Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey of Milk Storage Practices
- Do you store your expressed breast milk in the fridge?
- If yes, what is the usual amount of time that you store your milk in the fridge?
- Do you store your expressed breast milk in the freezer?
- If yes, what is the usual amount of time that you store your milk in the freezer?
2.2. Sample Collection and Storage
2.3. PMA Treatment
2.4. DNA Extraction
2.5. PacBio Sequencing
2.6. Sequence Processing
2.7. Statstistical Analysis
2.7.1. Survey Responses
2.7.2. DNA Quantification
2.7.3. Alpha Diversity
2.7.4. Beta Diversity
2.7.5. Relative Abundance Analysis
3. Results
3.1. Assessment of Milk Storage Practices
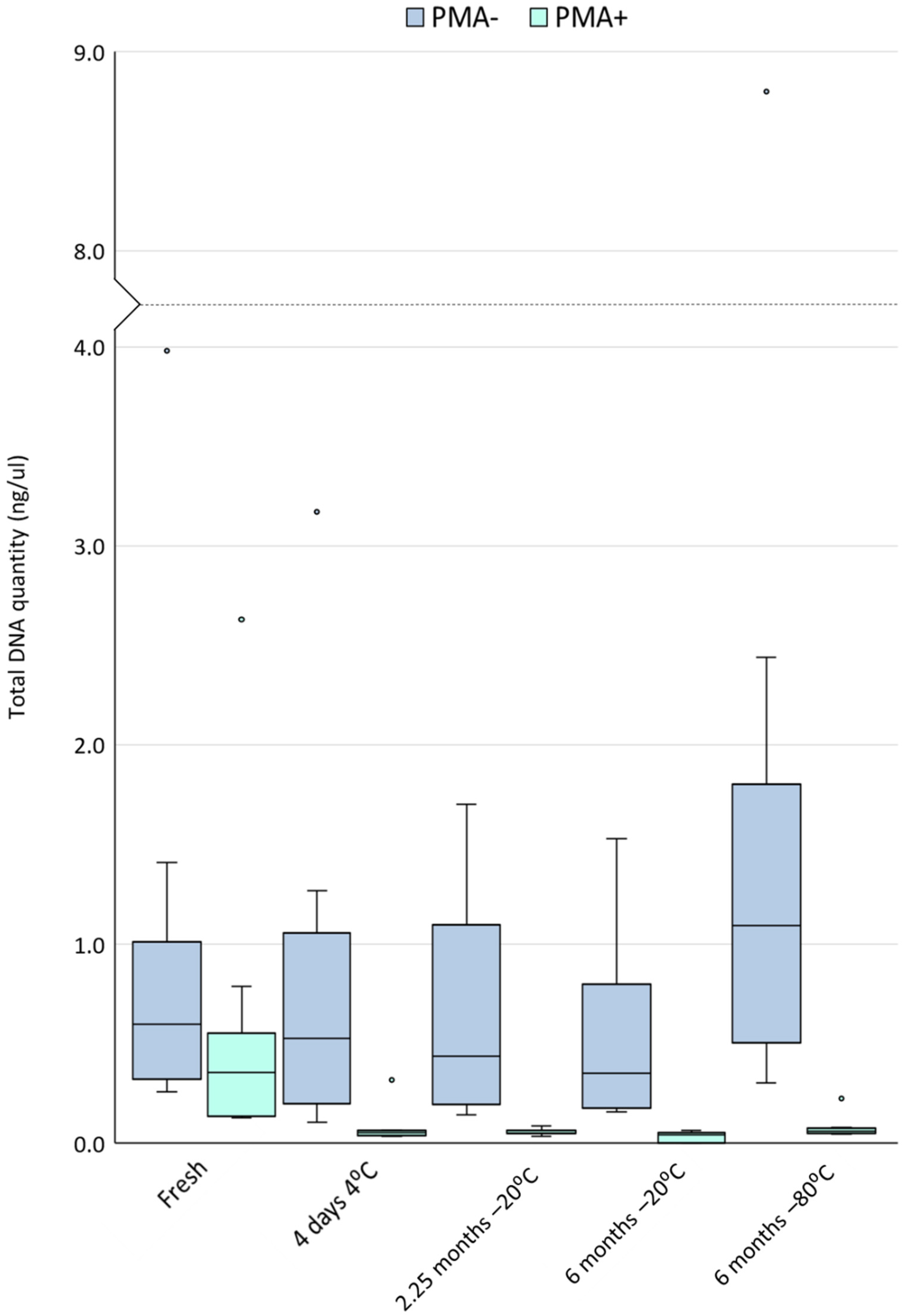
3.2. Cold Storage Reduces the Yield of DNA from Viable Cells
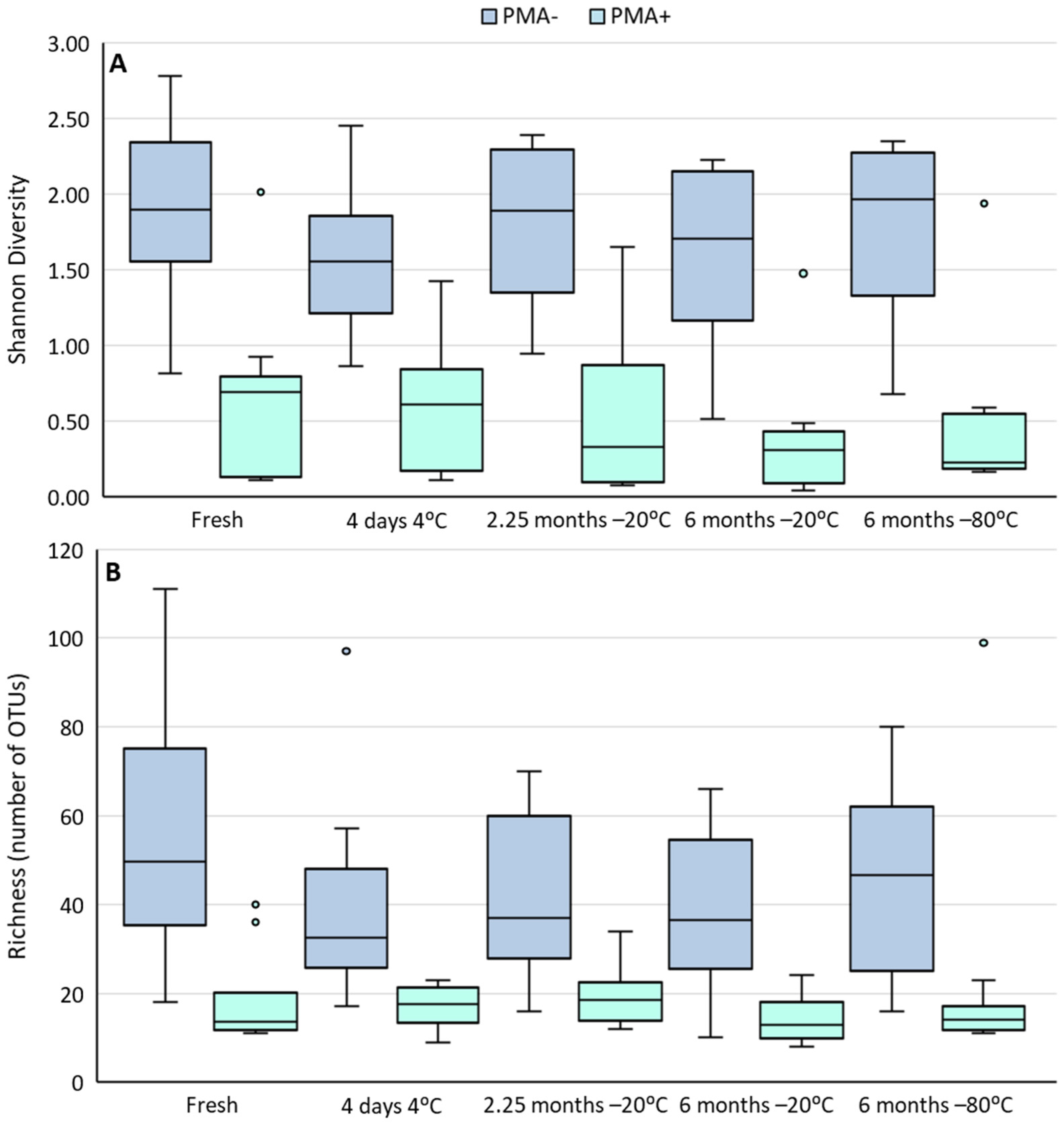
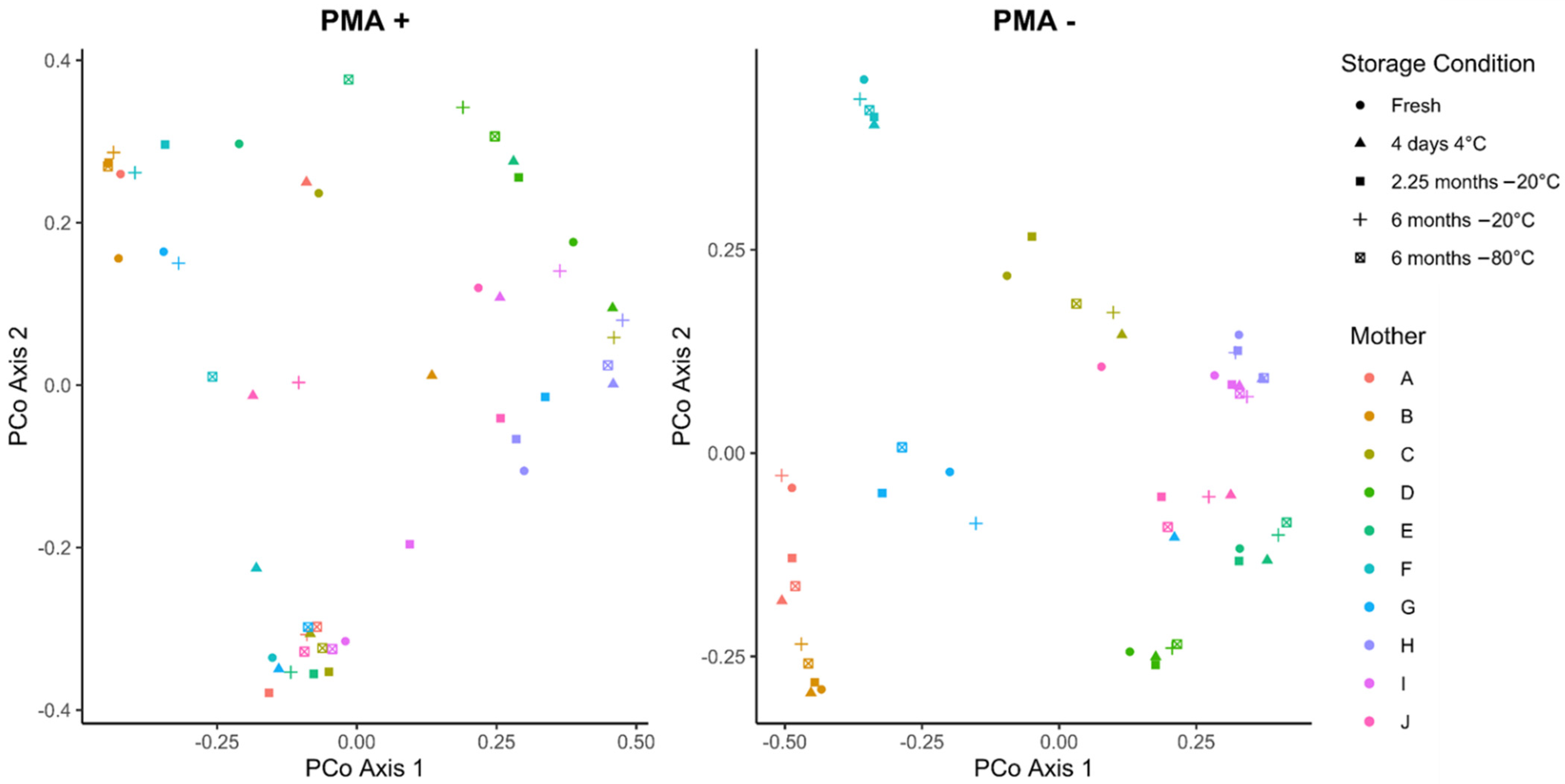
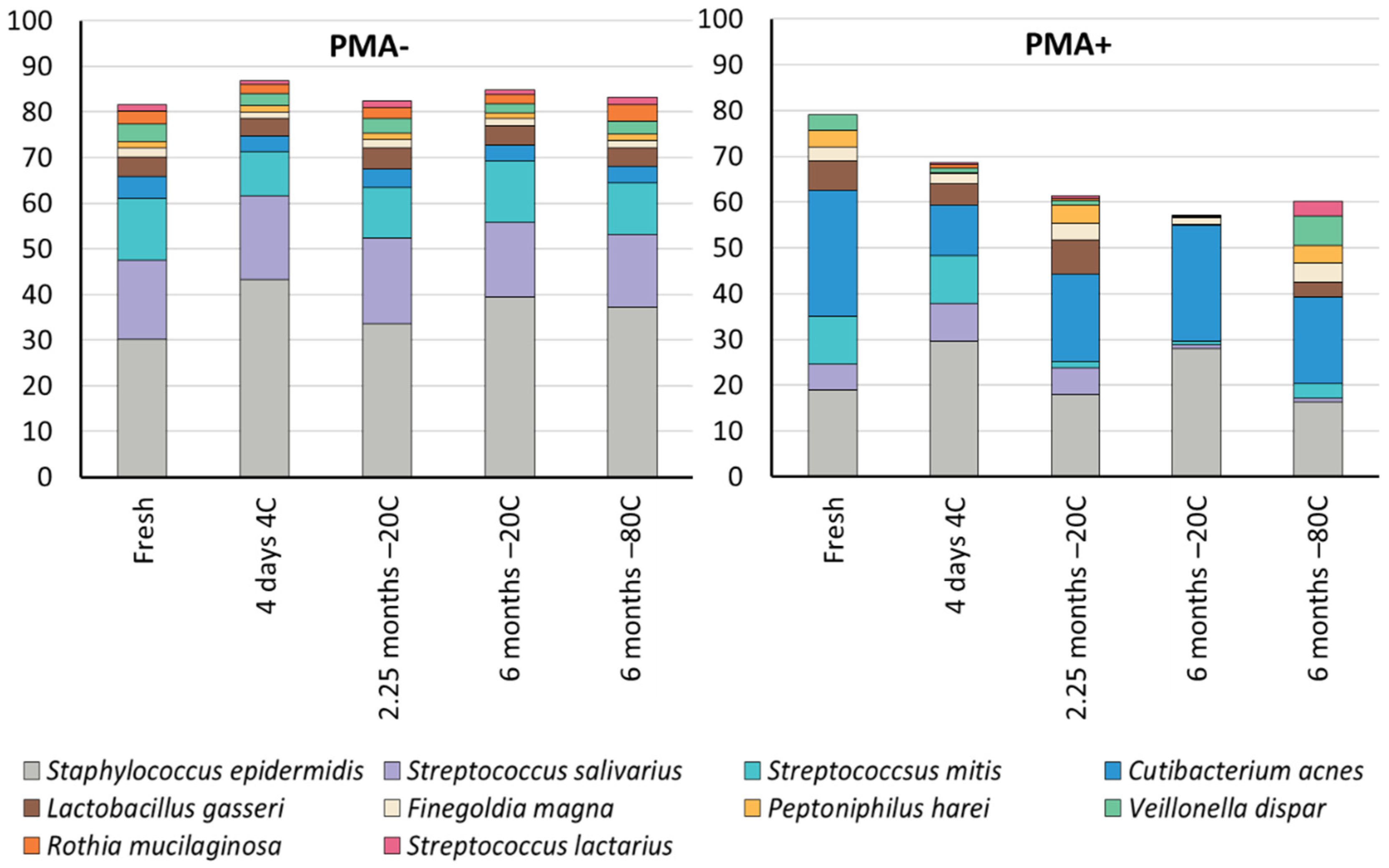
3.3. Cold Storage Alters the Composition of the Human Milk Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas, C.A.; Ivey, K.L.; Papanicolas, L.E.; Best, K.P.; Muhlhausler, B.S.; Rogers, G.B. DNA extraction approaches substantially influence the assessment of the human breast milk microbiome. Sci. Rep. 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Bacigalupe, R.; García-Carral, C.; Boix-Amoros, A.; Argüello, H.; Silva, C.B.; de Los Angeles Checa, M.; Mira, A.; Rodriguez, J.M. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci. Rep. 2019, 9, 8435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef] [Green Version]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Lugli, G.A.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef] [Green Version]
