Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Subjects
2.2. Intervention and Study Variables
2.3. Statistical Analysis
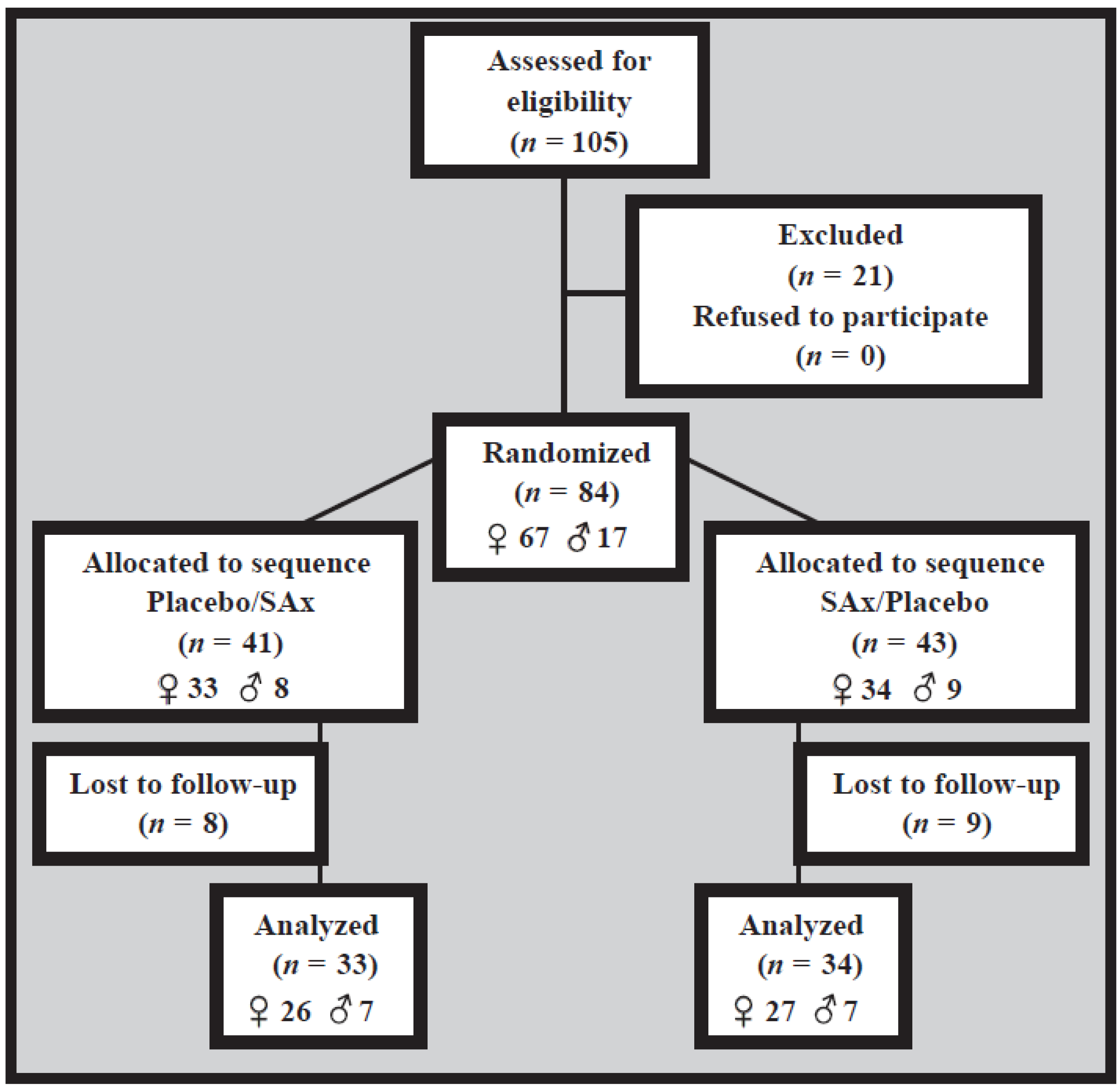
3. Results
3.1. Recruitment and Study Population
3.2. Baseline Characteristics
3.3. Dietary and Anthropometric Variables
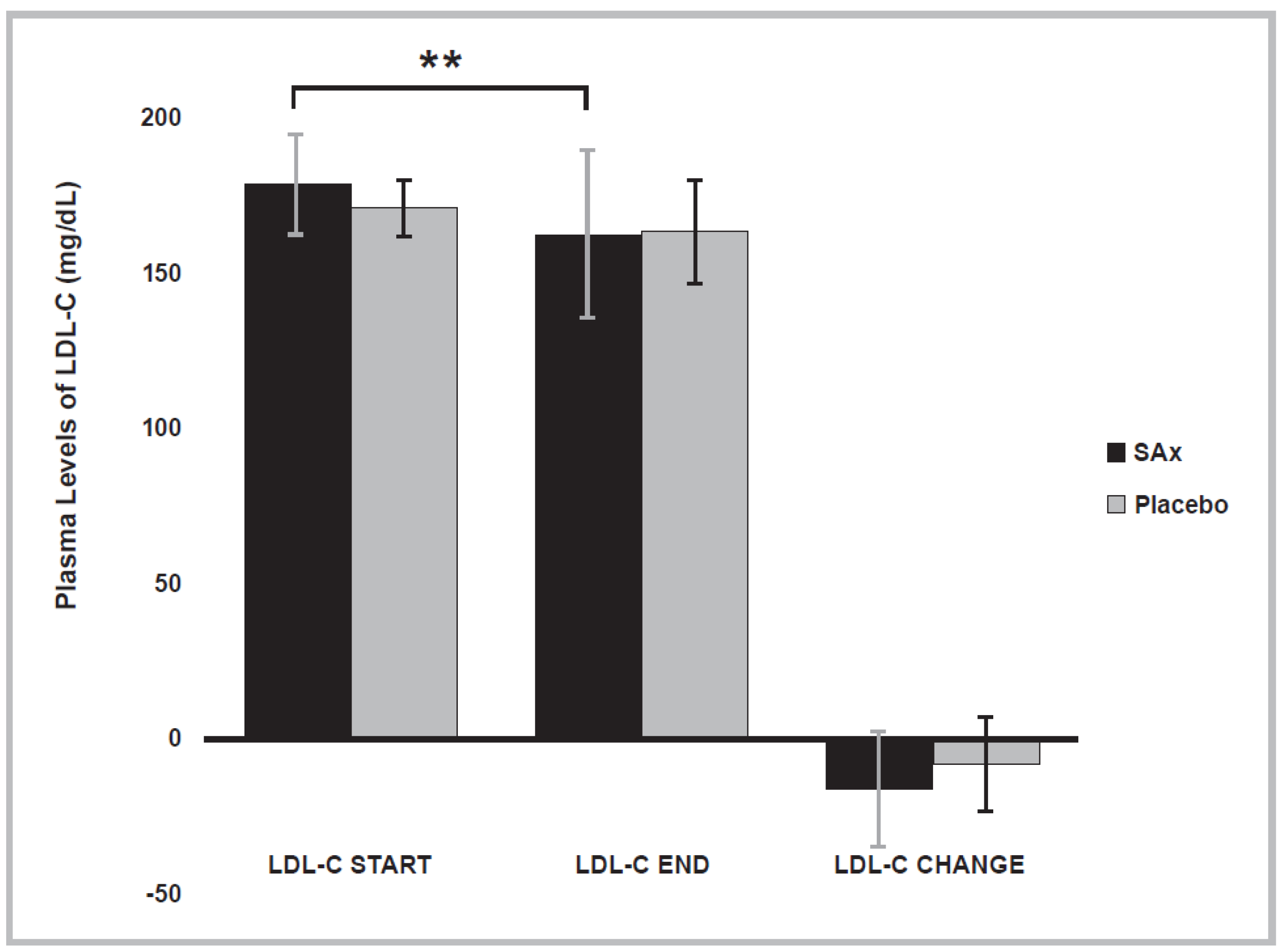
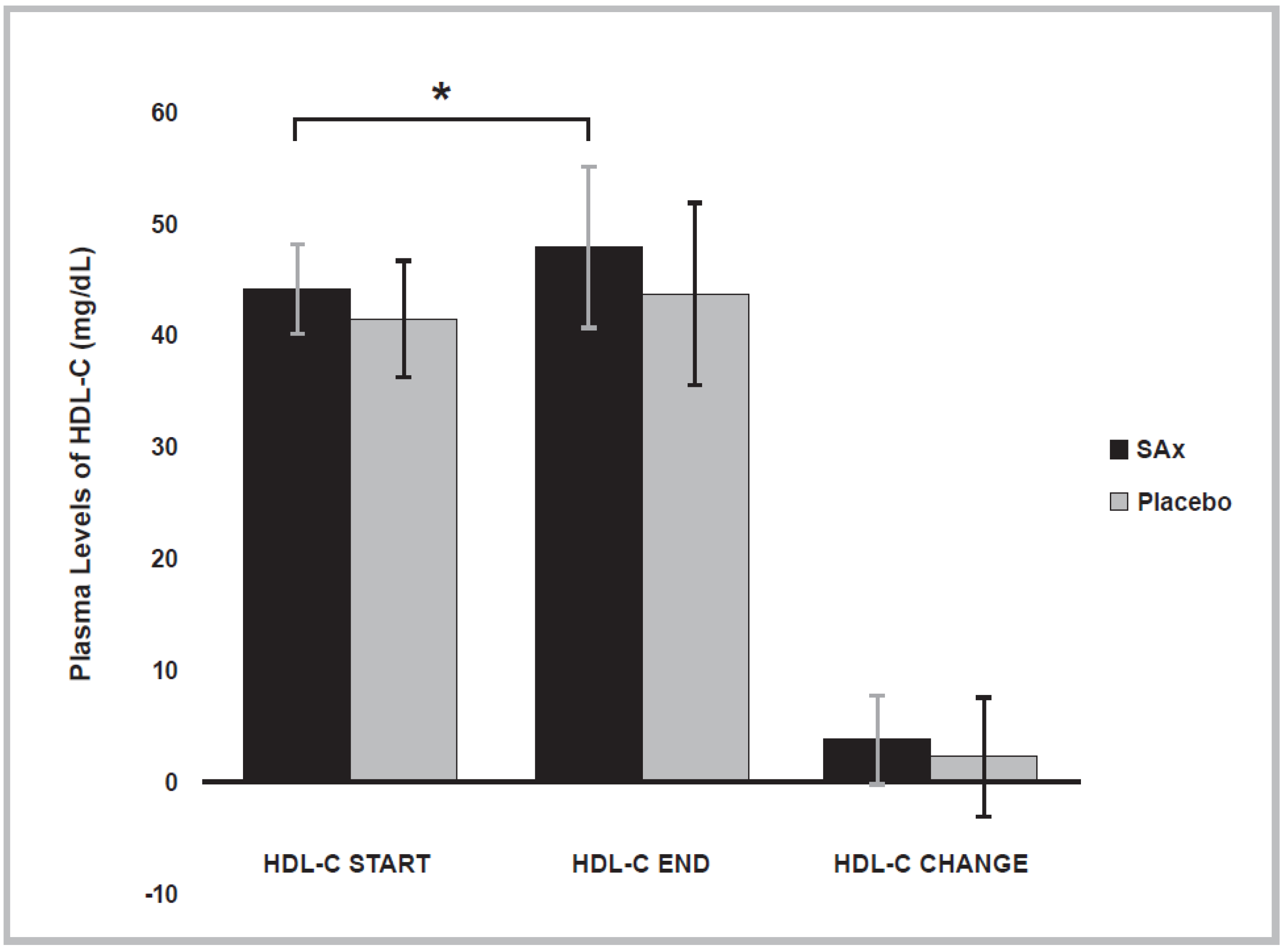
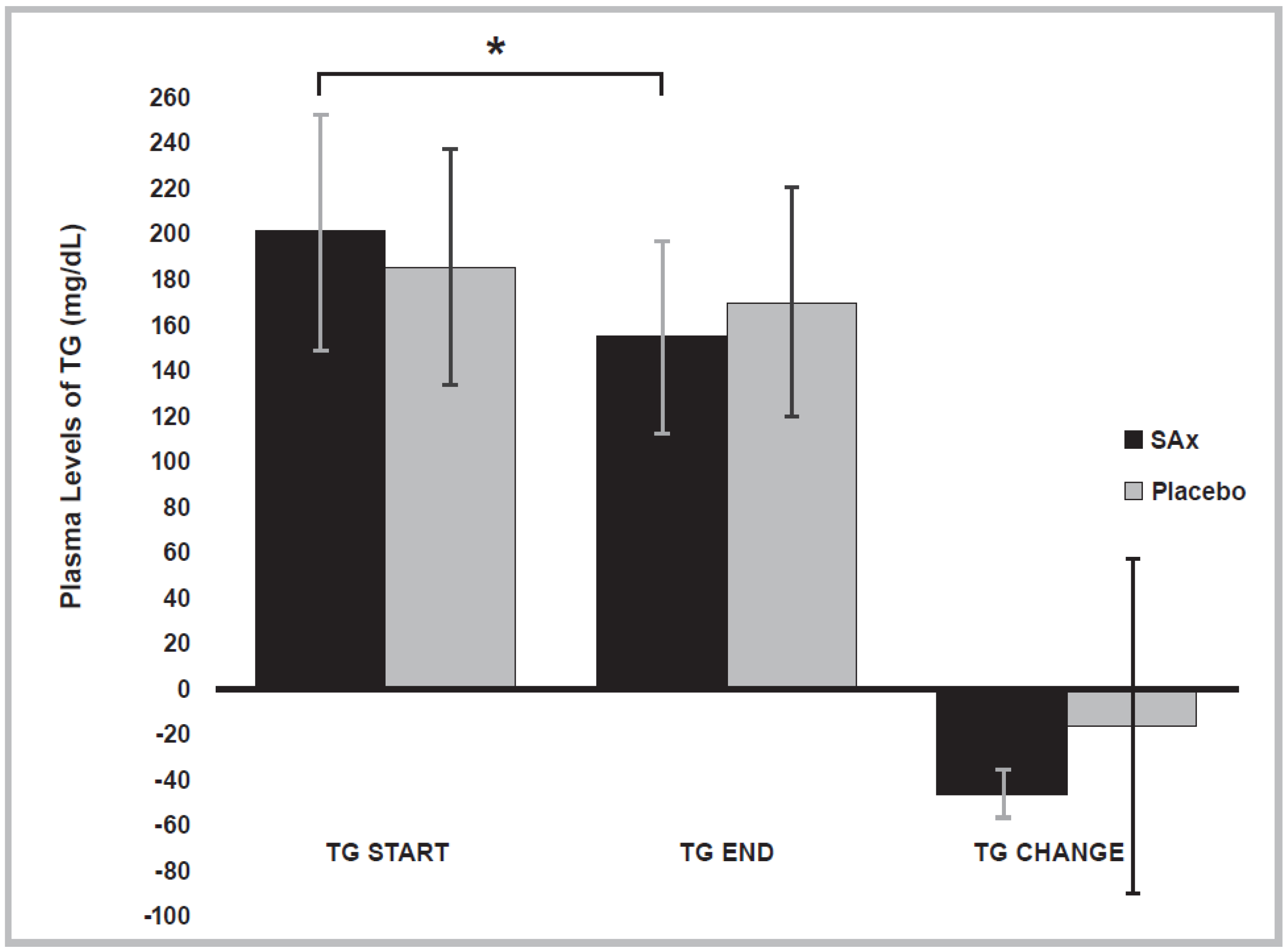
3.4. Lipid Profile Variables
3.5. Compliance and Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Patent
Abbreviations
| ABCA1 | ATP-binding membrane cassette transport protein A1 |
| ACAT | acyl-coenzyme a: cholesterol acyltransferase |
| ACS | acute coronary syndrome |
| ADMA | asymmetric dimethylarginine |
| AMPK | AMP-activated protein kinase |
| ApoAI | apolipoprotein A-I |
| ASVD | atherosclerotic vascular disease |
| BMI | body mass index |
| CPJ | concentrated pomegranate juice |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| db/db | mice with obesity and type 2 diabetes mellitus |
| DBP | diastolic blood pressure |
| FFM | fat-free mass |
| FM | fat mass |
| HDL-C | high-density lipoprotein cholesterol |
| HFD | high-fat-diet |
| HMGR | 3-hydroxy-3-methylglutaryl coenzyme A reductase |
| HR | heart rate |
| HT | hydroxytyrosol |
| HULP | University Hospital La Paz |
| JNK2 | c-Jun N-terminal kinase 2 |
| LDL-C | low-density lipoprotein cholesterol |
| LDLR | low-density lipoprotein receptor |
| LPL | lipoprotein lipase |
| MM | muscle mass |
| MiPo | microencapsulated pomegranate |
| MUFA | monounsaturated fatty acids |
| NADPH | nicotinamide adenine dinucleotide-phosphate |
| NF-κB | nuclear factor-kappa B |
| O2- | superoxide anion radical |
| oxLDL | oxidized low-density lipoprotein |
| PC | punicalagin |
| PLE | pomegranate leaf extract |
| PPAR-α | peroxisome proliferator-activated receptor-α |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| PUFA | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| SFA | saturated fatty acids |
| SREBP-1c | sterol regulatory element-binding protein 1c |
| FAS | fatty acid synthase |
| SREBP2 | sterol regulatory element-binding protein 2 |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| T2DM | type 2 diabetes mellitus |
| TC | total cholesterol |
| TG | triglycerides |
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 19 February 2022).
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 7 September 2016).
