L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. RNA Isolation, Transcription and RT-PCR
2.3. Western Blot for Protein Expression Measurement
2.4. Histology
2.5. Serum and Liver Hormones and Metabolites
2.6. Ex Vivo Lipolysis Assay
2.7. Primary Brown Adipocytes Culture
2.8. Cellular Respiration
2.9. Adipocyte Size Measurements
2.10. Statistical Analysis
3. Results
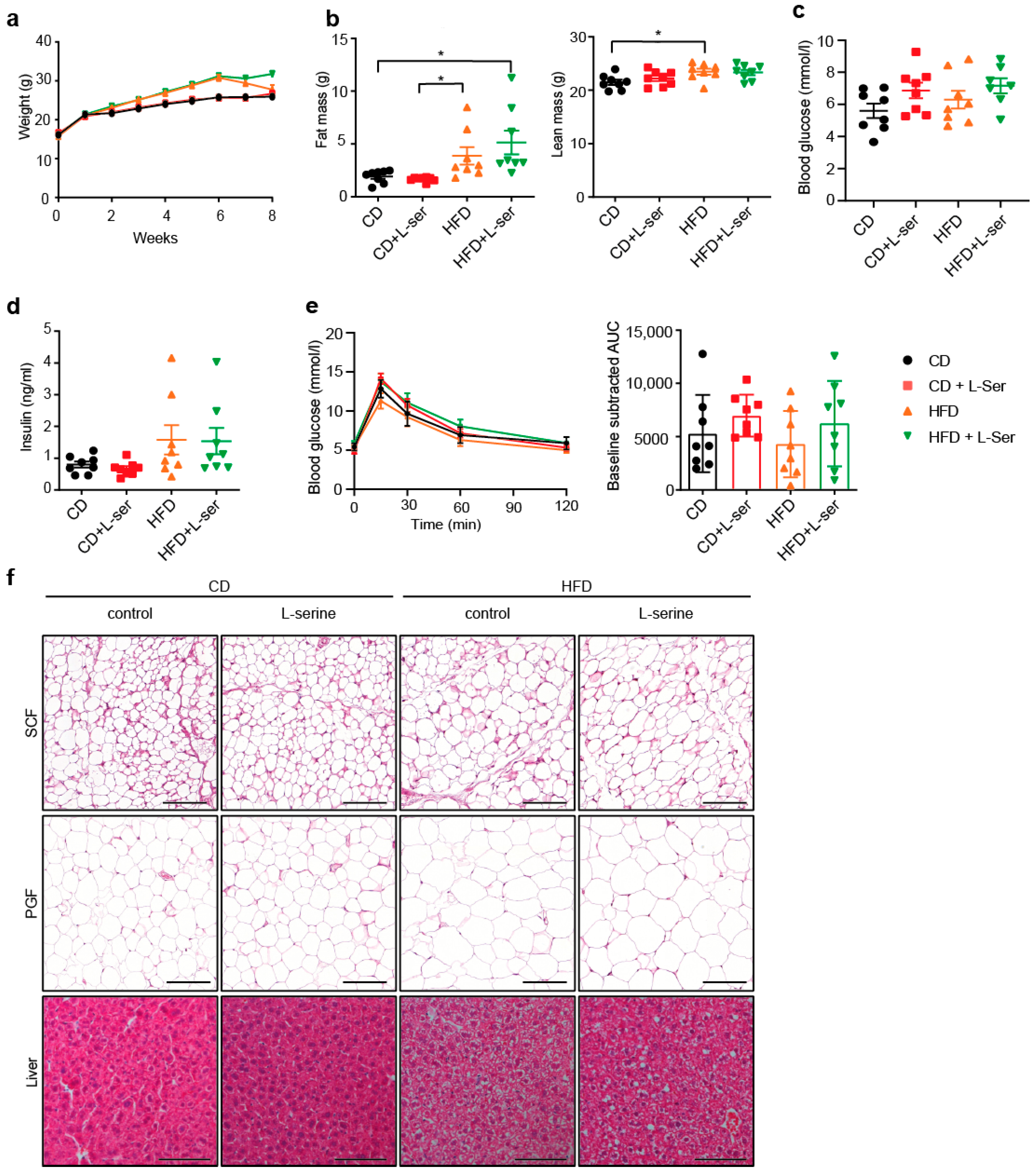
3.1. L-Serine Supplementation Does Not Alter Body Weight or Glucose Homeostasis in Ad Libitum-Fed Young Mice
3.2. L-Serine Supplementation Blunts Body Weight Regain after Repeated Overnight Fasting
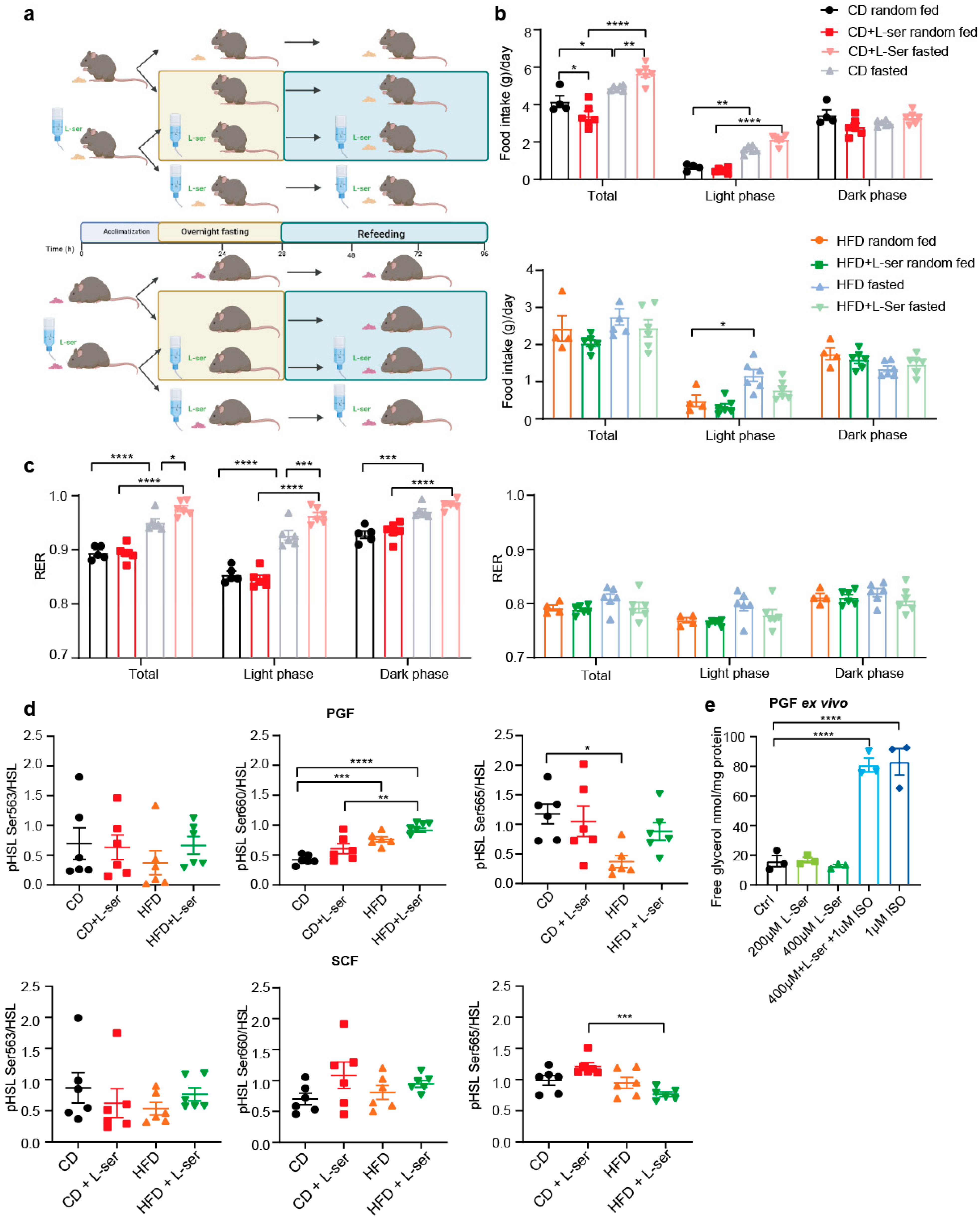
3.3. L-Serine Supplementation Increases Energy Expenditure following an Overnight Fast
3.4. L-Serine Supplementation Activates Brown Adipose Tissue Thermogenesis after Fasting
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Overview Complications Prevention and Control; World Health Organization: Geneva, Switzerland, 2020; Available online: www.who.int/health-topics/obesity (accessed on 10 September 2021).