- Labiner-Wolfe, J.; Fein, S.B.; Shealy, K.R.; Wang, C. Prevalence of Breast Milk Expression and Associated Factors. Pediatrics 2008, 122, S63–S68. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ingol, T.T.; Smith, K.; Oza-Frank, R.; Keim, S.A. Reliability of Maternal Recall of Feeding at the Breast and Breast Milk Expression 6 Years After Delivery. Breastfeed. Med. 2020, 15, 224–236. [Google Scholar] [CrossRef]
- Binns, C.W.; Win, N.N.; Zhao, Y.; Scott, J. Trends in the expression of breastmilk 1993–2003. Breastfeed. Rev. 2006, 14, 5–9. [Google Scholar]
- Clemons, S.N.; Amir, L.H. Breastfeeding Women’s Experience of Expressing: A Descriptive Study. J. Hum. Lact. 2010, 26, 258–265. [Google Scholar] [CrossRef]
- Eglash, A.; Simon, L.; Brodribb, W.; Reece-Stremtan, S.; Noble, L.; Brent, N.; Bunik, M.; Harrel, C.; Lawrence, R.A.; Lefort, Y.; et al. ABM Clinical Protocol #8: Human Milk Storage Information for Home Use for Full-Term Infants, Revised 2017. Breastfeed. Med. 2017, 12, 390–395. [Google Scholar] [CrossRef]
- Lawrence, R.A. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr. 1999, 88, 14–18. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L.; Mehta, N.R.; Hamosh, P.; Hamosh, M. Lipolysis of triglycerides of human milk during storage at low temperatures: A note of caution. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 521–524. [Google Scholar] [CrossRef]