- WHO. Global Atlas on Cardiovascular Disease Prevention and Control; The World Health Organization Press in Collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Daugherty, A. Atherosclerosis. Arter. Thromb Vasc Biol 2016, 35, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC95. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr. Drug Targets 2016, 18, 1477–1486. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.; Marshall, S.; Nakos, S.; Campbell, K.; Itsiopoulos, C. The effect of polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: A systematic review and meta-analysis. Nutrients 2017, 9, 1345. [Google Scholar] [CrossRef]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef]
- Yu, L.M.; Dong, X.; Xue, X.D.; Zhang, J.; Li, Z.; Wu, H.J.; Yang, Z.L.; Yang, Y.; Wang, H.S. Protection of the myocardium against ischemia/reperfusion injury by punicalagin through an SIRT1-NRF-2-HO-1-dependent mechanism. Chem. Biol. Interact. 2019, 306, 152–162. [Google Scholar] [CrossRef]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.M.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with hydroxytyrosol and punicalagin improves early atherosclerosis markers involved in the asymptomatic phase of atherosclerosis in the adult population: A randomized, placebo-controlled, crossover trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli Amirabadi, A.H.; Rahimi, R. Dietary polyphenols for atherosclerosis: A comprehensive review and future perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 547. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high- polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; Gomes, S.R.; Carmo, M.M.; Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.; Miron, A.; Trifan, A.; Luca, V.; Costache, I.-I. The Cardiovascular Effects of Cocoa Polyphenols—An Overview. Diseases 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer, and All Causes in Japan. The Ohsaki Study. JAMA-J. Am. Med. Assoc. 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef]
- Zhong, J.; Reece, E.A.; Yang, P. Punicalagin exerts protective effect against high glucose- induced cellular stress and neural tube defects. Biochem. Biophys. Res. Commun. 2015, 467, 179–184. [Google Scholar] [CrossRef]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Michael Nelson, D. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef]
- Tang, J.; Min, J.; Li, B.; Hong, S.; Liu, C.; Hu, M.; Li, Y.; Yang, J.; Hong, L. Therapeutic Effects of Punicalagin Against Ovarian Carcinoma Cells in Association With β-Catenin Signaling Inhibition. Int. J. Gynecol. Cancer 2016, 26, 1557–1563. [Google Scholar] [CrossRef]
