Abstract
Beetroot juice is a food high in nitrate and is associated with cardiometabolic health benefits and enhanced exercise performance through the production of nitric oxide in the nitrate–nitrite–nitric oxide pathway. Since various food components influence this pathway, the aim of this trial was to study the effect of beetroot juice alone and in conjunction with vitamin C or protein on the acute response to plasma nitrate and nitrite levels in healthy middle- to older-aged adults. In this cross-over trial, each participant received, in a randomized order, a single dose of Beet It Sport® alone; Beet It Sport®, plus a 200 mg vitamin C supplement; and Beet It Sport® plus 15 g of whey protein. Plasma levels of nitrate and nitrite were determined prior to and at 1 and 3 h after intervention. Log plasma nitrate and nitrite was calculated to obtain data that were normally distributed, and these data were analyzed using two-way within-factors ANOVA, with time and treatment as the independent factors. There were no statistically significant differences for log plasma nitrate (p = 0.308) or log plasma nitrite (p = 0.391) values across treatments. Log plasma nitrate increased significantly from pre-consumption levels after 1 h (p < 0.001) and 3 h (p < 0.001), but plasma nitrate was lower at 3 h than 1 h (p < 0.001). Log plasma nitrite increased from pre to 1 h (p < 0.001) and 3 h (p < 0.001) with log values at 3 h higher than at 1 h (p = 0.003). In this cohort, we observed no differences in log plasma nitrate and nitrite at 1 h and 3 h after co-ingesting beetroot juice with vitamin C or a whey protein supplement compared to beetroot juice alone. Further research needs to be undertaken to expand the blood-sampling time-frame and to examine factors that may influence the kinetics of the plasma nitrate to nitrite efficacy, such as differences in fluid volume and osmolarity between treatments employed.
1. Introduction
Inorganic nitrogen compounds, principally nitrate, are found in beetroot and many leafy green vegetables, such as spinach and lettuce [1]. Beetroot juice is a food high in nitrate and is associated with cardiometabolic health benefits and enhanced exercise performance through the production of nitric oxide in the nitrate–nitrite–nitric oxide pathway [2,3,4]. The physiological effects of nitrate are attributed to its endogenous nitric oxide formation, a molecule with important vascular and metabolic functions [5,6,7]. These actions include regulation of blood flow, muscle contractility, inhibition of platelet aggregation, prevention of adhesion of inflammatory signals and cells, improving endothelial barrier cell function, muscle glucose uptake, angiogenesis, decreased level of oxygen cost during exercise, and enhanced exercise performance [2,8,9,10,11,12,13], and are attributed to both cyclic guanosine monophosphate (cGMP)- dependent and cGMP-independent effects of nitric oxide [14,15,16,17,18,19]. Nitric oxide is formed through two pathways in the body. The first is through the oxidative reactions of nitric oxide synthase and arginine to form nitric oxide and citrulline [20]. The second method is through the nitrate–nitrite–nitric oxide pathway [21]. In this latter pathway, nitrate ingested from food is nearly 100% absorbed in the small intestine to the portal vein [7,22,23]. Twenty-five percent of this absorbed nitrate is then actively transported into the salivary glands, where the nitrate is reduced to nitrite by anerobic facultative bacteria located on the posterior aspect of the tongue [24,25]. The salivary nitrite is swallowed and absorbed into the circulation [7,26,27,28,29]. Acute and chronic intake of nitrate in its various forms, including beetroot juice, green leafy vegetables, or as a nitrate salt, substantially raises nitric oxide metabolites, such as plasma nitrate and nitrite levels [7,30], with the peak plasma response occurring from 1–3 h after ingestion of the nitrate source [31,32,33].
Endothelial dysfunction, manifested as a reduction in nitric oxide production via nitric oxide synthase pathway, is apparent with aging, primarily through prominent increases in inflammation and oxidative stress [34,35,36]. More specifically, older adults have lower levels of both L-arginine, the nitric oxide synthase substrate, and tetrahydrobiopterin, a cofactor for the reaction [37,38]. Additionally, aging is positively associated with increases in asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase [39]. Thus, optimizing nitric oxide generation through the alternative nitrate–nitrite–nitric oxide pathway is a reasonable goal to improve cardiovascular health [40,41,42].
