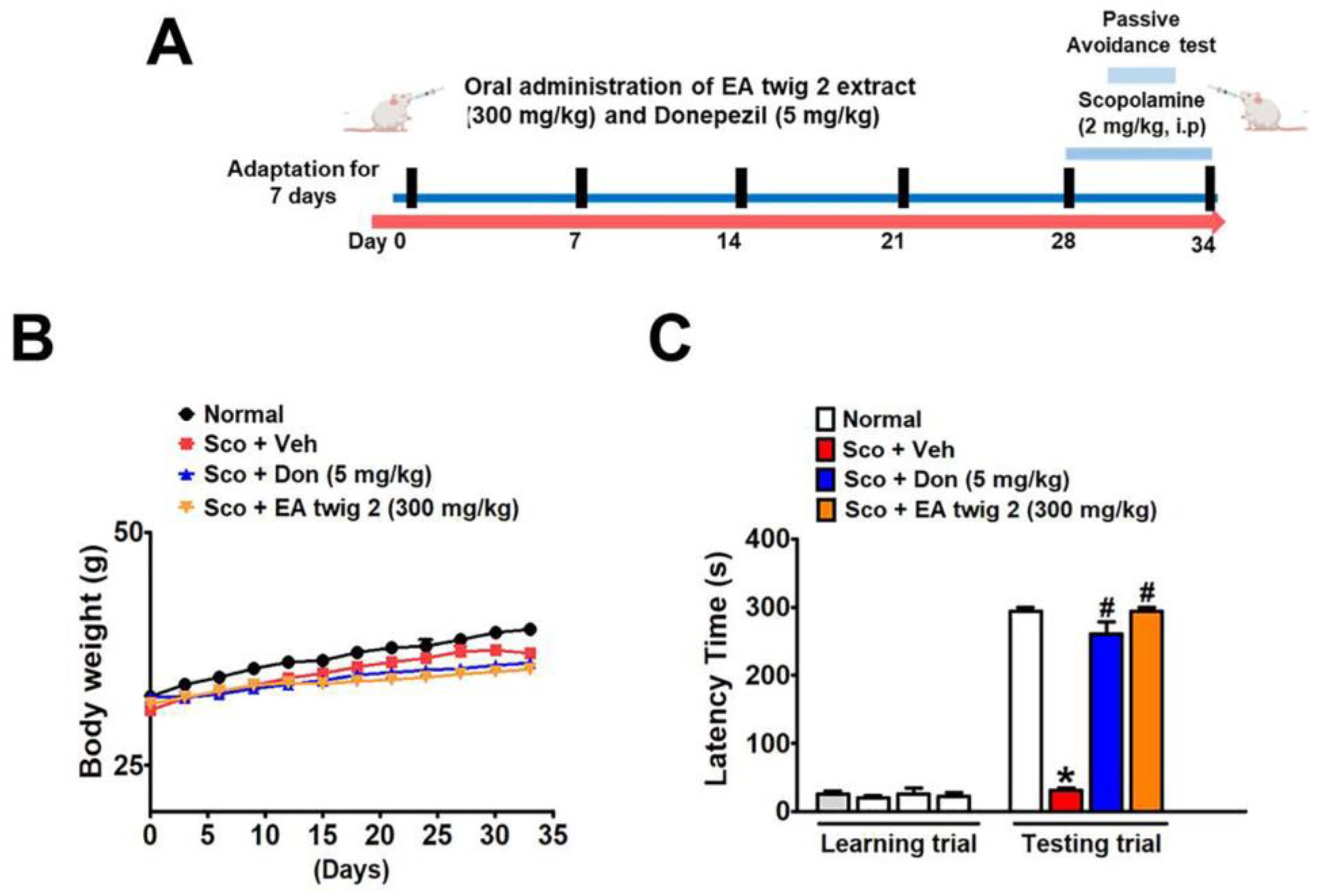

| Group 1 | Normal Mice |
|---|---|
| Group 2 | Scopolamine (2 mg/kg, i.p) |
| Group 3 | Scopolamine (2 mg/kg, i.p) + Donepezil (5 mg/kg, p.o) |
| Group 4 | Scopolamine (2 mg/kg, i.p) + EA twig 2 extract (300 mg/kg, p.o) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, P.; Shrestha, R.; Lim, J.; Thapa Magar, T.B.; Kim, H.-H.; Kim, Y.-W. Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways. Nutrients 2023, 15, 128. https://doi.org/10.3390/nu15010128
Gurung P, Shrestha R, Lim J, Thapa Magar TB, Kim H-H, Kim Y-W. Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways. Nutrients. 2023; 15(1):128. https://doi.org/10.3390/nu15010128
Chicago/Turabian StyleGurung, Pallavi, Rajeev Shrestha, Junmo Lim, Til Bahadur Thapa Magar, Han-Hyuk Kim, and Yong-Wan Kim. 2023. "Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways" Nutrients 15, no. 1: 128. https://doi.org/10.3390/nu15010128
APA StyleGurung, P., Shrestha, R., Lim, J., Thapa Magar, T. B., Kim, H.-H., & Kim, Y.-W. (2023). Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways. Nutrients, 15(1), 128. https://doi.org/10.3390/nu15010128








