Is the Brain Undernourished in Alzheimer’s Disease?
Abstract
1. Introduction
2. Methods
2.1. Procedures
2.1.1. Clinical Evaluation
2.1.2. Plasma and CSF AA Measurements
2.1.3. Assessment of AA Concentrations
2.1.4. Insulin and HOMA-IR
2.2. Objectives
2.3. Statistical Analysis
3. Results
3.1. Patients’ Clinical Characteristics
3.2. CSF and Plasma AA Levels in ADs after Stratification by Nutritional Status
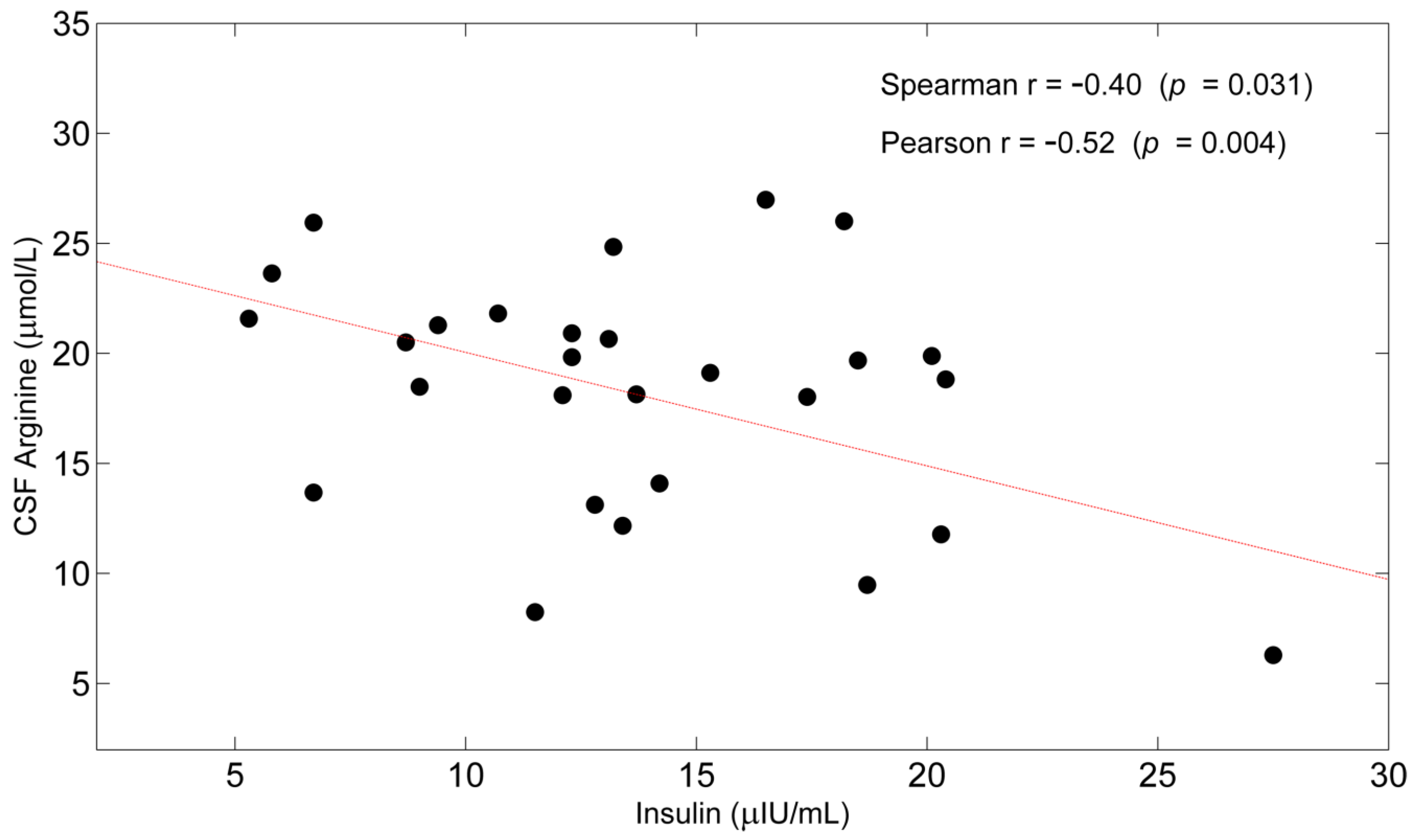
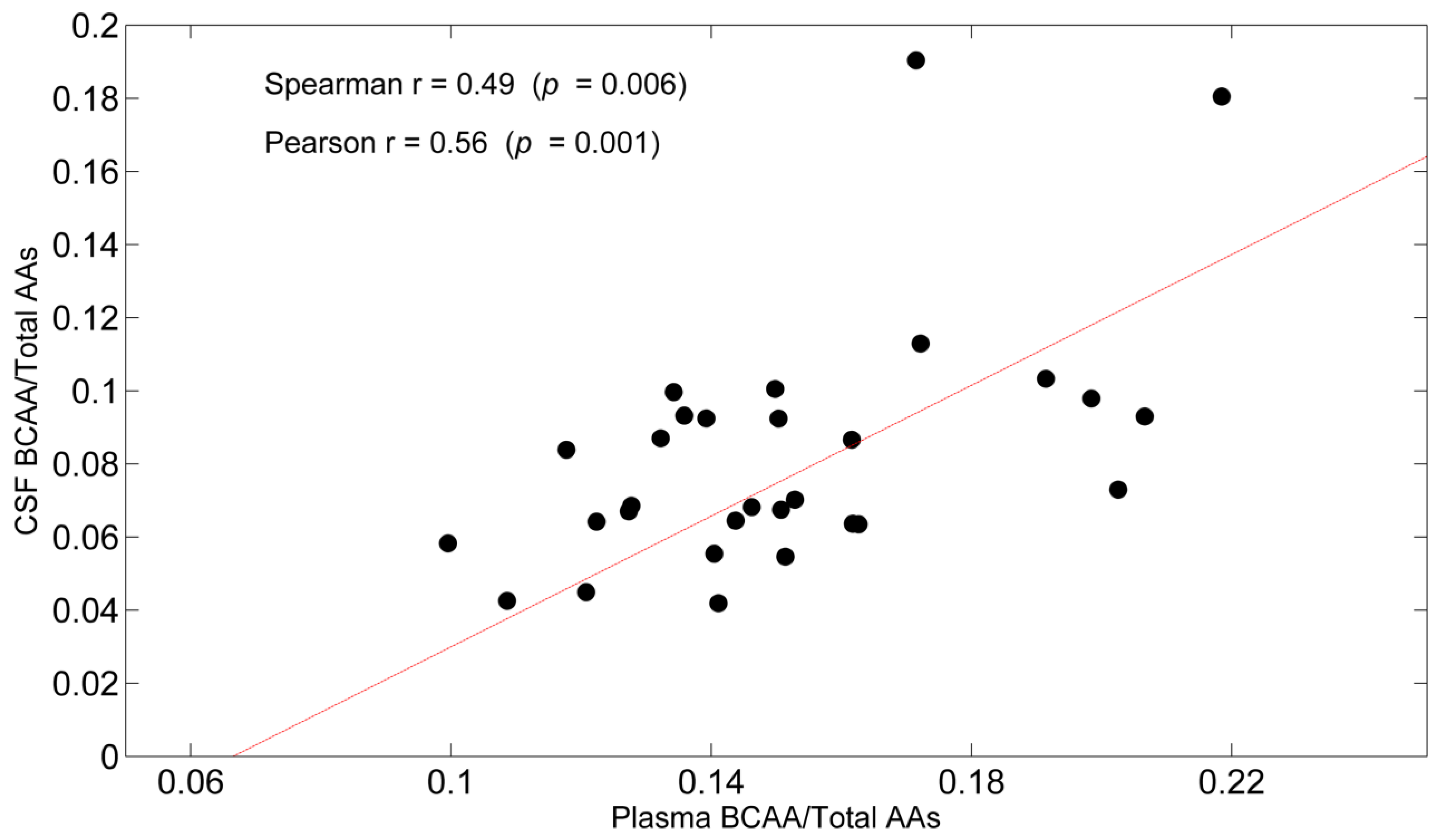
3.3. Correlations
4. Discussion
4.1. Some Potential General Mechanisms for Reduced CSF AA
4.2. Some Potential, Specific Mechanisms for Reduced CSF AA Levels Independently of Patients’ Nutritional Status
4.3. Some Potential, Specific Mechanisms Underlying Reduced CSF Levels in Nutritionally-Deteriorated AD
4.4. Were the Brains of AD Patients Undernourished? Suggestions for Clinical Practice
- Routine determinations of AA levels, both in plasma and CSF, in ADs.