- De Nigris, F.; Williams-Ignarro, S.; Sica, V.; Lerman, L.O.; D’Armiento, F.P.; Byrns, R.E.; Casamassimi, A.; Carpentiero, D.; Schiano, C.; Sumi, D.; et al. Effects of a Pomegranate Fruit Extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc. Res. 2007, 73, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC- 3 and LNCaP cells. Chem. Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Goodla, L.; Manubolu, M.; Tencomnao, T. Metabolic Alterations and the Protective Effect of Punicalagin Against Glutamate-Induced Oxidative Toxicity in HT22 Cells. Neurotox. Res. 2017, 31, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Fki, I.; Sahnoun, Z.; Sayadi, S. Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J. Agric. Food Chem. 2007, 55, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Martínez-López, S.; Arévalo, B.G.; Amigo-Benavent, M.; Sarriá, B.; Bravo- Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Sánchez de Ibargüen, L.; Cárdeno, A.; Alarcón de la Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally Occurring Hydroxytyrosol Derivatives: Hydroxytyrosyl Acetate and 3,4-Dihydroxyphenylglycol Modulate Inflammatory Response in Murine Peritoneal Macrophages. Potential Utility as New Dietary Supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Samson, A.O.; Khattib, A.; Vaya, J.; Khatib, S. Punicalagin Decreases Serum Glucose Levels and Increases PON1 Activity and HDL Anti-Inflammatory Values in Balb/c Mice Fed a High-Fat Diet. Oxid. Med. Cell. Longev. 2018, 2018, 2673076. [Google Scholar] [CrossRef]
- Liu, X.; Cao, K.; Lv, W.; Feng, Z.; Liu, J.; Gao, J.; Li, H.; Zang, W.; Liu, J. Punicalagin attenuates endothelial dysfunction by activating FoxO1, a pivotal regulating switch of mitochondrial biogenesis. Free Radic. Biol. Med. 2019, 135, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A New Possible Mechanism by Which Punicalagin Protects against Liver Injury Induced by Type 2 Diabetes Mellitus: Upregulation of Autophagy via the Akt/FoxO3a Signaling Pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-Derived Ellagitannin, Suppresses Obesity and Obesity-Induced Inflammatory Responses Via the Nrf2/Keap1 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, e1900574. [Google Scholar] [CrossRef] [PubMed]
- Giamogante, F.; Marrocco, I.; Cervoni, L.; Eufemi, M.; Chichiarelli, S.; Altieri, F. Punicalagin, an active pomegranate component, is a new inhibitor of PDIA3 reductase activity. Biochimie 2018, 147, 122–129. [Google Scholar] [CrossRef]
- Stefanon, B.; Colitti, M. Original Research: Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. 2016, 241, 1796–1802. [Google Scholar] [CrossRef]
- Xie, Y.D.; Chen, Z.Z.; Li, N.; Lu, W.F.; Xu, Y.H.; Lin, Y.Y.; Shao, L.H.; Wang, Q.T.; Guo, L.Y.; Gao, Y.Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Puri, K.S.; Suresh, K.R.; Gogtay, N.J.; Thatte, U.M. Declaration of Helsinki, 2008: Implications for stakeholders in research. J. Postgrad. Med. 2009, 55, 131–134. [Google Scholar] [CrossRef]
