Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Cell Implantation
2.3. Chemotherapy Intervention
2.4. Quercetin Administration
2.5. Dual-Energy X-Ray Absorptiometry
2.6. Muscle Histology
2.7. Myofiber Metabolic Phenotype
2.8. Electron Microscopy
2.9. Western Blotting
2.10. Real Time—Polymerase Chain Reaction (RT-PCR)
2.11. Plasma Interleukin-6 Analysis
2.12. Statistical Analyses
3. Results
3.1. Animal Characteristics
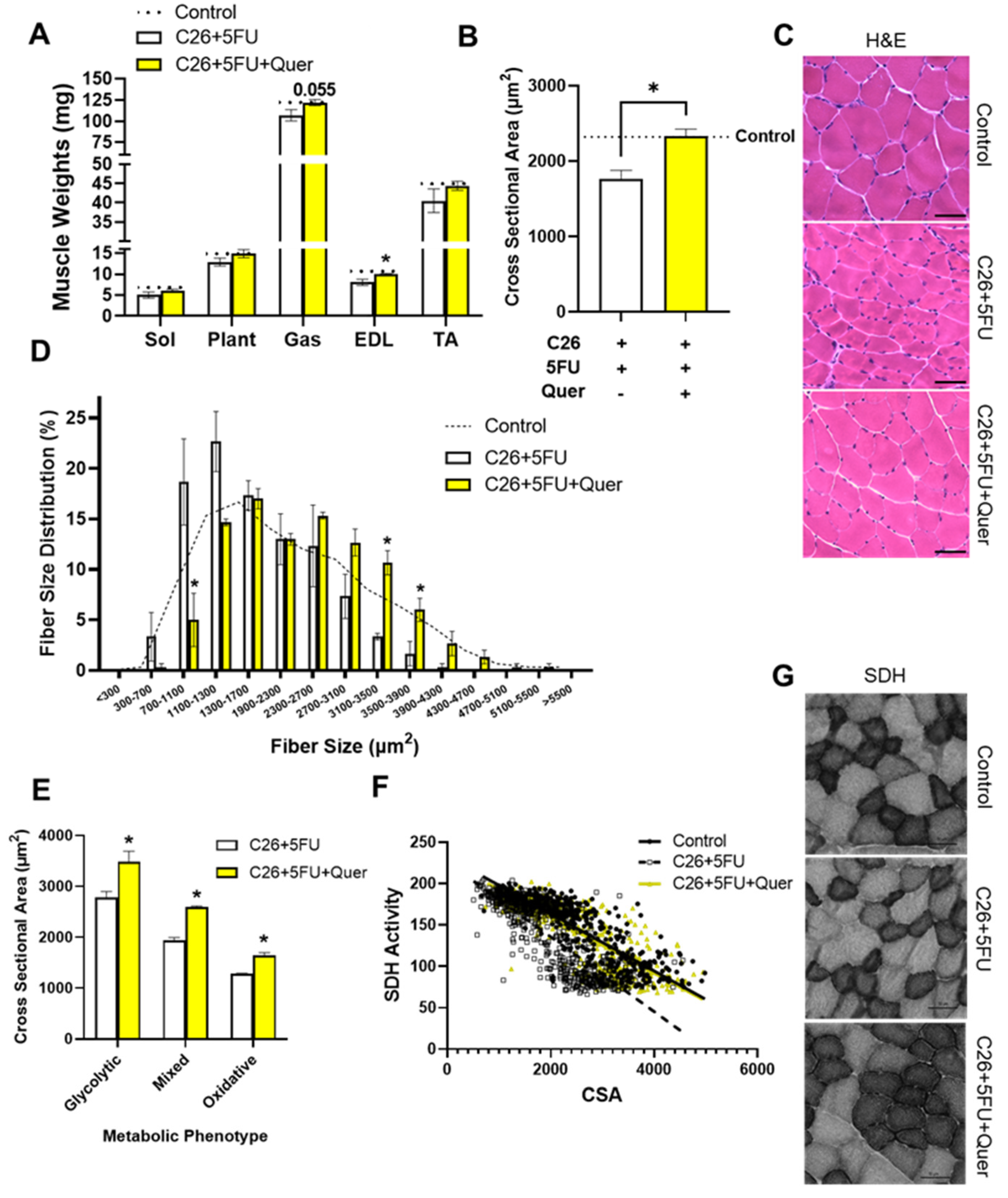
3.2. Quercetin Maintained Skeletal Muscle Cross Sectional Area
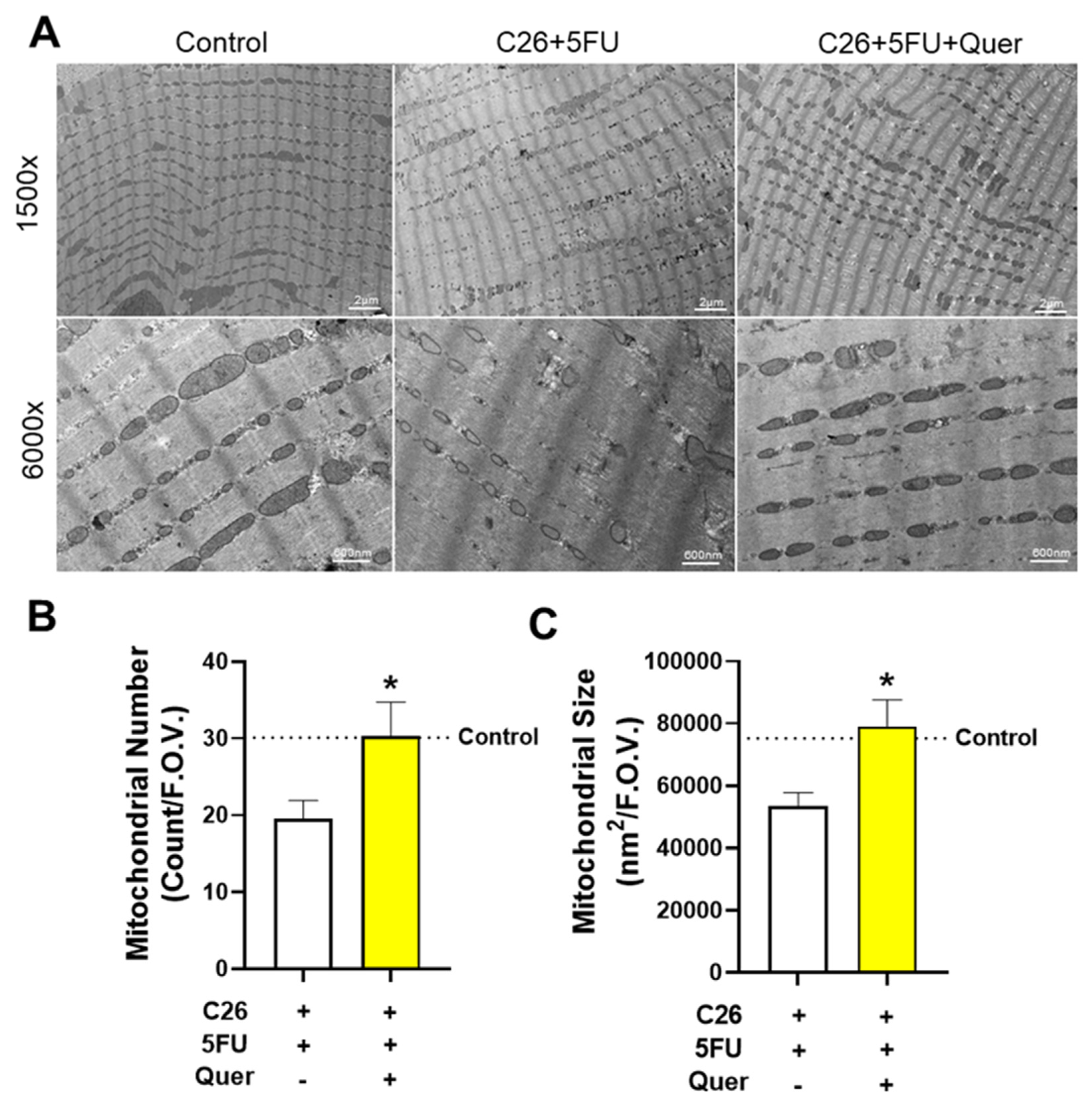
3.3. Quercetin Maintained Skeletal Muscle Mitochondrial Size and Number
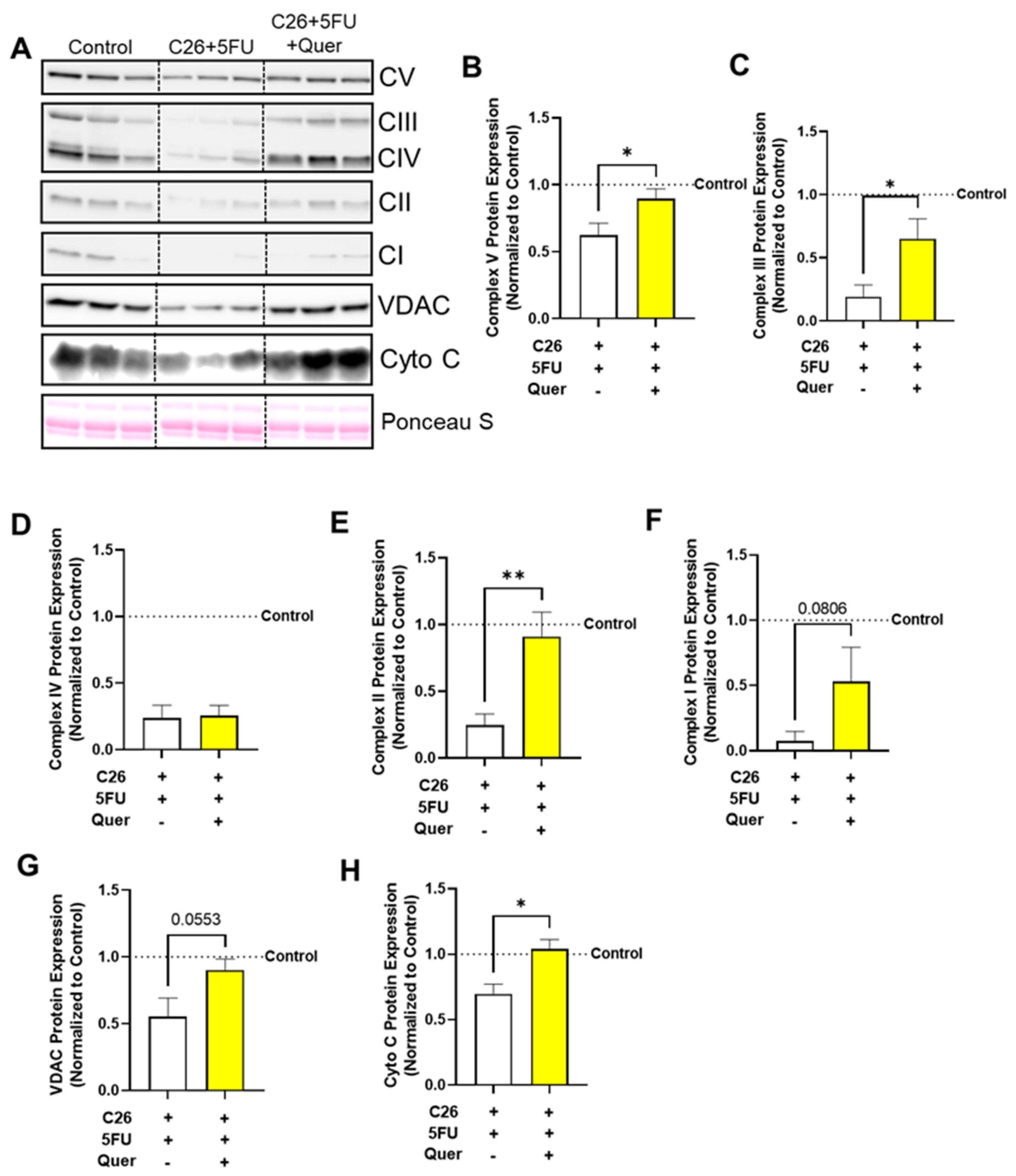
3.4. Quercetin Improved Mitochondrial Content-Associated Skeletal Muscle Proteins
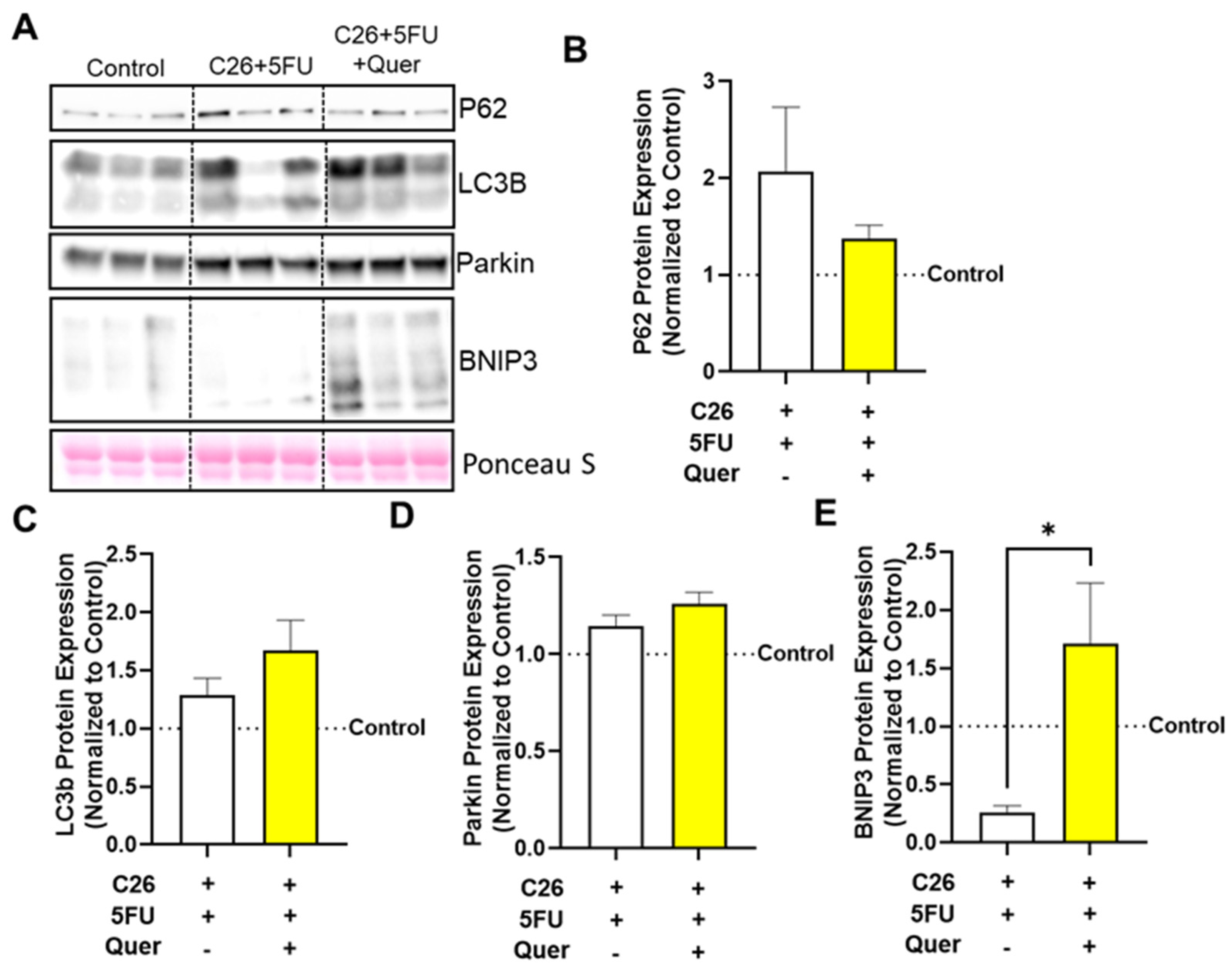
3.5. Quercetin Impacted Mitophagy-Associated Skeletal Muscle Proteins
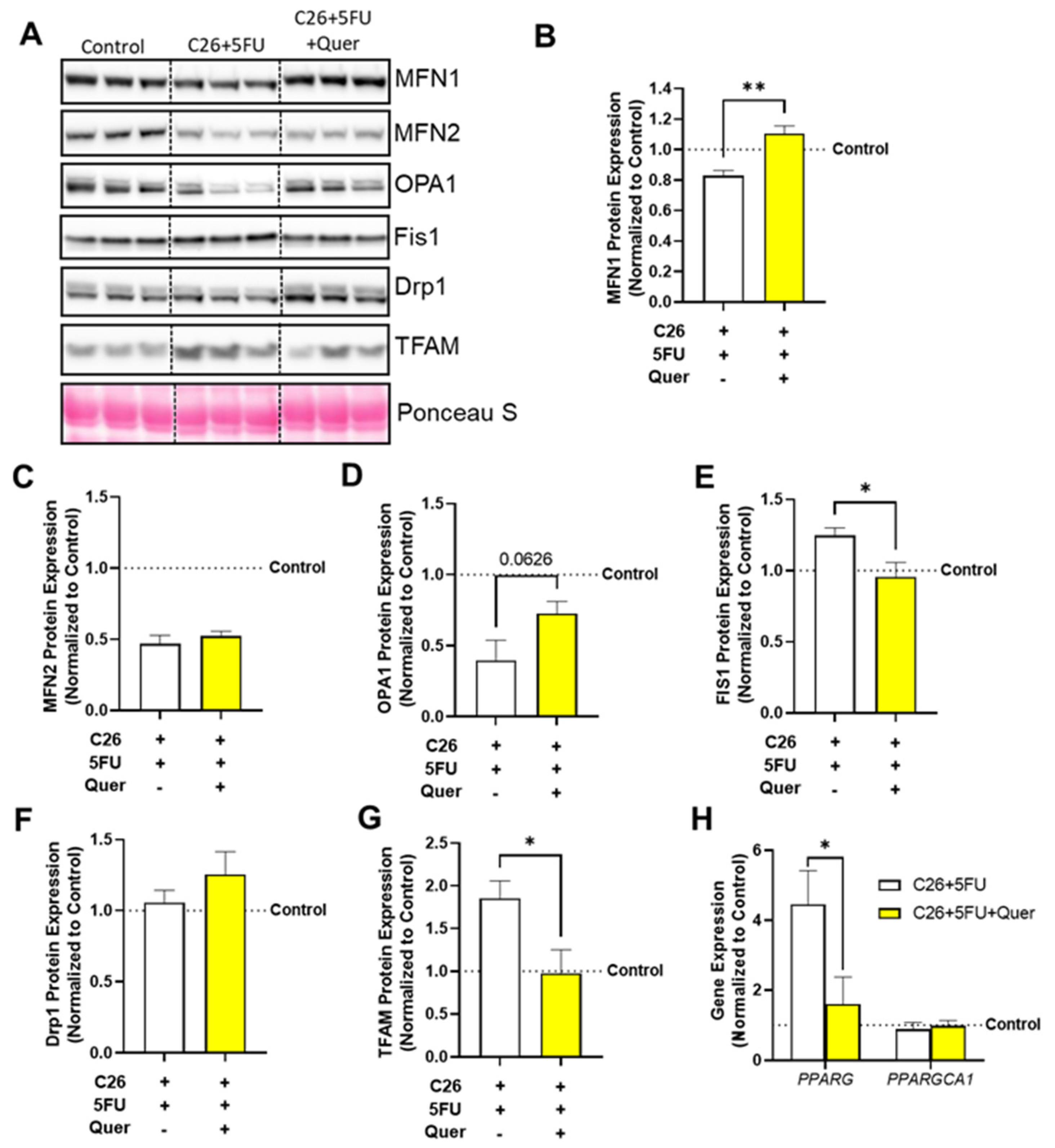
3.6. Quercetin Differentially Impacted Mitochondrial Biogenesis, Fission, and Fusion
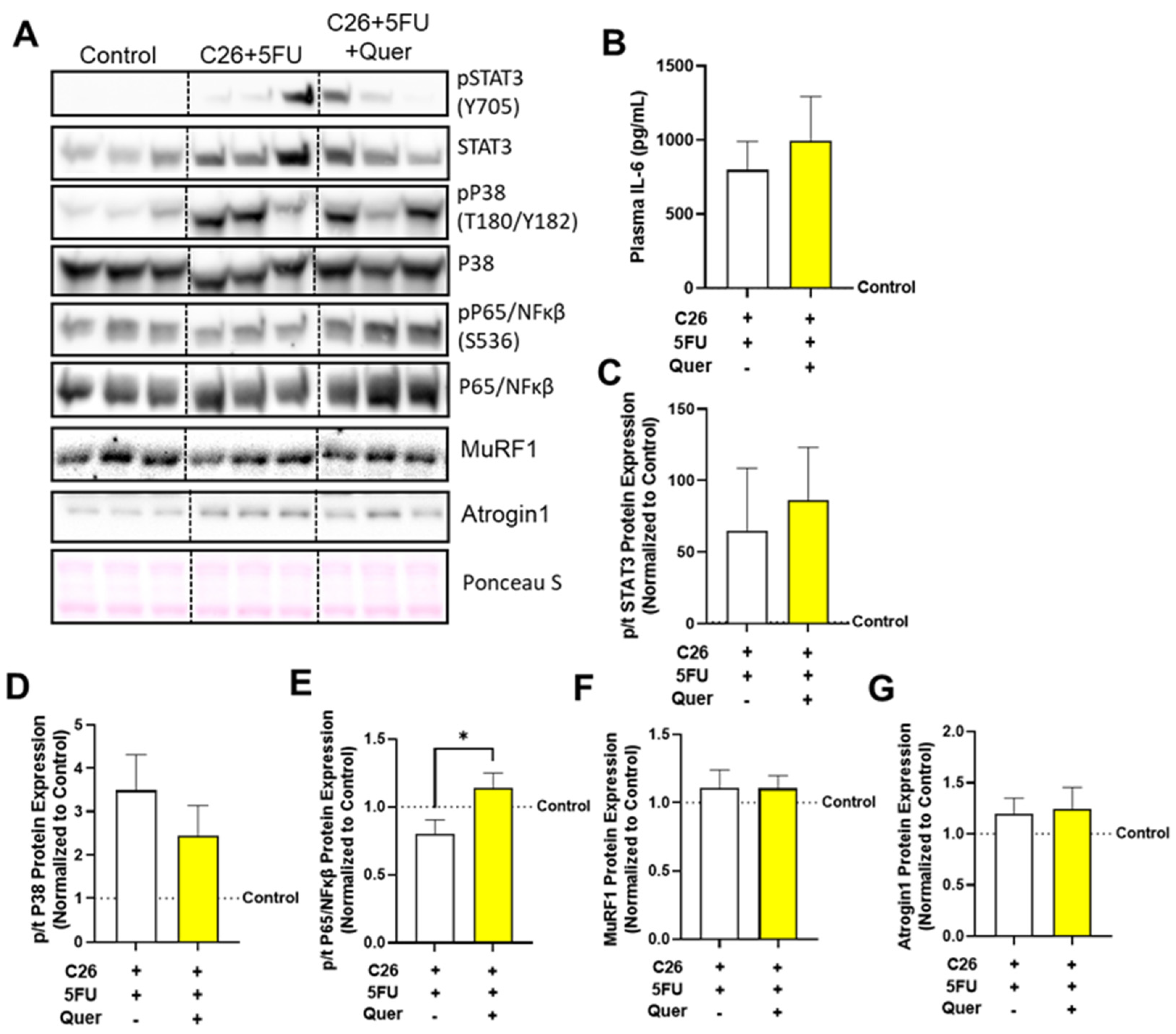
3.7. Quercetin Has a Modest Impact on Skeletal Muscle Inflammatory Signaling
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, S.T.; Noone, J.M.; Van Doren, B.A.; Roy, D.; Blanchette, C.M. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context 2014, 3, 212265. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 261–263. [Google Scholar] [CrossRef]
- Arthur, S.T.; Van Doren, B.A.; Roy, D.; Noone, J.M.; Zacherle, E.; Blanchette, C.M. Cachexia among US cancer patients. J Med. Econ. 2016, 19, 874–880. [Google Scholar] [CrossRef]
- Vigano, A.; Dorgan, M.; Buckingham, J.; Bruera, E.; Suarez-Almazor, M.E. Survival prediction in terminal cancer patients: A systematic review of the medical literature. Palliat. Med. 2000, 14, 363–374. [Google Scholar] [CrossRef]