Additionally, plasma nitrate and nitrite responses are known to be influenced by the consumption of components of foods, including polyphenols, antioxidants, and fatty acids [43,44,45]. Vitamin C has long been known to interact with the chemical reactions of nitrite [44]. Briefly, nitrite in an acidic environment, such as the stomach, can form nitrous acid (HNO2), which can be converted to nitrosamides (HN2O2) and nitrosamines (H2N2O) through dinitrogen trioxide (N2O3) as an intermediate [33,46]. Both nitrosamides and nitrosamines are carcinogenic. The presence of vitamin C in the stomach reduces the conversion of nitrite to N-nitroso compounds by reducing the nitrite to nitric oxide, which diffuses out of the gastric tract, influences gastric blood flow, and is reconverted to nitrite. Both protein and beetroot juice are frequently consumed supplements of athletes to enhance exercise performance. Since they may be consumed simultaneously, it is important and logical to assess if there is an interaction that would either potentiate or negate dietary nitrate’s effect on plasma nitrite. In an acidic environment of the stomach, the amine group in the amino acids of protein react with N2O3 to form nitrosamines [33,46]. This would potentially reduce plasma nitrite levels, as less nitrite would be available for absorption. Currently, research does not indicate that dietary protein affects the metabolism of nitrate in the nitrate–nitrite–nitric oxide pathway, such as reducing or increasing plasma levels of nitrate or nitrite. Thus, the aim of this trial was to study the effect of beetroot juice alone and in conjunction with vitamin C or protein on the acute response to plasma nitrate and nitrite levels in middle- to older-aged adults. It was hypothesized that compared to beetroot juice alone, the co-ingestion of vitamin C and beetroot juice would lead to higher plasma nitrite levels, and consuming protein along with beetroot juice would lead to lower plasma nitrite levels.
2. Methods
2.1. Participant Selection
Healthy adults (body mass index 18.5–30.0 kg/m2) 40–80 years of age were recruited from the Winston-Salem, North Carolina Community. A recruitment flyer was placed around the Wake Forest University campus and distributed to the Winston Salem community. Inclusion and exclusion criteria are presented in Table 1. All procedures followed were in accordance with the ethical standards of Wake Forest University. The study was approved by the institutional review board of Wake Forest University and all participants provided informed consent prior to participation in the study.

Table 1.
Inclusion and Exclusion Criteria.
2.2. Study Design
This study was designed as a cross-over trial, with each participant receiving, in a randomized order, a single dose of Beet It Sport® (James White Drinks Ltd.; Ipswich, United Kingdom) alone; Beet It Sport® plus vitamin C supplement; and Beet It Sport® plus a whey protein supplement. Each Beet It Sport contained 380 mg of nitrate.
2.3. Protocol
Participants reported to the Clinical Research Center of the Department of Health and Exercise Science at Wake Forest University on three separate occasions. Individuals were initially prescreened to determine eligibility. If eligible, they were instructed to report to a testing facility in an overnight fasted condition and to avoid high-nitrate-containing foods. At this first visit, informed consent was obtained. To determine eligibility, participants completed self-report questionnaires regarding health history and current health status, prescription and non-prescription medication use over the past 2 weeks, and recent dietary nitrate/nitrite intake for the past 24 h. A baseline blood draw was taken from the antecubital vein and stored in two 4 mL lithium heparin vials. Blood samples were immediately centrifuged at 1700× g for two min. Using a micropipette, two 0.4 mL aliquots of plasma were removed and stored in a -80 degree Celsius freezer. Plasma was thawed, treated with equal volume of methanol and centrifuged at 11,000× g for 10 min. Nitrate and nitrite concentrations were analyzed in supernatants by an ENO-20 nitric oxide analyzer (Amuza Inc. San Diego, CA, USA).
In randomized order, the participants then consumed 70 mL of Beet It Sport® drink containing 380 mg of nitrate, either alone, in combination with a 500 mg tablet of vitamin C or in combination with 15 g of whey protein supplement mixed with 180 mL of water. Participants were requested to consume intervention supplements within 15 min. At 1 h and 3 h after consuming the randomized intervention treatment, blood was drawn again from each participant, and processed and stored as described above. There was a minimum of 2 days and a maximum of 1 month of washout between each of the 3 treatments. Plasma nitrate and nitrite levels were examined at two different time points, 1 and 3 h after ingestion of the intervention products. This is based on the pharmokinetics of nitrate and nitrite showing peak responses occurring during this time after ingestion of a nitrate-containing product [33]. Based on increases in plasma nitrite achieved using tolerable volumes of beetroot juice by other studies [12,47,48,49,50], participants who had a 3 h plasma nitrite level of 0.50 mM or greater were deemed as a “responder” and a plasma nitrite level of less than 0.50 mM at 3 h were deemed as a “non-responder”. The nitrate and nitrite micromolar concentration was quantified in both the pre-consumption and post-consumption blood draws by a ENO-20 nitric oxide analyzer, as described earlier. The intraclass correlation coefficients for the day-to-day test–retest reliability in our laboratory was 0.987 for nitrate and 0.987 for nitrite.