- Hanna, N.; Ahmed, K.; Anwar, M.; Petrova, A.; Hiatt, M.; Hegyi, T. Effect of storage on breast milk antioxidant activity. Arch. Dis. Child. Fetal Neonatal. Ed. 2004, 89, F518–F520. [Google Scholar] [CrossRef] [Green Version]
- Marín, M.L.; Arroyo, R.; Jiménez, E.; Gómez, A.; Fernández, L.; Rodríguez, J.M. Cold Storage of Human Milk: Effect on Its Bacterial Composition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 343–348. [Google Scholar] [CrossRef]
- Ahrabi, A.F.; Handa, D.; Codipilly, C.N.; Shah, S.; Williams, J.E.; McGuire, M.A.; Potak, D.; Aharon, G.G.; Schanler, R.J. Effects of Extended Freezer Storage on the Integrity of Human Milk. J. Pediatr. 2016, 177, 140–143. [Google Scholar] [CrossRef]
- Slutzah, M.; Codipilly, C.N.; Potak, D.; Clark, R.M.; Schanler, R.J. Refrigerator Storage of Expressed Human Milk in the Neonatal Intensive Care Unit. J. Pediatr. 2010, 156, 26–28. [Google Scholar] [CrossRef]
- Nocker, A.; Sossa-Fernandez, P.; Burr, M.D.; Camper, A.K. Use of Propidium Monoazide for Live/Dead Distinction in Microbial Ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef] [Green Version]
- Stinson, L.F.; Trevenen, M.L.; Geddes, D.T. The Viable Microbiome of Human Milk Differs from the Metataxonomic Profile. Nutrients 2021, 13, 4445. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Mushajiang, S.; Luo, B.; Tian, F.; Ni, Y.; Yan, W. The Composition and Concordance of Lactobacillus Populations of Infant Gut and the Corresponding Breast-Milk and Maternal Gut. Front. Microbiol. 2020, 11, 597911. [Google Scholar] [CrossRef]