- Sealy, M.J.; Dechaphunkul, T.; van der Schans, C.P.; Krijnen, W.P.; Roodenburg, J.L.N.; Walker, J.; Jager-Wittenaar, H.; Baracos, V.E. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Dahele, M.; Skipworth, R.J.; Wall, L.; Voss, A.; Preston, T.; Fearon, K.C. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J. Pain Symptom. Manag. 2007, 33, 676–685. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxid Med. Cell Longev 2017, 2017, 3292087. [Google Scholar] [CrossRef]
- Barreto, R.; Mandili, G.; Witzmann, F.A.; Novelli, F.; Zimmers, T.A.; Bonetto, A. Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways. Front. Physiol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Barreto, R.; Waning, D.L.; Gao, H.; Liu, Y.; Zimmers, T.A.; Bonetto, A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016, 7, 43442–43460. [Google Scholar] [CrossRef]
- Morton, A.B.; Mor Huertas, A.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Christou, D.D.; Smuder, A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 2019, 45, 52–62. [Google Scholar] [CrossRef]
- Vanderveen, B.N.; Fix, D.K.; Counts, B.R.; Carson, J.A. The Effect of Wheel Exercise on Functional Indices of Cachexia in Tumor-bearing Mice. Med. Sci. Sport Exerc. 2020, 52, 2320–2330. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Hardee, J.P.; Fix, D.K.; Carson, J.A. Skeletal muscle function during the progression of cancer cachexia in the male Apc(Min/+) mouse. J. Appl. Physiol. 2018, 124, 684–695. [Google Scholar] [CrossRef]
- Counts, B.R.; Halle, J.L.; Carson, J.A. Early-Onset Physical Inactivity and Metabolic Dysfunction in Tumor-bearing Mice Is Associated with Accelerated Cachexia. Med. Sci Sport Exerc. 2022, 54, 77–88. [Google Scholar] [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2017. [Google Scholar] [CrossRef]