2.4. Analytic Plan
Plasma nitrate and nitrite at each time point for the three treatments were tested for normality using Shapiro–Wilk test. Since not all variables were normally distributed (p < 0.05), they were transformed by computing the log of the variable. The transformed data were tested for normality using Shapiro–Wilk, and all log-transformed variables were normally distributed (p < 0.05). Group means for log plasma nitrate and log plasma nitrite at pre- and 1 h and 3 h post-consumption were compared using two-way within-factors ANOVA, with time and treatment as the independent factors. Values for plasma nitrate and nitrite are presented in the text and table as unadjusted means ± SEM for ease of readers to understand values. Spearman correlations were performed between percent change in plasma nitrate and plasma nitrite, as not all of these variables were normally distributed as assessed using the Shapiro–Wilk test (p < 0.05). All analyses were performed using SPSS® version 28.0 (Chicago, IL, USA), and statistical significance was set at p < 0.05.
3. Results
A total of 12 participants (10 women and 2 men) consented and completed all three interventions and data collection at each time point. The mean age was 67.6 ± 6.5 years.
Unadjusted treatment means for plasma nitrate and plasma nitrite at each time point for each intervention are shown in Table 2, while the statistical analysis was performed on the log transformed values. Using a Greenhouse–Geisser correction, no significant interaction was found between time (i.e., pre, 1 h and 3 h) and treatments (BRJ, BRJ + Vit C, BRJ + Protein for log plasma nitrate (p = 0.920) or for log plasma nitrite (p = 0.743)). There were no statistically significant differences for log plasma nitrate (p = 0.308) or log plasma nitrite (p = 0.391) values across treatments. Time means (Table 2) for log plasma nitrate increased significantly from pre-consumption levels after 1 h (p < 0.001). At 3 h, log plasma nitrate values had decreased significantly from 1 h values (p < 0.001) but were still significantly greater than pre-consumption values (p < 0.001). Log plasma nitrite values were significantly greater at 1 h (p = 0.001) and 3 h (p < 0.001) compared to pre-consumption levels. Log plasma nitrite values were significantly greater at 3 h compared to 1 h (p = 0.003).

Table 2.
Non-transformed intervention means for plasma nitrate and nitrite at pre- and 1 h and 3 h post-consumption of the intervention. Values for pre, 1 h post and 3 h post are mean ± standard error of the mean. The Time Means and Treatment Means are estimated marginal means ± standard error of the mean. All analysis were performed on log-transformed values for plasma nitrate and nitrite, but the non-transformed values are presented for ease of reader interpretation. Values with different superscript letters within a column were statistically significantly different from each other.
Spearman correlations between percent change of plasma nitrate and nitrite from baseline values at 1 h and 3 h were examined to describe factors that influence plasma nitrate and nitrite responses. Since there were no differences between groups in the nitrate and nitrite responses, Pearson correlations were performed across all three interventions for percent change from pre- to 1 h and 3 h post-consumption in plasma nitrate and plasma nitrite. A greater percentage change in plasma nitrate at both 1 h and 3 h post-consumption was statistically significantly correlated with a greater percentage change in plasma nitrite at 3 h (rs = 0.435; p = 0.008 for 1 h and rs =0.461; p = 0.005 for 3 h), but not for plasma nitrite at 1 h.
Individual plasma nitrite levels at pre and at 1 h and 3 h post-consumption are depicted in Figure 1a–c for beetroot juice alone, with vitamin C and with protein, respectively. Two of the twelve participants within each treatment were deemed non-responders (3 h plasma nitrite level < 0.50 μM), with 10 classified as responders (3 h plasma nitrite level > 500 μM). Categorizing an individual as a responder or a non-responder was based on the definition employed in our study. Two separate participants failed to achieve the 0.50 μM level at 3 h for two of the treatments, and two others failed to achieve the 0.50 mM level on just one of the treatments. It is interesting to note the variability in the figures within and between individuals for the various treatments.
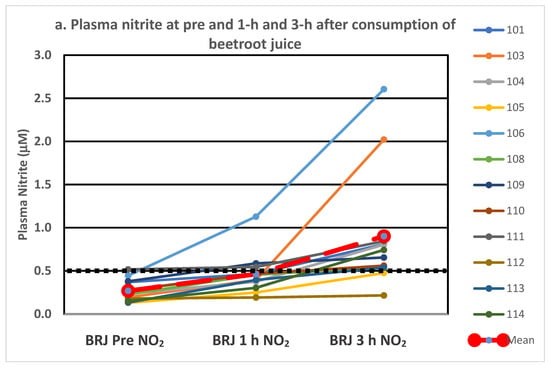
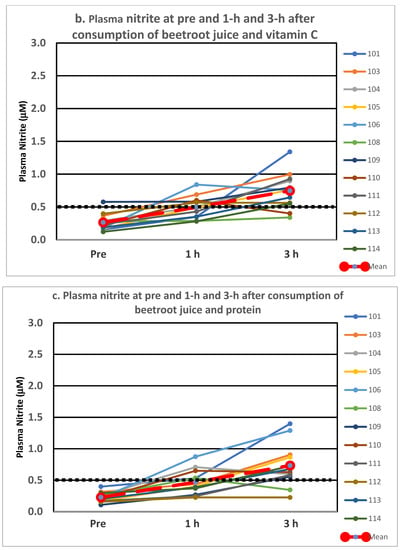
Figure 1.
