Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
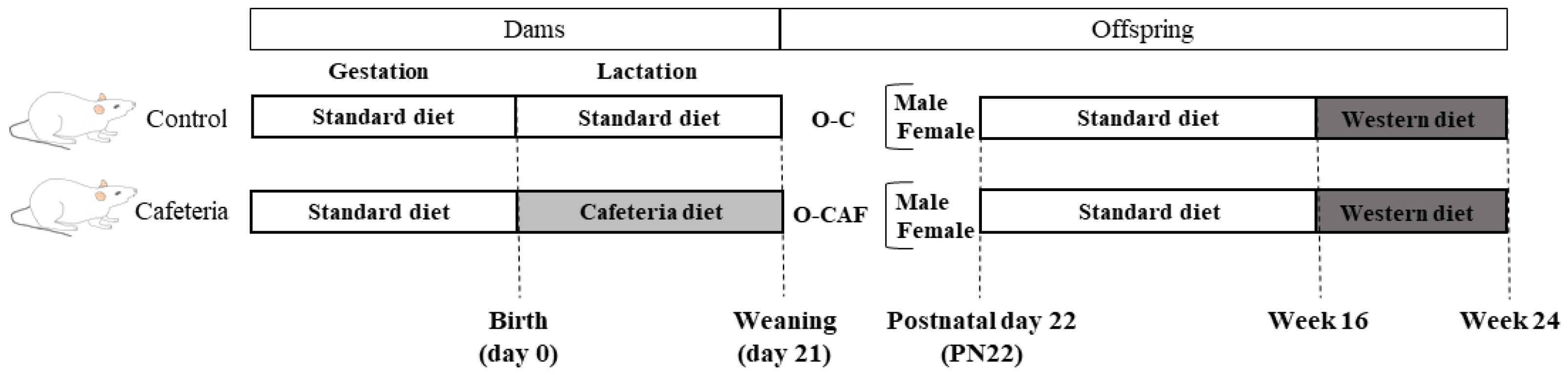
2.2. Experimental Design
2.3. Analysis of Blood Parameters
2.4. RNA Extraction and Gene Expression Analysis
2.5. UCP1 Protein Levels
2.6. Statistical Analysis
3. Results
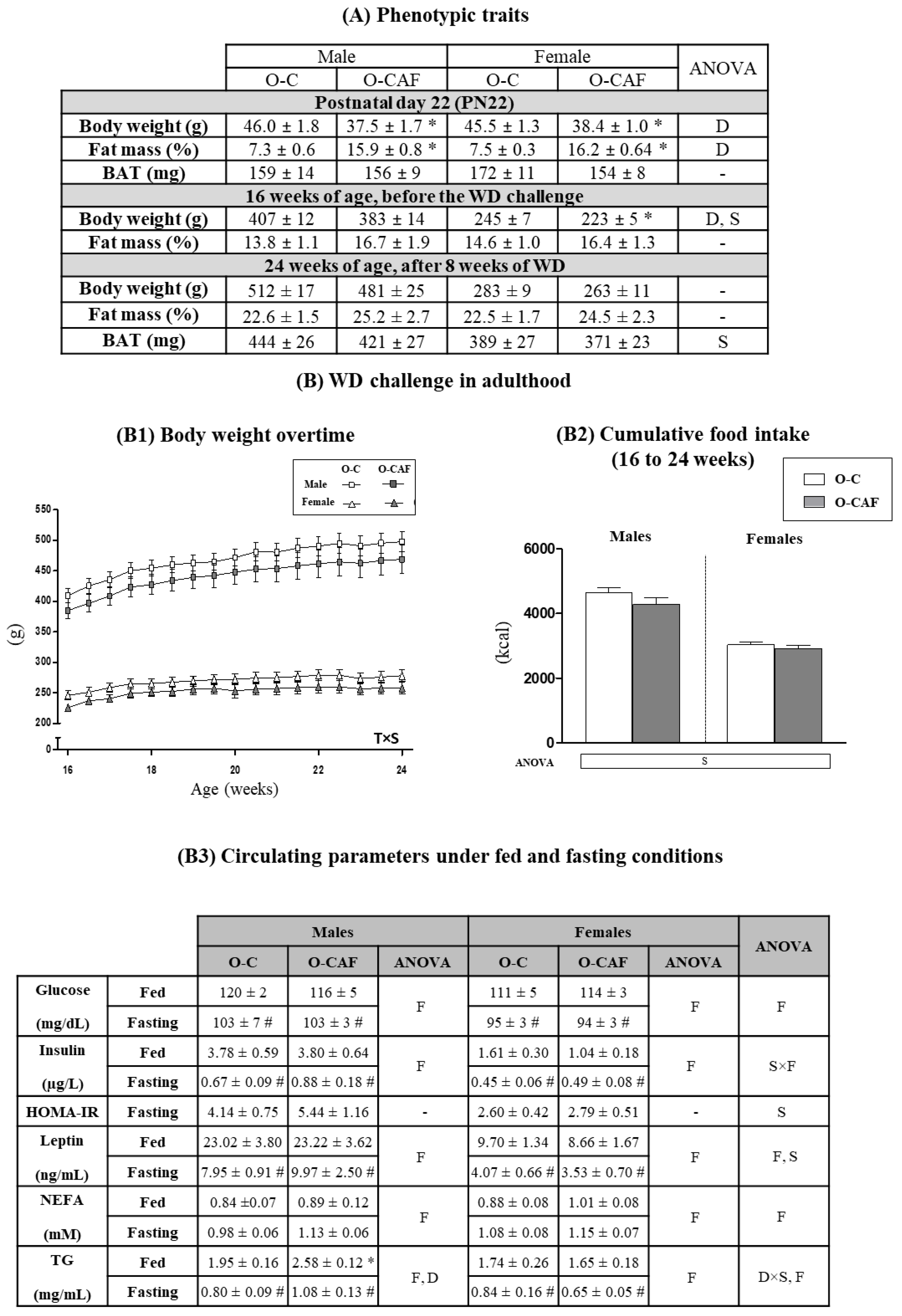
3.1. Effects of Maternal Cafeteria Diet Intake during Lactation on Phenotypic Parameters and Food Intake in the Offspring
3.2. Effects of Maternal Exposure to a Cafeteria Diet during Lactation on the Expression of Selected Genes in BAT of the Offspring
3.2.1. Results in Young Animals (PN22)
3.2.2. Results in Adult Animals (24 Weeks) after Exposure to Western Diet
3.2.3. Results in Adult Animals (24 Weeks) That Were Maintained under Standard Diet
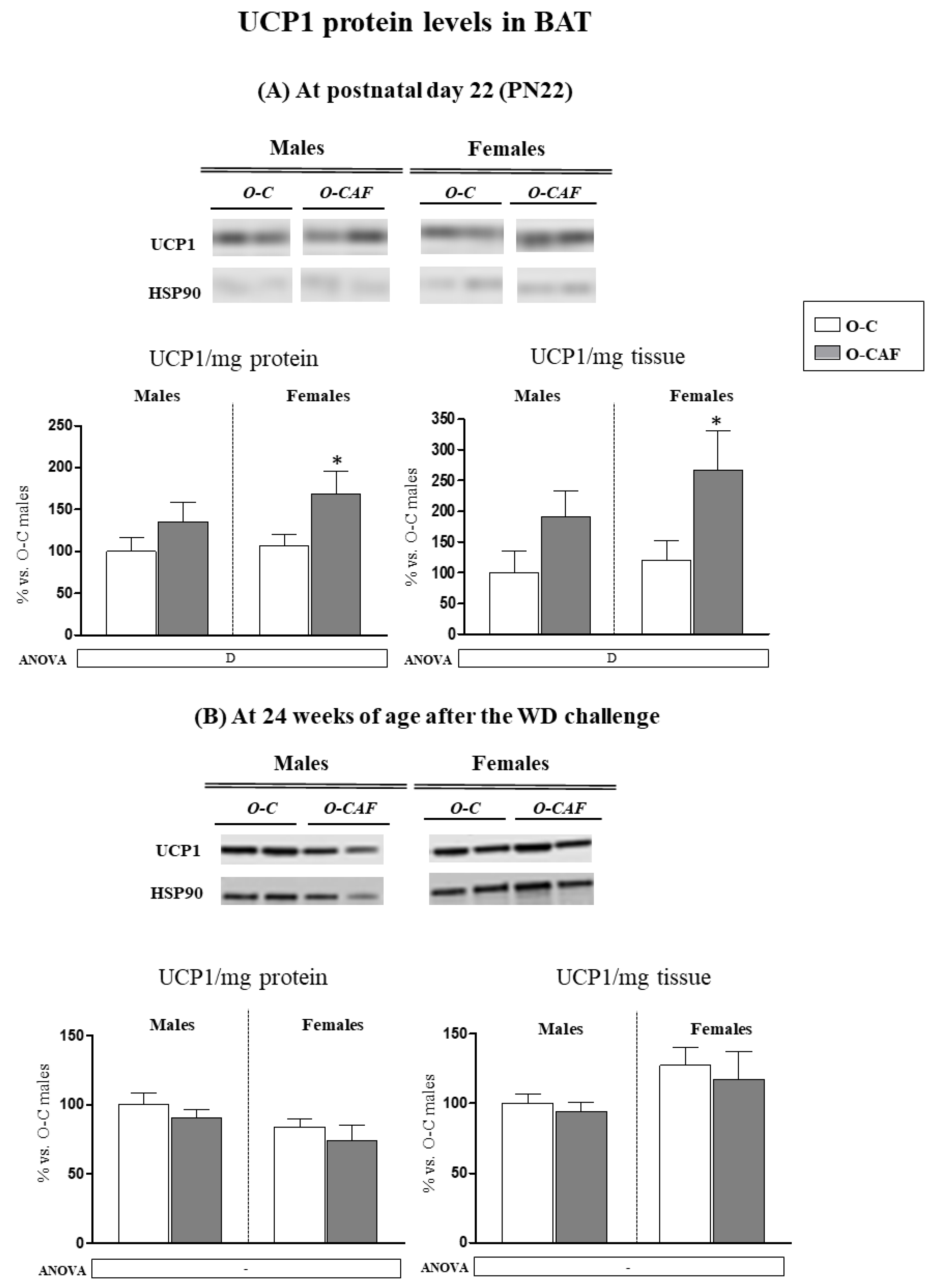
3.3. Western Blot Analysis of UCP1 in BAT in Young Animals (PN22) and in Adult Animals (24 Weeks) after Exposure to Western Diet
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Langley-Evans, S.C. Nutrition in early life and the programming of adult disease: A review. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [Green Version]
- Ohta, T.; Toriniwa, Y.; Ryumon, N.; Inaba, N.; Hirao, T.; Yamanaka, S.; Maeno, T.; Sakakibara, W.; Sumikawa, M.; Chiba, K.; et al. Maternal high-fat diet promotes onset of diabetes in rat offspring. Anim. Sci. J. 2017, 88, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ainge, H.; Thompson, C.; Ozanne, S.E.; Rooney, K.B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int. J. Obes. 2011, 35, 325–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picó, C.; Reis, F.; Egas, C.; Mathias, P.; Matafome, P. Lactation as a programming window for metabolic syndrome. Eur. J. Clin. Investig. 2021, 51, e13482. [Google Scholar] [CrossRef]
- Kruse, M.; Seki, Y.; Vuguin, P.M.; Du, X.Q.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef]
- Palou, A.; Picó, C.; Bonet, M.L.; Oliver, P. The uncoupling protein, thermogenin. Int. J. Biochem. Cell Biol. 1998, 30, 7–11. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. Brown adipose tissue as a heat-producing thermoeffector. Handb. Clin. Neurol. 2018, 156, 137–152. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef]