- Wijler, L.A.; Raats, D.A.E.; Elias, S.G.; Dijk, F.J.; Quirindongo, H.; May, A.M.; Furber, M.J.W.; Dorresteijn, B.; van Dijk, M.; Kranenburg, O. Specialized nutrition improves muscle function and physical activity without affecting chemotherapy efficacy in C26 tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2021, 12, 796–810. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric Food Chem 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sport Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Velazquez, K.T.; Enos, R.T.; Narsale, A.A.; Puppa, M.J.; Davis, J.M.; Murphy, E.A.; Carson, J.A. Quercetin supplementation attenuates the progression of cancer cachexia in ApcMin/+ mice. J. Nutr. 2014, 144, 868–875. [Google Scholar] [CrossRef]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P. Flavonoids as antagonists at A1 adenosine receptors. Phytother. Res. 2006, 20, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Bartekova, M.; Radosinska, J.; Pancza, D.; Barancik, M.; Ravingerova, T. Cardioprotective effects of quercetin against ischemia-reperfusion injury are age-dependent. Physiol. Res. 2016, 65 (Suppl. 1), S101–S107. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Davis, J.; McClellan, J.; Enos, R.; Carson, J.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.; Altomare, D.; Creek, K.; et al. Dose-dependent benefits of quercetin on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Cancer Biol. 2014, 15, 1456–1467. [Google Scholar] [CrossRef]
- Murphy, E.A.; Davis, J.M.; McClellan, J.L.; Carmichael, M.D. Quercetin's effects on intestinal polyp multiplicity and macrophage number in the Apc(Min/+) mouse. Nutr. Cancer 2011, 63, 421–426. [Google Scholar] [CrossRef]
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.A.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharm. 2016, 84, 559–568. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Gangemi, J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R505–R509. [Google Scholar] [CrossRef]