(a–c). Individual responses in plasma nitrite following consumption of beetroot juice alone (a), with vitamin C (b) and with protein (c). The treatment mean is depicted in the large dashed red line and the black dotted line at 0.5 mM line shows the level marking responders vs. non-responders.
4. Discussion
This analysis compared the effect of beetroot juice alone or in combination with either vitamin C or protein in a randomized cross-over trial of healthy middle aged and older adults. These comparisons were based on the premise that: (1) vitamin C alters nitrite metabolism; and (2) both beetroot juice and protein are popular supplements for athletes, and are therefore likely to be consumed simultaneously. Our results suggest that in this cohort and conditions of the interventions and study design, neither vitamin C nor protein co-ingestion with beetroot juice alters plasma nitrate and nitrite response compared to beetroot juice alone.
Consistent with our findings, Ashor et al. found no differences in plasma nitrate and nitrite levels measured 3 h after consuming 0.1 mmol potassium nitrate/kg body weight alone or with a 200 mg dose of vitamin C in both younger and older adults [51]. Interestingly, they observed a synergistic physiologically effect with regard to improvements in arterial stiffness and blood pressure in older adults compared to vitamin C or nitrate alone. This suggests that although the biomarker for nitric oxide (plasma nitrite) is not changed with the co-ingestion of vitamin C and a dietary nitrate source, the physiological action of nitric oxide is enhanced with vitamin C consumption. Our study did not measure any physiological effects, such as blood pressure, from the treatments; therefore, it is not known if vitamin C plus beetroot juice in our study led to enhanced physiological actions. In contrast, others found greater change in fasting levels of plasma nitrate from baseline to 4 weeks of supplementation with co-administration of 1000 mg of vitamin C and a 6.4 mmol dose of nitrate in beetroot juice compared to nitrate alone [52]. Surprisingly, the change Basaqr et al. found in fasting plasma nitrite was greater for the nitrate supplement alone. It is interesting to note they report fasting levels for plasma nitrite 2–3-fold higher (0.4–0.5 mM) than values frequently found in the literature [12,26,49,53,54]. Furthermore, Basaqr et al. also showed that the combined levels of both nitrate and nitrite (NOx) in plasma correlated significantly with plasma vitamin C levels after the combination treatment [52]. In this group of hypercholesterolemic middle-to-older aged adults, the combined treatment of vitamin C and beetroot juice reduced blood lipids and oxidized low-density lipoproteins [52]. Together, this supports the epidemiological evidence that consumption of fruits and vegetables, which are high sources of vitamin C and nitrate, lowers the risk of cardiovascular disease [3,4].
Vitamin C’s action in the nitrite metabolism includes blunting the formation of N-nitroso compounds [46,55,56] through stimulating the conversion of nitrite into nitric oxide [44]. Nitrous acid forms dinitrogen trioxide (N2O3), which reacts with amines to form nitrosamines [33,46]. Vitamin C undergoes nitrosation in the presence of nitrous acid (HNO2) and forms nitric oxide. Thus, with vitamin C there is less nitrous acid and less nitrosamine formation. Reducing the formation of these carcinogenic nitrosamines is considered advantageous [44]. Increasing the nitric oxide pool with vitamin C and nitrate supplementation may explain the improvement in blood pressure and arterial stiffness previously observed [51].
Currently, research does not indicate that dietary protein affects the metabolism of nitrate in the nitrate–nitrite–nitric oxide pathway, such as reducing or increasing plasma levels of nitrate or nitrite. It is recognized that amines in amino acids react with N2O3 to form nitrosamines [33,46] in an acidic environment in the stomach, which in theory would reduce the level of plasma nitrite, since there would be less nitrite available for absorption. The current results do not support this reduction as plasma nitrite levels were similar among beetroot juice alone and in combination with a protein supplement.
As shown in Figure 1a–c, 2 of the 12 participants (~17%) in each treatment were classified as non-responders based on our definition employed in this study as they did not achieve a plasma nitrite level of at least 0.50 μM at the 3 h time-point. This frequency is similar to our earlier work, which showed approximately 20% of participants had suboptimal responses [53,57]. Several factors are known to affect the nitrate–nitrite–nitric oxide metabolism, including composition and abundance of oral and gut microbes, use of hexedine-containing mouthwash, spitting, saliva flow rate, smoking, source of nitrate, dietary components (vitamin C, antioxidants, polyphenols and thiocyanates), digestion of the food bound nitrate to free inorganic nitrate, and stomach pH [58,59,60,61,62]. Efforts were made to minimize several of these factors through inclusion/exclusion criteria (non-tobacco users, no active gastrointestinal disorders, not taking medications suspected to alter gut pH), instructions to avoid use of mouthwash and maintaining a similar diet for the different treatments. Furthermore, the treatment order was randomized, and a washout period was employed in the research design.