- Puigserver, P.; Picó, C.; Stock, M.J.; Palou, A. Effect of selective beta-adrenoceptor stimulation on UCP synthesis in primary cultures of brown adipocytes. Mol. Cell. Endocrinol. 1996, 117, 7–16. [Google Scholar] [CrossRef]
- Collins, S. β-Adrenergic Receptors and Adipose Tissue Metabolism: Evolution of an Old Story. Annu. Rev. Physiol. 2022, 84, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Jacobsson, A.; Rehnmark, S.; Nedergaard, J. Signal transduction in brown adipose tissue recruitment: Noradrenaline and beyond. Int. J. Obes. Relat. Metab. Disord. 1996, 20 (Suppl. 3), S36–S42. [Google Scholar] [PubMed]
- Lowell, B.B.; Susulic, V.S.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Villarroya, F. Mitochondrial Uncoupling and the Regulation of Glucose Homeostasis. Curr. Diabetes Rev. 2017, 13, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef]
- Palou, M.; Priego, T.; Romero, M.; Szostaczuk, N.; Konieczna, J.; Cabrer, C.; Remesar, X.; Palou, A.; Pico, C. Moderate calorie restriction during gestation programs offspring for lower BAT thermogenic capacity driven by thyroid and sympathetic signaling. Int. J. Obes. 2015, 39, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Yang, Q.; Zhang, L.; Maricelli, J.W.; Rodgers, B.D.; Zhu, M.J.; Du, M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016, 6, 34345. [Google Scholar] [CrossRef] [Green Version]
- Lettieri Barbato, D.; Tatulli, G.; Vegliante, R.; Cannata, S.M.; Bernardini, S.; Ciriolo, M.R.; Aquilano, K. Dietary fat overload reprograms brown fat mitochondria. Front. Physiol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomar, C.A.; van Nes, R.; Sánchez, J.; Picó, C.; Keijer, J.; Palou, A. Maternal consumption of a cafeteria diet during lactation in rats leads the offspring to a thin-outside-fat-inside phenotype. Int. J. Obes. 2017, 41, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.A.; Castro, H.; Picó, C.; Palou, A.; Sánchez, J. Maternal Overfeeding during Lactation Impairs the Metabolic Response to Fed/Fasting Changing Conditions in the Postweaning Offspring. Mol. Nutr. Food Res. 2019, 63, e1900504. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Pomar, C.A.; Picó, C.; Sánchez, J.; Palou, A. Cafeteria diet overfeeding in young male rats impairs the adaptive response to fed/fasted conditions and increases adiposity independent of body weight. Int. J. Obes. 2015, 39, 430–437. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [Green Version]
- Lidell, M.E. Brown Adipose Tissue in Human Infants. Handb. Exp. Pharmacol. 2019, 251, 107–123. [Google Scholar] [CrossRef]
- Giralt, M.; Martin, I.; Iglesias, R.; Viñas, O.; Villarroya, F.; Mampel, T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Unaltered iodothyronine 5′-deiodinase activity is necessary for the response to environmental temperature at birth. Eur. J. Biochem. 1990, 193, 297–302. [Google Scholar] [CrossRef]
- Van Baak, M.A. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 2008, 94, 178–186. [Google Scholar] [CrossRef]
- Landsberg, L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol. Neurobiol. 2006, 26, 497–508. [Google Scholar] [CrossRef]
- Fromme, T.; Klingenspor, M. Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1–R8. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Reynés, B.; Díaz-Rúa, R.; Ceresi, E.; Oliver, P.; Palou, A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Waldén, T.B.; Hansen, I.R.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Recruited vs. nonrecruited molecular signatures of brown, “brite”, and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E19–E31. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Sanchez-Gurmaches, J.; Guertin, D.A. Brown Adipose Tissue Development and Metabolism. Handb. Exp. Pharmacol. 2019, 251, 3–36. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J.; Trayhurn, P. Reduced lipogenesis in cafeteria-fed rats exhibiting diet-induced thermogenesis. Biosci. Rep. 1983, 3, 217–224. [Google Scholar] [CrossRef]
- Priego, T.; Sánchez, J.; García, A.P.; Palou, A.; Picó, C. Maternal dietary fat affects milk Fatty Acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids 2013, 48, 481–495. [Google Scholar] [CrossRef]