- Mahoney, S.E.; Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Pena, M.M. Dietary quercetin reduces chemotherapy-induced fatigue in mice. Integr. Cancer 2014, 13, 417–424. [Google Scholar] [CrossRef]
- Levolger, S.; van den Engel, S.; Ambagtsheer, G.; Ijzermans, J.N.M.; de Bruin, R.W.F. Quercetin supplementation attenuates muscle wasting in cancer-associated cachexia in mice. Nutr. Healthy Aging 2021, 6, 35–47. [Google Scholar] [CrossRef]
- Cunningham, P.; Patton, E.; VanderVeen, B.N.; Unger, C.; Aladhami, A.; Enos, R.T.; Madero, S.; Chatzistamou, I.; Fan, D.; Murphy, E.A.; et al. Sub-chronic oral toxicity screening of quercetin in mice. BMC Complement Med. 2022, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 Carcinoma Tumor-bearing Mouse as a Model for the Study of Cancer Cachexia. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Cardaci, T.D.; McDonald, S.J.; Madero, S.S.; Unger, C.A.; Bullard, B.M.; Enos, R.T.; Velazquez, K.T.; Kubinak, J.L.; Fan, D.; et al. Obesity reduced survival with 5-fluorouracil and did not protect against chemotherapy-induced cachexia or immune cell cytotoxicity in mice. Cancer Biol. 2022, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Sougiannis, A.T.; Velazquez, K.T.; Carson, J.A.; Fan, D.; Murphy, E.A. The Acute Effects of 5 Fluorouracil on Skeletal Muscle Resident and Infiltrating Immune Cells in Mice. Front. Physiol. 2020, 11, 593468. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Cardaci, T.D.; Madero, S.S.; McDonald, S.J.; Bullard, B.M.; Price, R.L.; Carson, J.A.; Fan, D.; Murphy, E.A. 5-Fluorouracil disrupts skeletal muscle immune cells and impairs skeletal muscle repair and remodeling. J. Appl. Physiol. 