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.J.; Buschard, K. L-Serine: A neglected amino acid with a potential therapeutic role in diabetes. Apmis 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salari, N.; Jafari, S.; Darvishi, N.; Valipour, E.; Mohammadi, M.; Mansouri, K.; Shohaimi, S. The best drug supplement for obesity treatment: A systematic review and network meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Parham, J.S.; Pendse, J.; Alemán, J.O. Treatment of Obesity in Mitigating Metabolic Risk. Circ. Res. 2020, 126, 1646–1665. [Google Scholar] [CrossRef]
- Fischer, I.P.; Irmler, M.; Meyer, C.W.; Sachs, S.J.; Neff, F.; de Angelis, M.H.; Beckers, J.; Tschöp, M.H.; Hofmann, S.; Ussar, S. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int. J. Obes. 2017, 42, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K.; American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; He, L.; Wu, X.; Yin, Y. Long-Term l-Serine Administration Reduces Food Intake and Improves Oxidative Stress and Sirt1/NFκB Signaling in the Hypothalamus of Aging Mice. Front. Endocrinol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Holm, L.J.; Haupt-Jorgensen, M.; Larsen, J.; Giacobini, J.D.; Bilgin, M.; Buschard, K. L-serine supplementation lowers diabetes incidence and improves blood glucose homeostasis in NOD mice. PLoS ONE 2018, 13, e0194414. [Google Scholar] [CrossRef] [Green Version]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated with Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Parker, S.J.; Metallo, C.M. Chasing One-Carbon Units to Understand the Role of Serine in Epigenetics. Mol. Cell 2016, 61, 185–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalhan, S.C.; Hanson, R.W. Resurgence of Serine: An Often Neglected but Indispensable Amino Acid. J. Biol. Chem. 2012, 287, 19786–19791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Stenson, L.; Sulvucci, M.; Sumayao, R.; Krause, M. Amino Acid Metabolism, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar]
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine restriction alters sphingolipid diversity to constrain tumor growth. Nature 2021, 586, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. L-Serine GRAS 172.320. Substances Added to Food (formerly EAFUS), Food Ingredient and Packaging Inventories. 2021. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=SERINE (accessed on 20 January 2022).