- Martin, V.; Maldonado-Barragan, A.; Moles, L.; Rodriguez-Banos, M.; Campo, R.D.; Fernandez, L.; Rodriguez, J.M.; Jimenez, E. Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact. 2012, 28, 36–44. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; et al. Breastmilk Feeding Practices Are Associated with the Co-Occurrence of Bacteria in Mothers’ Milk and the Infant Gut: The CHILD Cohort Study. Cell Host Microbe 2020, 28, 285–297. [Google Scholar] [CrossRef]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.C.; Tan, S.; van de Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.M.; Nolte-’t Hoen, E.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3, 24215. [Google Scholar] [CrossRef] [Green Version]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Kontopoulou, E.; Strachan, S.; Reinhardt, K.; Kunz, F.; Walter, C.; Walkenfort, B.; Jastrow, H.; Hasenberg, M.; Giebel, B.; von Neuhoff, N.; et al. Evaluation of dsDNA from extracellular vesicles (EVs) in pediatric AML diagnostics. Ann. Hematol. 2020, 99, 459–475. [Google Scholar] [CrossRef]
- Bewicke-Copley, F.; Mulcahy, L.A.; Jacobs, L.A.; Samuel, P.; Akbar, N.; Pink, R.C.; Carter, D.R.F. Extracellular vesicles released following heat stress induce bystander effect in unstressed populations. J. Extracell. Vesicles 2017, 6, 1340746. [Google Scholar] [CrossRef]