2022, 133, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Zogby, A.M.; Dayanidhi, S.; Chambers, H.G.; Schenk, S.; Lieber, R.L. Skeletal muscle fiber-type specific succinate dehydrogenase activity in cerebral palsy. Muscle Nerve 2017, 55, 122–124. [Google Scholar] [CrossRef]
- Hardee, J.P.; Mangum, J.E.; Gao, S.; Sato, S.; Hetzler, K.L.; Puppa, M.J.; Fix, D.K.; Carson, J.A. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J. Appl. Physiol. 2016, 120, 29–37. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Cranford, T.L.; Enos, R.T.; Velazquez, K.T.; McDonald, S.; Bader, J.E.; Chatzistamou, I.; Fan, D.; Murphy, E.A. Impact of weight loss and partial weight regain on immune cell and inflammatory markers in adipose tissue in male mice. J. Appl. Physiol. 2020, 129, 909–919. [Google Scholar] [CrossRef]
- Min, K.; Kwon, O.S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.K.; Hwang, M.H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef]
- Molinari, F.; Pin, F.; Gorini, S.; Chiandotto, S.; Pontecorvo, L.; Penna, F.; Rizzuto, E.; Pisu, S.; Musaro, A.; Costelli, P.; et al. The mitochondrial metabolic reprogramming agent trimetazidine as an 'exercise mimetic' in cachectic C26-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 954–973. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, R.N.; Doerr, V.; Min, K.; Szeto, H.H.; Smuder, A.J. Doxorubicin-induced oxidative stress differentially regulates proteolytic signaling in cardiac and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R227–R233. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Hardee, J.P.; VanderVeen, B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin. Cell Dev. Biol. 2016, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, X.; Luo, Y.; You, J.; Yin, D.; Xu, Q.; He, H.; He, M. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3gamma. Toxicol. Mech. Methods 2019, 29, 344–354. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vasc. Pharm. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Waseem, M.; Kaushik, P.; Dutta, S.; Chakraborty, R.; Hassan, M.I.; Parvez, S. Modulatory Role of Quercetin in Mitochondrial Dysfunction in Titanium Dioxide Nanoparticle-Induced Hepatotoxicity. ACS Omega 2022, 7, 3192–3202. [Google Scholar] [CrossRef]
- Zymone, K.; Benetis, R.; Trumbeckas, D.; Baseviciene, I.; Trumbeckaite, S. Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function-Uncoupling, Cytochrome C Reducing and Antioxidant Activity. Molecules 2022, 27, 6377. [Google Scholar] [CrossRef]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial biogenesis and fragmentation as regulators of protein degradation in striated muscles. J. Mol. Cell Cardiol. 2013, 55, 64–72. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, L.; Li, L.; Tang, C.; Wan, Z.; Wang, R.; Tan, J.; Tan, Y.; Han, H.; Tian, R.; et al. BNIP3 Protein Suppresses PINK1 Kinase Proteolytic Cleavage to Promote Mitophagy. J. Biol. Chem. 2016, 291, 21616–21629. [Google Scholar] [CrossRef] [PubMed]
- Manan, M.; Saleem, U.; Akash, M.S.H.; Qasim, M.; Hayat, M.; Raza, Z.; Ahmad, B. Antiarthritic Potential of Comprehensively Standardized Extract of Alternanthera bettzickiana: In Vitro and In Vivo Studies. ACS Omega 2020, 5, 19478–19496. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Cittadini, M.C.; Soria, E.A. Pharmacological Activity of Quercetin and 5 Caffeoylquinic Acid Oral Intake in Male Balb/c Mice with Lung Adenocarcinoma. Arch. Med. Res. 2020, 51, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.; Derbre, F.; Lefeuvre-Orfila, L.; Rebillard, A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Radic. Biol. Med. 2016, 91, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; da Silva, M.E.; da Silva, R.A.; Justo, G.Z.; Gomes-Marcondes, M.C.; Aoyama, H. Inhibition of tumor growth by quercetin with increase of survival and prevention of cachexia in Walker 256 tumor-bearing rats. Biochem. Biophys. Res. Commun. 