- Pomar, C.A.; Kuda, O.; Kopecky, J.; Rombaldova, M.; Castro, H.; Picó, C.; Sánchez, J.; Palou, A. Maternal diet, rather than obesity itself, has a main influence on milk triacylglycerol profile in dietary obese rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158556. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Williams, S.M.; Grayson, B.E.; Glavas, M.M.; Cowley, M.A.; Smith, M.S.; Grove, K.L. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology 2007, 148, 4150–4159. [Google Scholar] [CrossRef]
- Bachman, E.S.; Dhillon, H.; Zhang, C.Y.; Cinti, S.; Bianco, A.C.; Kobilka, B.K.; Lowell, B.B. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Inokuma, K.; Okamatsu-Ogura, Y.; Omachi, A.; Matsushita, Y.; Kimura, K.; Yamashita, H.; Saito, M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1014–E1021. [Google Scholar] [CrossRef]
- Fisher, M.H.; Amend, A.M.; Bach, T.J.; Barker, J.M.; Brady, E.J.; Candelore, M.R.; Carroll, D.; Cascieri, M.A.; Chiu, S.H.; Deng, L.; et al. A selective human beta3 adrenergic receptor agonist increases metabolic rate in rhesus monkeys. J. Clin. Investig. 1998, 101, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Goldgof, M.; Gavrilova, O.; Reitman, M.L. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 °C. Obesity 2015, 23, 1450–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priego, T.; Sánchez, J.; Picó, C.; Palou, A. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity 2008, 16, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomar, C.A.; Picó, C.; Palou, A.; Sánchez, J. Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood. Nutrients 2022, 14, 1958. https://doi.org/10.3390/nu14091958
Pomar CA, Picó C, Palou A, Sánchez J. Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood. Nutrients. 2022; 14(9):1958. https://doi.org/10.3390/nu14091958
Chicago/Turabian StylePomar, Catalina Amadora, Catalina Picó, Andreu Palou, and Juana Sánchez. 2022. "Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood" Nutrients 14, no. 9: 1958. https://doi.org/10.3390/nu14091958
APA StylePomar, C. A., Picó, C., Palou, A., & Sánchez, J. (2022). Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood. Nutrients, 14(9), 1958. https://doi.org/10.3390/nu14091958