- FAO; UNICEF; WHO. Methodology of nutritional surveillance. Report of a Joint FAO/UNICEF/WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1976, 593, 1–66. [Google Scholar]
- Sorensen, K.E.; Celermajer, D.S.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Thomas, O.; Deanfield, J.E. Non-invasive measurement of human endothelium dependent arterial responses: Accuracy and reproducibility. Br. Heart J. 1995, 74, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yebyo, H.G.; Aschmann, H.E.; Kaufmann, M.; Puhan, M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am. Heart J. 2019, 210, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, S. Statin induced myotoxicity. Eur. J. Intern. Med. 2012, 23, 317–324. [Google Scholar] [CrossRef]
- Schandelmaier, S.; Briel, M.; Saccilotto, R.; Olu, K.K.; Arpagaus, A.; Hemkens, L.G.; Nordmann, A.J. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst. Rev. 2017, 6, CD009744. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Li, L.; Lu, D.; Yazdanparast, A.; Wang, L.; Kreutz, R.P.; Whipple, E.C.; Schleyer, T.K. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur. J. Clin. Pharmacol. 2018, 74, 1099–1109. [Google Scholar] [CrossRef]
- Okopień, B.; Bułdak, Ł.; Bołdys, A. Benefits and risks of the treatment with fibrates—A comprehensive summary. Expert Rev. Clin. Pharmacol. 2018, 11, 1099–1112. [Google Scholar] [CrossRef]
- Squizzato, A.; Galli, M.; Romualdi, E.; Dentali, F.; Kamphuisen, P.W.; Guasti, L.; Venco, A.; Ageno, W. Statins, fibrates, and venous thromboembolism: A meta-analysis. Eur. Heart J. 2010, 31, 1248–1256. [Google Scholar] [CrossRef]
- Tabernero, M.; Sarriá, B.; Largo, C.; Martínez-López, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Renzo, L.D.; Romeo, F. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression: A nutrigenomic approach for cardiovascular prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Perez, R.; Betanzos-Cabrera, G.; Dorantes- Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef]
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54. [Google Scholar] [CrossRef]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Khatib, S.; Sela, S.; Vaya, J.; Samson, A.O. Punicalagin Induces Serum Low- Density Lipoprotein Influx to Macrophages. Oxid. Med. Cell. Longev. 2016, 2016, 7124251. [Google Scholar] [CrossRef]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Concentrated pomegranate juice improves lipid profiles in diabetic patients with hyperlipidemia. J. Med. Food 2004, 7, 305–308. [Google Scholar] [CrossRef]