- Bitto, N.J.; Cheng, L.; Johnston, E.L.; Pathirana, R.; Phan, T.K.; Poon, I.K.H.; O’Brien-Simpson, N.M.; Hill, A.F.; Stinear, T.P.; Kaparakis-Liaskos, M. Staphylococcus aureus membrane vesicles contain immunostimulatory DNA, RNA and peptidoglycan that activate innate immune receptors and induce autophagy. J. Extracell. Vesicles 2021, 10, e12080. [Google Scholar] [CrossRef]
| Fridge | Freezer | |
|---|---|---|
| Average (SD) | 1.8 (1.2) days | 2.25 (1.74) months |
| Minimum | 5 h | 4 days |
| Maximum | 6 days | 6 months |
| Non-PMA Treated Samples | Fresh | 4 Days 4 °C | 2 Months −20 °C | 6 Months −20 °C | 6 Months −80 °C |
|---|---|---|---|---|---|
| Staphylococcus epidermidis | 30.2 a,b | 43.2 a | 33.6 | 39.5 b | 37.2 |
| Streptococcus salivarius | 17.4 | 18.3 | 18.8 a | 16.3 | 16.0 a |
| Streptococcus mitis | 13.4 | 9.8 a | 10.9 | 13.4 a | 11.2 |
| Cutibacterium acnes | 4.8 | 3.4 | 4.2 | 3.5 | 3.7 |
| Lactobacillus gasseri | 4.4 | 3.8 a,b,c | 4.6 a,d,e | 4.2 b,d,f | 4.0 c,e,f |
| Finegoldia magna | 2.0 a,b | 1.4 a,c | 1.9 b,c | 1.6 * | 1.6 |
| Peptoniphilus harei | 1.5 a,b,c | 1.4 a,d,e | 1.3 b,d,f | 1.3 * | 1.4 c,e,f |
| Veillonella dispar | 3.8 a | 2.6 | 3.3 b | 2.0 a,b | 2.7 |
| Rothia mucilaginosa | 2.8 | 2.2 a | 2.4 b | 1.9 c | 3.6 a,b,c |
| Streptococcus lactarius | 1.5 | 0.7 | 1.4 | 1.1 | 1.6 |
| PMA treated samples | Fresh | 4 days 4 °C | 2 months −20 °C | 6 months −20 °C | 6 months −80 °C |