It is recognized that several individual biological factors and physical and chemical characteristics of the treatments (beetroot juice, vitamin C + beetroot juice, and protein + beetroot juice) potentially influence plasma nitrite levels. The composition of the oral microbes, use of antibacterial mouthwash, spitting, saliva flow rate, smoking, source of nitrate, dietary components that interact with nitrate metabolism (vitamin C, antioxidants, polyphenols, and thiocyanates), digestion of the food bound nitrate to free inorganic nitrate, gut microbiome composition and abundance, stomach pH [33,59,60,61,62,63,64,65]. Gastric emptying rate is also a key factor, especially in a time course study such as this. The speed at which the stomach empties its content into the duodenum is influenced by several factors, including the fluid volume, osmolality of the food bolus and age [66,67,68]. While the inclusion and exclusion criteria and study design were developed to minimize several of these factors, attempts were not made to equalize the treatments based on differences in fluid volume and osmolarity, which may have influenced the results. Additionally, age is also a likely contributing factor to the plasma nitrate and nitrite response to nitrate consumption [36,38,69,70]. In the wide age spread of this cohort (40–80 years old), this also may have implications in interpreting the results.
5. Conclusions
Thus, considering the study design and interventions delivered, these results demonstrate in this cohort of middle-to-older aged healthy adults, the co-ingestion of beetroot juice with a 200 mg dose of vitamin C or 15 g of a protein supplement does not alter the efficacy of converting dietary nitrate to plasma nitrite within the 1 and 3 h sampling employed. Further research needs to be undertaken to expand the blood-sampling time-frame and to examine factors that may influence the kinetics of the plasma nitrate to nitrite efficacy, such as differences in fluid volume and osmolarity between treatments employed.
Author Contributions
Conceptualization, G.D.M., D.B.K.-S. and M.J.B.; methodology, G.D.M., B.A.N. and S.B.; validation, G.D.M., D.B.K.-S. and S.B.; formal analysis, M.J.B.; resources, D.B.K.-S. and S.B.; data curation, B.A.N. and D.B.K.-S.; writing-original draft, G.D.M.; writing-review and edition, B.A.N., D.B.K.-S., S.B. and M.J.B.; project administration, B.A.N.; funding acquisiton, G.D.M. and D.B.K.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Translational Science Center at Wake Forest University and by the National Institute of Health (Grant # HL098032).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Wake Forest University (protocol number IRB 00021971and approved 2 March 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Daniel B. Kim-Shapiro is listed as a co-inventor on several patent applications related to use of nitrite in cardiovascular conditions and/or treatment of hemolysis. Daniel B. Kim-Shapiro owns stock in and serves on the scientific advisory board for Beverage Operations LLC, which has licensed Wake Forest University intellectual properties and thus has a financial interest in Beverage Operations LLC.
References
- Hord, N.G. Dietary Nitrates, Nitrites, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2011, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute L-Arginine Supplementation Reduces the O2 Cost of Moderate-Intensity Exercise and Enhances High-Intensity Exercise Tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, J.; Liu, Y.-J.; Cai, L.-B.; Xu, F.-R.; Xie, T.; He, Q.-Q. Fruit and Vegetable Consumption and Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D.; He, Y. Fruit and Vegetables Consumption and Incident Hypertension: Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Hum. Hypertens. 2016, 30, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute Ingestion of Beetroot Bread Increases Endothelium-Independent Vasodilation and Lowers Diastolic Blood Pressure in Healthy Men: A Randomized Controlled Trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Machha, A.; Schechter, A.N. Dietary Nitrite and Nitrate: A Review of Potential Mechanisms of Cardiovascular Benefits. Eur. J. Nutr. 2011, 50, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Jackson, J.; Patterson, A.J.; MacDonald-Wicks, L.; McEvoy, M. The Role of Inorganic Nitrate and Nitrite in CVD. Nutr. Res. Rev. 2017, 30, 247–264. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Gladwin, M.; Coleman, G.D.; Hord, N.; Howard, G.; Kim-Shapiro, D.B.; Lajous, M.; Larsen, F.J.; Lefer, D.J.; McClure, L.A.; et al. Dietary Nitrate and the Epidemiology of Cardiovascular Disease: Report From a National Heart, Lung, and Blood Institute Workshop. J. Am. Heart Assoc. 2016, 5, e003402. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Rosselli, M.; Keller, P.J.; Dubey, R.K. Role of Nitric Oxide in the Biology, Physiology and Pathophysiology of Reproduction. Hum. Reprod. Update 1998, 4, 3–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary Nitrate Supplementation Reduces the O2 Cost of Low-Intensity Exercise and Enhances Tolerance to High-Intensity Exercise in Humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon III, R.O.; et al. Nitrite Infusion in Humans and Nonhuman Primates: Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, D.T.; Matsumoto, A.; Kim, S.-O.; Marshall, H.E.; Stamler, J.S. Protein S-Nitrosylation: Purview and Parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milsom, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite Is a Signaling Molecule and Regulator of Gene Expression in Mammalian Tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- West, M.B.; Hill, B.G.; Xuan, Y.-T.; Bhatnagar, A. Protein Glutathiolation by Nitric Oxide: An Intracellular Mechanism Regulating Redox Protein Modification. FASEB J. 2006, 20, 1715–1717. [Google Scholar] [CrossRef] [Green Version]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Melino, G.; Bernassola, F.; Knight, R.A.; Corasaniti, M.T.; Nistico, G.; Finazzi-Agro, A. S-Nitrosylation Regulates Apoptosis. Nature 1997, 388, 432–433. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular Endothelial Cells Synthesize Nitric Oxide from L-Arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Schechter, A.N.; Kim-Shapiro, D.B.; Patel, R.P.; Hogg, N.; Shiva, S.; Cannon III, R.O.; Kelm, M.; Wink, D.A.; Espey, M.G.; et al. The Emerging Biology of the Nitrite Anion. Nat. Chem. Biol. 2005, 1, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical Evidence Demonstrating the Utility of Inorganic Nitrate in Cardiovascular Health. Nitric Oxide Biol. Chem. 2014, 38, 45–57. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, A.G.; Sips, A.J.A.M.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The Oral Bioavailability of Nitrate from Nitrate-Rich Vegetables in Humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Carlstörm, M.; Larsen, F.J.; Weitzberg, E. Roles of Dietary Inorganic Nitrate in Cardiovascular Health and Disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood Pressure-Lowering Effects of Beetroot Juice and Novel Beetroot- Enriched Bread Products in Normotensive Male Subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and Nitrite in Biology, Nutrition and Therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef]
- Kim, J.; Moore, D.J.; Maurer, D.G.; Kim-shapiro, D.B.; Basu, S.; Flanagan, M.P.; Skulas-ray, A.C.; Kris-etherton, P.; Proctor, D.N. Acute Dietary Nitrate Supplementation Does Not Augment Submaximal Forearm Exercise Hyperemia in Healthy Young Men. Appl. Physiol. Nutr. Metab. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Lundberg, J.M.; Alving, K. Intragastric Nitric Oxide Production in Humans: Measurements in Expelled Air. Gut 1994, 35, 1543–1546. [Google Scholar] [CrossRef] [Green Version]
- Babateen, A.M.; Shannon, O.M.; O’Brien, G.M.; Okello, E.; Khan, A.A.; Rubele, S.; Wightman, E.; Smith, E.; McMahon, N.; Olgacer, D.; et al. Acceptability and Feasibility of a 13-Week Pilot Randomised Controlled Trial Testing the Effects of Incremental Doses of Beetroot Juice in Overweight and Obese Older Adults. Nutrients 2021, 13, 769. [Google Scholar] [CrossRef]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zand, J.; Lanza, F.; Garg, H.K.; Bryan, N.S. All-Natural Nitrite and Nitrate Containing Dietary Supplement Promotes Nitric Oxide Production and Reduces Triglycerides in Humans. Nutr. Res. 2011, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- James, P.E.; Willis, G.R.; Allen, J.D.; Winyard, P.G.; Jones, A.M. Nitrate Pharmacokinetics: Taking Note of the Difference. Nitric Oxide Biol. Chem. 2015, 48, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Mauriello, A.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Cardillo, C.; Melino, G.; Di Daniele, N. Arterial Ageing: From Endothelial Dysfunction to Vascular Calcification. J. Intern. Med. 2017, 281, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashor, A.W.; Chowdhury, S.; Oggioni, C.; Qadir, O.; Brandt, K.; Ishaq, A.; Mathers, J.C.; Saretzki, G.; Siervo, M. Inorganic Nitrate Supplementation in Young and Old Obese Adults Does Not Affect Acute Glucose and Insulin Responses but Lowers Oxidative Stress. J. Nutr. 2016, 146, 2224–2232. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Lara, J.; Jajja, A.; Sutyarjoko, A.; Ashor, A.W.; Brandt, K.; Qadir, O.; Mathers, J.C.; Benjamin, N.; Winyard, P.G.; et al. Ageing Modifies the Effects of Beetroot Juice Supplementation on 24-Hour Blood Pressure Variability: An Individual Participant Meta-Analysis. Nitric Oxide Biol. Chem. 2015, 47, 97–105. [Google Scholar] [CrossRef]