- Rouhi, S.Z.T.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Daw. BMC Complement. Altern. Med. 2017, 17, 156. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.; Brennan, C.; Biggs, J.; Morgan, P.; Hodgkinson, S.; Hofman, P.; Cutfield, W. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle- aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Uydu, H.A.; Bostan, M.; Atak, M.; Yilmaz, A.; Demir, A.; Akçan, B.; Sümer, F.; Baltaş, N.; Karadaǧ, Z.; Uǧurlu, Y.; et al. Cholesterol forms and traditional lipid profile for projection of atherogenic dyslipidemia: Lipoprotein subfractions and erythrocyte membrane cholesterol. J. Membr. Biol. 2014, 247, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Arellano, L.E.; Muñoz-Valle, J.F.; De la Cruz-Mosso, U.; Salgado-Bernabé, A.B.; Castro-Alarcón, N.; Parra-Rojas, I. Circulating CD36 and oxLDL levels are associated with cardiovascular risk factors in young subjects. BMC Cardiovasc. Disord. 2014, 14, 54. [Google Scholar] [CrossRef]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Van de Werf, F. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 844–848. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low- density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Borén, J.; John Chapman, M.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef]
- Tsutsui, T.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Hayashi, M.; Ohnishi, M.; Kinoshita, M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 957–962. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Assmann, G.; Schulte, H.; Cullen, P.; Seedorf, U. Assessing risk of myocardial infarction and stroke: New data from the Prospective Cardiovascular Münster (PROCAM) study. Eur. J. Clin. Investig. 2007, 37, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Cumplido, A.S.; Nuñez-Cortés, J.M.; Rodríguez de Miguel, M.; Orera-Peña, M.L. The application of atherogenic dyslipidaemia consensus recommendations in the primary care setting. DAT-AP study. Clin. Res. Arterioscler. 2017, 29, 178–184. [Google Scholar] [CrossRef]
- Wan Ahmad, W.N.H.; Sakri, F.; Mokhsin, A.; Rahman, T.; Mohd Nasir, N.; Abdul-Razak, S.; Yasin, M.M.; Mohd Ismail, A.; Ismail, Z.; Nawawi, H. Low serum high density lipoprotein cholesterol concentration is an independent predictor for enhanced inflammation and endothelial activation. PLoS ONE 2015, 10, e0116867. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Ortiz-Rodriguez, M.A.; Medina-Briseño, L.; Carreón-Torres, E.; Izquierdo- Vega, J.A.; Sharma, A.; Cancino-Díaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current therapies focused on high-density lipoproteins associated with cardiovascular disease. Molecules 2018, 23, 2730. [Google Scholar] [CrossRef]