| Staphylococcus epidermidis | 19.0 | 29.6 a | 17.9 | 27.9 | 16.2 a |
| Streptococcus salivarius | 5.6 a,b,c,d | 8.3 a,e,f | 5.8 b,g,h | 0.9 c,e,g,i | 0.9 d,f,h,i |
| Streptococcus mitis | 10.3 a,b,c | 10.3 d,e,f | 1.5 a,d,g,h | 0.8 b,e,g | 3.2 c,f,h |
| Cutibacterium acnes | 27.6 a | 11.1 b,c | 19.1 | 25.2 b,d | 19.0 a,c,d |
| Lactobacillus gasseri | 6.3 a,b | 4.6 * | 7.4 a,c | 0.3 b,c | 3.1 * |
| Finegoldia magna | 3.2 * | 2.3 * | 3.7 | 1.3 * | 4.4 |
| Peptoniphilus harei | 3.6 * | 0.2 * | 4.0 a | 0.3 * | 3.8 a |
| Veillonella dispar | 3.3 a,b | 0.9 * | 1.0 a,c | 0.1 * | 6.4 b,c |
| Rothia mucilaginosa | 0.0 * | 0.8 * | 0.5 | 0.0 * | 0.0 * |
| Streptococcus lactarius | 0.0 * | 0.3 * | 0.5 a | 0.1 * | 3.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stinson, L.F.; Trevenen, M.L.; Geddes, D.T. Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk. Nutrients 2022, 14, 1875. https://doi.org/10.3390/nu14091875
Stinson LF, Trevenen ML, Geddes DT. Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk. Nutrients. 2022; 14(9):1875. https://doi.org/10.3390/nu14091875
Chicago/Turabian StyleStinson, Lisa F., Michelle L. Trevenen, and Donna T. Geddes. 2022. "Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk" Nutrients 14, no. 9: 1875. https://doi.org/10.3390/nu14091875
APA StyleStinson, L. F., Trevenen, M. L., & Geddes, D. T. (2022). Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk. Nutrients, 14(9), 1875. https://doi.org/10.3390/nu14091875