- Delp, M.D.; Behnke, B.J.; Spier, S.A.; Wu, G.; Muller-Delp, J.M. Ageing Diminishes Endothelium-Dependent Vasodilatation and Tetrahydrobiopterin Content in Rat Skeletal Muscle Arterioles. J. Physiol. 2008, 586, 1161–1168. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Kellum, J.A.; Blanchard, E.J.; Bacon, E.E.; Wesley, A.J.; Kruckeberg, W.C. Changes in Nitric Oxide Precursor, L-Arginine, and Metabolites, Nitrate and Nitrite, with Aging. Life Sci. 1994, 55, 1895–1902. [Google Scholar] [CrossRef]
- Sverdlov, A.L.; Ngo, D.T.M.; Chan, W.P.A.; Chirkov, Y.Y.; Horowitz, J.D. Aging of the Nitric Oxide System: Are We as Old as Our NO? J. Am. Heart Assoc. 2014, 3, e000973. [Google Scholar] [CrossRef] [Green Version]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients: A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Hypertens. Dallas Tex 2015, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary Nitrate Improves Vascular Function in Patients with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’El-Rei, J.; Cunha, A.R.; Trindade, M.; Neves, M.F. Beneficial Effects of Dietary Nitrate on Endothelial Function and Blood Pressure Levels. Int. J. Hypertens. 2016, 2016, 6791519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughan, K.S.; Wendell, S.G.; Delmastro-Greenwood, M.; Helbling, N.; Corey, C.; Bellavia, L.; Potti, G.; Grimes, G.; Goodpaster, B.; Kim-Shapiro, D.B.; et al. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertens. Dallas Tex 2017, 70, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; McColl, K.E.L. Alterations in Intragastric Nitrite and Vitamin C Levels during Acid Inhibitory Therapy. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 523–537. [Google Scholar] [CrossRef]
- Rocha, B.S.; Nunes, C.; Pereira, C.; Barbosa, R.M.; Laranjinha, J. A Shortcut to Wide-Ranging Biological Actions of Dietary Polyphenols: Modulation of the Nitrate-Nitrite-Nitric Oxide Pathway in the Gut. Food Funct. 2014, 5, 1646–1652. [Google Scholar] [CrossRef]
- Berends, J.E.; van den Berg, L.M.M.; Guggeis, M.A.; Henckens, N.F.T.; Hossein, I.J.; de Joode, M.E.J.R.; Zamani, H.; van Pelt, K.A.A.J.; Beelen, N.A.; Kuhnle, G.G.; et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-Nitroso Compounds in Humans. Int. J. Mol. Sci. 2019, 20, 2277. [Google Scholar] [CrossRef] [Green Version]
- Lansley, K.E.; Winyard, P.G.; Fulford, J.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Dimenna, F.J.; Gilchrist, M.; Benjamin, N.; Jones, A.M.; et al. Dietary Nitrate Supplementation Reduces the O2 Cost of Walking and Running: A Placebo-Controlled Study. J. Appl. Physiol 2011, 110, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Dimenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and Chronic Effects of Dietary Nitrate Supplementation on Blood Pressure and the Physiological Responses to Moderate-Intensity and Incremental Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.D.; Marsh, A.P.; Dove, R.W.; Beavers, D.; Presley, T.; Helms, C.; Bechtold, E.; King, S.B.; Kim-Shapiro, D. Plasma Nitrate and Nitrite Are Increased by a High-Nitrate Supplement but Not by High-Nitrate Foods in Older Adults. Nutr. Res. N. Y. N 2012, 32, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.J.; Miller, G.D.; Kim-Shapiro, D.B.; Fletcher, M.S.; Jones, C.G.; Gauthier, Z.D.; Collins, S.L.; Basu, S.; Heinrich, T.M. A Randomized Controlled Trial of Nitrate Supplementation in Well-Trained Middle and Older-Aged Adults. PLoS ONE 2020, 15, e0235047. [Google Scholar] [CrossRef]