- Lawler, P.R.; Kotrri, G.; Koh, M.; Goodman, S.G.; Farkouh, M.E.; Lee, D.S.; Austin, P.C.; Udell, J.A.; Ko, D.T. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur. Heart J. 2020, 41, 86–94. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Rämet, M.E.; Patel, A.R.; Pandian, N.G.; Mendelsohn, M.E.; Karas, R.H. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002, 144, 165–172. [Google Scholar] [CrossRef][Green Version]
- Ansar, S.; Koska, J.; Reaven, P.D. Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: Focus on incretins. Cardiovasc. Diabetol. 2011, 10, 61. [Google Scholar] [CrossRef]
- Nagashima, H.; Endo, M. Pitavastatin prevents postprandial endothelial dysfunction via reduction of the serum triglyceride level in obese male subjects. Heart Vessel. 2011, 26, 428–434. [Google Scholar] [CrossRef]
- Badimon, L.; Storey, R.F.; Vilahur, G. Update on lipids, inflammation and atherothrombosis. Thromb. Haemost. 2011, 105, 34–42. [Google Scholar] [CrossRef]
- Helkin, A.; Stein, J.J.; Lin, S.; Siddiqui, S.; Maier, K.G.; Gahtan, V. Dyslipidemia Part 1—Review of Lipid Metabolism and Vascular Cell Physiology. Vasc. Endovasc. Surg. 2016, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.; Wang, J.; Zhang, C.; Cai, H. Positive correlation between in vivo oxidized LDL and LDL immune complexes. Clin. Biochem. 2004, 37, 72–75. [Google Scholar] [CrossRef]
- Marin, M.T.; Dasari, P.S.; Tryggestad, J.B.; Aston, C.E.; Teague, A.M.; Short, K.R. Oxidized HDL and LDL in adolescents with type 2 diabetes compared to normal weight and obese peers. J. Diabetes Complicat. 2015, 29, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Osto, E.; Matter, C.M.; Kouroedov, A.; Malinski, T.; Bachschmid, M.; Camici, G.G.; Kilic, U.; Stallmach, T.; Boren, J.; Iliceto, S.; et al. c-Jun N-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation 2008, 118, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Warnholtz, A.; Mollnau, H.; Oelze, M.; Wendt, M.; Münzel, T. Antioxidants and endothelial dysfunction in hyperlipidemia. Curr. Hypertens. Rep. 2001, 3, 53–60. [Google Scholar] [CrossRef]
- Böger, R.H. Association of Asymmetric Dimethylarginine and Endothelial Dysfunction. Clin. Chem. Lab. Med. 2003, 41, 1467–1472. [Google Scholar] [CrossRef]
- Vasconcelos, E.M.A.; Degasperi, G.R.; de Oliveira, H.C.F.; Vercesi, A.E.; de Faria, E.C.; Castilho, L.N. Reactive oxygen species generation in peripheral blood monocytes and oxidized LDL are increased in hyperlipidemic patients. Clin. Biochem. 2009, 42, 1222–1227. [Google Scholar] [CrossRef]