- Suwandhi, L.; Hausmann, S.; Braun, A.; Gruber, T.; Heinzmann, S.S.; Gálvez, E.J.; Buck, A.; Legutko, B.; Israel, A.; Feuchtinger, A.; et al. Chronic d-serine supplementation impairs insulin secretion. Mol. Metab. 2018, 16, 191–202. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, R.; Zhang, B.; Zhang, X.; Yang, H.; Zhou, X. Serine Alleviates Dextran Sulfate Sodium-Induced Colitis and Regulates the Gut Microbiota in Mice. Front. Microbiol. 2018, 9, 3062. [Google Scholar] [CrossRef] [Green Version]
- Heijboer, A.C.; Donga, E.; Voshol, P.J.; Dang, Z.-C.; Havekes, L.M.; Romijn, J.A.; Corssmit, E.P.M. Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J. Lipid Res. 2005, 46, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.L.; Kiersgaard, M.K.; Sørensen, D.B.; Mikkelsen, L.F. Fasting of mice: A review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef]
- Pedroso, J.A.; Wasinski, F.; Donato, J. Prolonged fasting induces long-lasting metabolic consequences in mice. J. Nutr. Biochem. 2020, 84, 108457. [Google Scholar] [CrossRef]
- Felig, P.; Owen, O.E.; Wahren, J.; Cahill, G.F. Amino acid metabolism during prolonged starvation Amino Acid Metabolism during Prolonged Starvation. J. Clin. Investig. 1969, 48, 584–594. [Google Scholar] [CrossRef]
- Adibi, A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J. Appl. Physiol. 1968, 25, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary L-Arginine Supplementation Reduces Fat Mass in Zucker Diabetic Fatty Rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.; Li, X.; Yin, Y.; Wu, Z.; Liu, C.; Tekwe, C.D.; Wu, G. Regulatory roles for L-arginine in reducing white adipose tissue. Front. Biosci. 2012, 17, 2237–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial effects of a long-term oral l-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am. J. Physiol. Metab. 2006, 291, E906–E912. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, X.; Hu, L.; Chen, J.; Zhu, J.; Shan, A. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2279–2290. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Zuo, S.; Zhang, Y.; Wan, D.; Long, C.; Huang, P.; Wu, X.; Wu, C.; Liu, G.; et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 488–498. [Google Scholar] [CrossRef]
- Sim, W.-C.; Yin, H.-Q.; Choi, H.-S.; Choi, Y.-J.; Kwak, H.C.; Kim, S.-K.; Lee, B.-H. L-Serine Supplementation Attenuates Alcoholic Fatty Liver by Enhancing Homocysteine Metabolism in Mice and Rats. J. Nutr. 2014, 145, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Fridman, V.; Suriyanarayanan, S.; Novak, P.; David, W.; Macklin, E.A.; McKenna-Yasek, D.; Walsh, K.; Aziz-Bose, R.; Oaklander, A.L.; Brown, R.; et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019, 92, e359–e370. [Google Scholar] [CrossRef]
- Garofalo, K.; Penno, A.; Schmidt, B.; Lee, H.-J.; Frosch, M.P.; Von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral l-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Labs, B.C. Phase IIa L-serine Trial for ALzheimer’s Disease (LSPI-2). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03062449 (accessed on 20 January 2022).
- De Déu, S.J. Tolerability and Efficacy of L-Serine in Patients with GRIN-Related Encephalopathy. 2020. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04646447 (accessed on 20 January 2022).
- Demetrius, L. Of mice and men. EMBO Rep. 2005, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gonzales, E.; Lehmann, L.; Ruiz-Ojeda, F.J.; Hernández-Bautista, R.; Altun, I.; Onogi, Y.; Khalil, A.E.; Liu, X.; Israel, A.; Ussar, S. L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis. Nutrients 2022, 14, 1922. https://doi.org/10.3390/nu14091922
López-Gonzales E, Lehmann L, Ruiz-Ojeda FJ, Hernández-Bautista R, Altun I, Onogi Y, Khalil AE, Liu X, Israel A, Ussar S. L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis. Nutrients. 2022; 14(9):1922. https://doi.org/10.3390/nu14091922
Chicago/Turabian StyleLópez-Gonzales, Elena, Lisa Lehmann, Francisco Javier Ruiz-Ojeda, René Hernández-Bautista, Irem Altun, Yasuhiro Onogi, Ahmed Elagamy Khalil, Xue Liu, Andreas Israel, and Siegfried Ussar. 2022. "L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis" Nutrients 14, no. 9: 1922. https://doi.org/10.3390/nu14091922
APA StyleLópez-Gonzales, E., Lehmann, L., Ruiz-Ojeda, F. J., Hernández-Bautista, R., Altun, I., Onogi, Y., Khalil, A. E., Liu, X., Israel, A., & Ussar, S. (2022). L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis. Nutrients, 14(9), 1922. https://doi.org/10.3390/nu14091922