2011, 406, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Francaux, M.; Deldicque, L. Using polyphenol derivatives to prevent muscle wasting. Curr. Opin. Clin. Nutr. Metab. Care. 2018, 21, 159–163. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Fix, D.K.; Montalvo, R.N.; Counts, B.R.; Smuder, A.J.; Murphy, E.A.; Koh, H.J.; Carson, J.A. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp. Physiol. 2019, 104, 385–397. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Sato, S.; Gao, S.; Price, R.L.; Baynes, J.W.; Kostek, M.C.; Matesic, L.E.; Carson, J.A. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle 2012, 2, 14. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Davis, J.M.; Muga, S.J.; Carson, J.A. Interleukin-6 and cachexia in ApcMin/+ mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R393–R401. [Google Scholar] [CrossRef] [PubMed]
- Narsale, A.A.; Carson, J.A. Role of interleukin-6 in cachexia: Therapeutic implications. Curr. Opin. Support Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and Exercise against Muscle Wasting during Cancer Cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef] [PubMed]

| Protein/Gene | Vendor | Catalog Number# |
|---|---|---|
| Total OXPHOS Cocktail | Abcam | Ab110413 |
| VDAC | Cell Signaling Technology | 4661 |
| Cytochrome C | Cell Signaling Technology | 11940 |
| P62 | Cell Signaling Technology | 23214 |
| LC3 | Cell Signaling Technology | 43566 |
| Parkin | Cell Signaling Technology | 2132 |
| BNIP3 | Cell Signaling Technology | 3769 |
| MFN1 | Abcam | ab221661 |