- Holvoet, P.; Harris, T.B.; Tracy, R.P.; Verhamme, P.; Newman, A.B.; Rubin, S.M.; Simonsick, E.M.; Colbert, L.H.; Kritchevsky, S.B. Association of High Coronary Heart Disease Risk Status with Circulating Oxidized LDL in the Well-Functioning Elderly. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1444–1448. [Google Scholar] [CrossRef]
- Kopprasch, S.; Pietzsch, J.; Kuhlisch, E.; Fuecker, K.; Temelkova-Kurktschiev, T.; Hanefeld, M.; Kühne, H.; Julius, U.; Graessler, J. In vivo evidence for increased oxidation of circulating LDL in impaired glucose tolerance. Diabetes 2002, 51, 3102–3106. [Google Scholar] [CrossRef]
| Placebo/SAx (n = 33) | SAx/Placebo (n = 34) | ||
|---|---|---|---|
| Gender | (Female %, n) | 78.79 (26) | 79.41 (27) |
| Age | (years) | 53.21 ± 4.2 | 52.79 ± 4.8 |
| Smoking | (Smokers %, n) | 18.18 (6) | 26.47 (9) |
| Weight | (kg) | 66.26 ± 11.8 | 64.08 ± 10.9 |
| BMI | (kg/m2) | 24.64 ± 2.9 | 24.56 ± 3.2 |
| Waist circumference | (cm) | 80.51 ± 9.2 | 82.58 ± 9.8 |
| FM | (%) | 29.18 ± 6.7 | 28.76 ± 6.4 |
| FFM | (%) | 70.82 ± 6.7 | 71.24 ± 6.4 |
| MM | (%) | 48.03 ± 7.7 | 47.87 ± 5.5 |
| SBP | (mmHg) | 110.3 ± 13.1 | 110.9 ± 12.9 |
| DBP | (mmHg) | 74.06 ± 10.8 | 73.75 ± 9.5 |
| HR | (bpm) | 67.36 ± 8.9 | 70.41 ± 7.5 |
| TC | (mg/dL) | 226.7 ± 29.6 | 224.6 ± 35.4 |
| LDL-C | (mg/dL) | 144.3 ± 23.9 | 145.3 ± 28.6 |
| HDL-C | (mg/dL) | 66.25 ± 12.9 | 62.00 ± 12.6 |
| TG | (mg/dL) | 80.56 ± 24.6 | 86.52 ± 44.0 |
| SAx (n = 67) | Placebo (n = 67) | |||
|---|---|---|---|---|
| Energy | (kcal/day) | Start | 1923 ± 513.6 | 1864 ± 471.9 |
| End | 1891 ± 549.4 | 1881 ± 569.5 | ||
| Change | −31.88 ± 463.2 | 17.07 ± 355.5 | ||
| Carbohydrates | (%) | Start | 38.06 ± 6.5 | 38.46 ± 8.9 |
| End | 37.62 ± 6.3 | 39.06 ± 6.5 | ||
| Change | −0.439 ± 5.7 | 0.598 ± 8.1 | ||
| Proteins | (%) | Start | 17.24 ± 3.7 | 17.44 ± 2.9 |
| End | 17.43 ± 3.6 | 17.60 ± 3.1 | ||
| Change | 0.193 ± 4.4 | 0.164 ± 3.5 | ||
| Lipids | (%) | Start | 41.42 ± 6.3 | 40.48 ± 8.8 |
| End | 41.36 ± 5.7 | 40.19 ± 5.9 | ||
| Change | −0.067 ± 5.3 | −0.287 ± 8.0 | ||
| SFA | (%) | Start | 12.33 ± 2.9 | 12.46 ± 4.0 |
| End | 12.38 ± 2.8 | 12.03 ± 2.9 | ||
| Change | 0.049 ± 2.7 | −0.433 ± 4.2 | ||
| MUFA | (%) | Start | 19.10 ± 3.6 | 18.82 ± 4.9 |
| End | 19.56 ± 4.0 | 19.29 ± 3.5 | ||
| Change | 0.453 ± 3.8 | 0.470 ± 4.2 | ||
| PUFA | (%) | Start | 6.45 ± 2.3 | 5.60 ± 1.6 |
| End | 5.98 ± 1.7 | 5.46 ± 1.8 | ||
| Change | −0.470 ± 2.4 | −0.134 ± 1.4 | ||
| Total Cholesterol | (mg/dL) | Start | 350.5 ± 172.8 | 303.9 ± 150.4 |