- Ashor, A.W.; Shannon, O.M.; Werner, A.-D.; Scialo, F.; Gilliard, C.N.; Cassel, K.S.; Seal, C.J.; Zheng, D.; Mathers, J.C.; Siervo, M. Effects of Inorganic Nitrate and Vitamin C Co-Supplementation on Blood Pressure and Vascular Function in Younger and Older Healthy Adults: A Randomised Double-Blind Crossover Trial. Clin. Nutr. Edinb. Scotl. 2020, 39, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Basaqr, R.; Skleres, M.; Jayswal, R.; Thomas, D.T. The Effect of Dietary Nitrate and Vitamin C on Endothelial Function, Oxidative Stress and Blood Lipids in Untreated Hypercholesterolemic Subjects: A Randomized Double-Blind Crossover Study. Clin. Nutr. Edinb. Scotl. 2021, 40, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute Effect of a High Nitrate Diet on Brain Perfusion in Older Adults. Nitric. Oxide. 2011, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary Nitrate Supplementation Enhances Muscle Contractile Efficiency during Knee-Extensor Exercise in Humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednar, C.; Kies, C. Nitrate and Vitamin C from Fruits and Vegetables: Impact of Intake Variations on Nitrate and Nitrite Excretions of Humans. Plant Foods Hum. Nutr. Dordr. Neth. 1994, 45, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wolff, I.A.; Wasserman, A.E. Nitrates, Nitrites, and Nitrosamines. Science 1972, 177, 15–19. [Google Scholar] [CrossRef]
- Berry, M.J.; Justus, N.W.; Hauser, J.I.; Case, A.H.; Helms, C.C.; Basu, S.; Rogers, Z.; Lewis, M.T.; Miller, G.D. Dietary Nitrate Supplementation Improves Exercise Performance and Decreases Blood Pressure in COPD Patients. Nitric Oxide Biol. Chem. 2015, 48, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The Increase in Plasma Nitrite after a Dietary Nitrate Load Is Markedly Attenuated by an Antibacterial Mouthwash. Nitric. Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Hyde, E.R.; Andrade, F.; Vaksman, Z.; Parthasarathy, K.; Jiang, H.; Parthasarathy, D.K.; Torregrossa, A.C.; Tribble, G.; Kaplan, H.B.; Petrosino, J.F.; et al. Metagenomic Analysis of Nitrate-Reducing Bacteria in the Oral Cavity: Implications for Nitric Oxide Homeostasis. PLoS ONE 2014, 9, e88645. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J. Effect of Diet and Gut Environment on the Gastrointestinal Formation of N-Nitroso Compounds: A Review. Nitric Oxide Biol. Chem. 2018, 73, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary Nitrate Metabolism and the Microbiome: Intersection of Microbial Metabolism, Nitric Oxide and Diet in Cardiac and Pulmonary Vascular Health. Free Radic. Biol. Med. 2017, 105, 48–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.M.; Wu, Z.F.; Pang, B.X.; Jin, L.Y.; Qin, L.Z.; Wang, S.L. From Nitrate to Nitric Oxide: The Role of Salivary Glands and Oral Bacteria. J. Dent. Res. 2016, 95, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary Flavonoids and Nitrate: Effects on Nitric Oxide and Vascular Function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst-Trigg, R.; Yeates, T.; Blackwell, J.R.; Thompson, C.; Linoby, A.; Morgan, P.T.; Clarke, I.; Connolly, L.J.; Wylie, L.J.; Winyard, P.G.; et al. Lowering of Blood Pressure after Nitrate-Rich Vegetable Consumption Is Abolished with the Co-Ingestion of Thiocyanate-Rich Vegetables in Healthy Normotensive Males. Nitric Oxide Biol. Chem. 2018, 74, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björne, H.; Weitzberg, E.; Lundberg, J.O. Intragastric Generation of Antimicrobial Nitrogen Oxides from Saliva-Physiological and Therapeutic Considerations. Free Radic. Biol. Med. 2006, 41, 1404–1412. [Google Scholar] [CrossRef]
- O’Donovan, D.; Hausken, T.; Lei, Y.; Russo, A.; Keogh, J.; Horowitz, M.; Jones, K.L. Effect of Aging on Transpyloric Flow, Gastric Emptying, and Intragastric Distribution in Healthy Humans--Impact on Glycemia. Dig. Dis. Sci. 2005, 50, 671–676. [Google Scholar] [CrossRef]
- Shi, X.; Osterberg, K.L.; Petrie, H.; Stofan, J.R.; Murray, R. Effect of Different Osmolalities, CHO Types, and [CHO] on Gastric Emptying in Humans. Med. Sci. Sports Exerc. 2017, 49, 1015–1021. [Google Scholar] [CrossRef]
- Okabe, T.; Terashima, H.; Sakamoto, A. Determinants of Liquid Gastric Emptying: Comparisons between Milk and Isocalorically Adjusted Clear Fluids. Br. J. Anaesth. 2015, 114, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Fulford, J.; Vanhatalo, A.; Blackwell, J.R.; French, O.; Bailey, S.J.; Gilchrist, M.; Winyard, P.G.; Jones, A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R73–R83. [Google Scholar] [CrossRef] [Green Version]
- Tiefenbacher, C.P. Tetrahydrobiopterin: A Critical Cofactor for ENOS and a Strategy in the Treatment of Endothelial Dysfunction? Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2484–H2488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).