| MFN2 | Cell Signaling Technology | 9482 |
| OPA1 | Cell Signaling Technology | 80471 |
| FIS1 | Abcam | ab229969 |
| DRP1 | Cell Signaling Technology | 8570 |
| TFAM | Abcam | ab252432 |
| pSTAT3 (Y705) | Cell Signaling Technology | 9145 |
| STAT3 | Cell Signaling Technology | 4904 |
| pP38 (T180/Y182) | Cell Signaling Technology | 4511 |
| P38 | Cell Signaling Technology | 8690 |
| pP65/NFκβ (S536) | Cell Signaling Technology | 3033 |
| P65/NFκβ | Cell Signaling Technology | 8242 |
| MuRF1 | Abcam | ab172479 |
| Atrogin1 | Abcam | ab168372 |
| Anti-rabbit IgG -HRP linked | Cell Signaling Technology | 7074 |
| Anti-mouse IgG -HRP linked | Cell Signaling Technology | 7076 |
| PPARGCA1 | Applied Biosystems | 4351372 |
| PPARG | Applied Biosystems | 4331182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanderVeen, B.N.; Cardaci, T.D.; Cunningham, P.; McDonald, S.J.; Bullard, B.M.; Fan, D.; Murphy, E.A.; Velázquez, K.T. Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia. Nutrients 2023, 15, 102. https://doi.org/10.3390/nu15010102
VanderVeen BN, Cardaci TD, Cunningham P, McDonald SJ, Bullard BM, Fan D, Murphy EA, Velázquez KT. Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia. Nutrients. 2023; 15(1):102. https://doi.org/10.3390/nu15010102
Chicago/Turabian StyleVanderVeen, Brandon N., Thomas D. Cardaci, Patrice Cunningham, Sierra J. McDonald, Brooke M. Bullard, Daping Fan, E. Angela Murphy, and Kandy T. Velázquez. 2023. "Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia" Nutrients 15, no. 1: 102. https://doi.org/10.3390/nu15010102
APA StyleVanderVeen, B. N., Cardaci, T. D., Cunningham, P., McDonald, S. J., Bullard, B. M., Fan, D., Murphy, E. A., & Velázquez, K. T. (2023). Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia. Nutrients, 15(1), 102. https://doi.org/10.3390/nu15010102