- Quantification of dietary protein/EAA intakes since the first diagnosis and frequent monitoring over time.
- Improving physical activity and exercise, aiming at the preservation of/increase in skeletal muscle mass, strength, and function, since muscle tissue is the main body store of AAs.
- In nutritionally deteriorated patients (MNA < 24 scores), who represented more than half of the ADs considered in this study, a supplementation of EAAs to restore their plasma levels may be needed as EAAs are also vital for maintenance of the structures and functions of extracerebral organs and tissues. Of note, supplementation of the diet with L-serine prevents synaptic loss and behavioral deficits in AD mice [92]. Future studies will address the question of whether, in addition to EAA supplementation, supplying lactate, pyruvate, and ketone bodies may reduce the brain’s need for AAs to generate energy.
4.5. Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C.P.G.M. Literature Review on the Role of Dietary Protein and Amino Acids in Cognitive Functioning and Cognitive Decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Apelt, J.; Mehlhorn, G.; Schliebs, R. Insulin-Sensitive GLUT4 Glucose Transporters Are Colocalized with GLUT3-Expressing Cells and Demonstrate a Chemically Distinct Neuron-Specific Localization in Rat Brain. J. Neurosci. Res. 1999, 57, 693–705. [Google Scholar] [CrossRef]
- Freude, S.; Schilbach, K.; Schubert, M. The Role of IGF-1 Receptor and Insulin Receptor Signaling for the Pathogenesis of Alzheimer’s Disease: From Model Organisms to Human Disease. Curr. Alzheimer Res. 2009, 6, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Decreased Glucose Transporters Correlate to Abnormal Hyperphosphorylation of Tau in Alzheimer Disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bigl, M.; Apelt, J.; Eschrich, K.; Schliebs, R. Cortical Glucose Metabolism Is Altered in Aged Transgenic Tg2576 Mice That Demonstrate Alzheimer Plaque Pathology. J. Neural Transm. 2003, 110, 77–94. [Google Scholar] [CrossRef]
- Mazzola, J.L.; Sirover, M.A. Subcellular Alteration of Glyceraldehyde-3-Phosphate Dehydrogenase in Alzheimer’s Disease Fibroblasts. J. Neurosci. Res. 2003, 71, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.C.; Schubert, D. Amyloid-Beta Induces Disulfide Bonding and Aggregation of GAPDH in Alzheimer’s Disease. FASEB J. 2005, 19, 2060–2062. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lange, M.L.B. Multifunctional Roles of Enolase in Alzheimer’s Disease Brain: Beyond Altered Glucose Metabolism. J. Neurochem. 2009, 111, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Stadtman, E.R.; Floyd, R.A.; Markesbery, W.R. Excess Brain Protein Oxidation and Enzyme Dysfunction in Normal Aging and in Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10540–10543. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread Peroxynitrite-Mediated Damage in Alzheimer’s Disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef]
- Good, P.F.; Werner, P.; Hsu, A.; Olanow, C.W.; Perl, D.P. Evidence of Neuronal Oxidative Damage in Alzheimer’s Disease. Am. J. Pathol. 1996, 149, 21–28. [Google Scholar] [PubMed]
- Mosconi, L.; Pupi, A.; De Leon, M.J. Brain Glucose Hypometabolism and Oxidative Stress in Preclinical Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2008, 1147, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Santos, M.S.; Seiça, R.; Oliveira, C.R. Brain Mitochondrial Dysfunction as a Link between Alzheimer’s Disease and Diabetes. J. Neurol. Sci. 2007, 257, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Duarte, A.I.; Santos, M.S.; Rego, A.C.; Oliveira, C.R. An Integrative View of the Role of Oxidative Stress, Mitochondria and Insulin in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 16, 741–761. [Google Scholar] [CrossRef]