| End | 328.1 ± 133.8 | 323.0 ± 115.9 | ||
| Change | −22.35 ± 210.8 | 19.04 ± 130.1 | ||
| Fiber | (g/d) | Start | 21.95 ± 7.8 | 21.71 ± 8.0 |
| End | 21.68 ± 8.9 | 20.47 ± 7.3 | ||
| Change | −0.275 ± 8.2 | −1.234 ± 6.5 | ||
| Weight | (kg) | Start | 65.10 ± 11.3 | 65.10 ± 11.2 |
| End | 64.93 ± 11.2 | 64.85 ± 11.3 | ||
| Change | −0.173 ± 1.3 | −0.249 ± 1.0 | ||
| BMI | (kg/m2) | Start | 24.58 ± 3.0 | 24.63 ± 3.0 |
| End | 24.51 ± 3.0 | 24.48 ± 3.0 | ||
| Change | −0.068 ± 0.5 | −0.151 ± 0.6 | ||
| Waist Circumference | (cm) | Start | 81.85 ± 9.0 | 81.24 ± 9.8 |
| End | 81.82 ± 9.6 | 81.25 ± 9.6 | ||
| Change | −0.034 ± 2.9 | 0.008 ± 3.7 | ||
| FM | (%) | Start | 29.16 ± 6.6 | 28.90 ± 6.5 |
| End | 29.56 ± 6.8 | 29.79 ± 7.2 | ||
| Change | 0.400 ± 2.8 | 0.891 ± 3.7 | ||
| FFM | (%) | Start | 70.84 ± 6.6 | 71.10 ± 6.5 |
| End | 70.44 ± 6.8 | 70.21 ± 7.2 | ||
| Change | −0.400 ± 2.8 | −0.891 ± 3.7 | ||
| MM | (%) | Start | 47.63 ± 6.2 | 47.52 ± 6.5 |
| End | 46.67 ± 5.7 | 46.44 ± 5.8 | ||
| Change | −0.970 ± 5.1 | −1.082 ± 5.8 |
| SAx (n = 67) | Placebo (n = 67) | |||
|---|---|---|---|---|
| TC | (mg/dL) (n = 49) | Start End Change | 237.6 ± 26.0 234.9 ± 25.1 −2.776 ± 18.8 | 238.4 ± 20.0 233.0 ± 22.8 −5.388 ± 18.8 |
| LDL-C | (mg/dL) | Start | 179.1 ± 16.2 | 171.6 ± 9.1 |
| (n = 16) | End | 162.9 ± 27.1 ** | 163.6 ± 16.9 | |
| Change | −16.20 ± 18.5 | −8.063 ± 15.1 | ||
| HDL-C | (mg/dL) | Start | 44.25 ± 4.0 | 41.50 ± 5.2 |
| (n = 8) | End | 48.00 ± 7.3 * | 43.75 ± 8.3 | |
| Change | 3.750 ± 4.0 | 2.250 ± 5.4 | ||
| TG | (mg/dL) | Start | 200.7 ± 51.4 | 186.0 ± 51.5 |
| (n = 4) | End | 155.3 ± 42.4 * | 170.5 ± 50.3 | |
| Change | −45.33 ± 10.5 | −15.50 ± 73.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.M.; Palma Milla, S.; Zangara, A.; Candela, C.G. Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial. Nutrients 2022, 14, 1879. https://doi.org/10.3390/nu14091879
Quirós-Fernández R, López-Plaza B, Bermejo LM, Palma Milla S, Zangara A, Candela CG. Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial. Nutrients. 2022; 14(9):1879. https://doi.org/10.3390/nu14091879
Chicago/Turabian StyleQuirós-Fernández, Rebeca, Bricia López-Plaza, Laura M. Bermejo, Samara Palma Milla, Andrea Zangara, and Carmen Gómez Candela. 2022. "Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial" Nutrients 14, no. 9: 1879. https://doi.org/10.3390/nu14091879
APA StyleQuirós-Fernández, R., López-Plaza, B., Bermejo, L. M., Palma Milla, S., Zangara, A., & Candela, C. G. (2022). Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial. Nutrients, 14(9), 1879. https://doi.org/10.3390/nu14091879