- Gibson, G.E.; Sheu, K.F.; Blass, J.P. Abnormalities of Mitochondrial Enzymes in Alzheimer Disease. J. Neural Transm. (Vienna) 1998, 105, 855–870. [Google Scholar] [CrossRef]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial Abnormalities in Alzheimer Brain: Mechanistic Implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef]
- Ferrer, I. Altered Mitochondria, Energy Metabolism, Voltage-Dependent Anion Channel, and Lipid Rafts Converge to Exhaust Neurons in Alzheimer’s Disease. J. Bioenerg Biomembr. 2009, 41, 425–431. [Google Scholar] [CrossRef]
- Brooks, W.M.; Lynch, P.J.; Ingle, C.C.; Hatton, A.; Emson, P.C.; Faull, R.L.M.; Starkey, M.P. Gene Expression Profiles of Metabolic Enzyme Transcripts in Alzheimer’s Disease. Brain Res. 2007, 1127, 127–135. [Google Scholar] [CrossRef]
- Liang, W.S.; Reiman, E.M.; Valla, J.; Dunckley, T.; Beach, T.G.; Grover, A.; Niedzielko, T.L.; Schneider, L.E.; Mastroeni, D.; Caselli, R.; et al. Alzheimer’s Disease Is Associated with Reduced Expression of Energy Metabolism Genes in Posterior Cingulate Neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 4441–4446. [Google Scholar] [CrossRef]
- Takuma, K.; Yao, J.; Huang, J.; Xu, H.; Chen, X.; Luddy, J.; Trillat, A.-C.; Stern, D.M.; Arancio, O.; Yan, S.S. ABAD Enhances Abeta-Induced Cell Stress via Mitochondrial Dysfunction. FASEB J. 2005, 19, 597–598. [Google Scholar] [CrossRef]
- Kruman, I.I.; Mattson, M.P. Pivotal Role of Mitochondrial Calcium Uptake in Neural Cell Apoptosis and Necrosis. J. Neurochem. 1999, 72, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Development of Alzheimer-Related Neurofibrillary Changes in the Neocortex Inversely Recapitulates Cortical Myelogenesis. Acta Neuropathol. 1996, 92, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Delacourte, A.; David, J.P.; Sergeant, N.; Buée, L.; Wattez, A.; Vermersch, P.; Ghozali, F.; Fallet-Bianco, C.; Pasquier, F.; Lebert, F.; et al. The Biochemical Pathway of Neurofibrillary Degeneration in Aging and Alzheimer’s Disease. Neurology 1999, 52, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Storandt, M.; McKeel, D.W.; Rubin, E.H.; Price, J.L.; Grant, E.A.; Berg, L. Cerebral Amyloid Deposition and Diffuse Plaques in “Normal” Aging: Evidence for Presymptomatic and Very Mild Alzheimer’s Disease. Neurology 1996, 46, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Mattson, M.P.; Pedersen, W.A.; Duan, W.; Culmsee, C.; Camandola, S. Cellular and Molecular Mechanisms Underlying Perturbed Energy Metabolism and Neuronal Degeneration in Alzheimer’s and Parkinson’s Diseases. Ann. N. Y. Acad. Sci. 1999, 893, 154–175. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s Disease from Perturbed Cerebral Glucose Metabolism: Implications for Diagnostic and Therapeutic Strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- Lin, A.P.; Shic, F.; Enriquez, C.; Ross, B.D. Reduced Glutamate Neurotransmission in Patients with Alzheimer’s Disease—An in Vivo (13)C Magnetic Resonance Spectroscopy Study. MAGMA 2003, 16, 29–42. [Google Scholar] [CrossRef]
- Pavlov, P.F.; Hansson Petersen, C.; Glaser, E.; Ankarcrona, M. Mitochondrial Accumulation of APP and Abeta: Significance for Alzheimer Disease Pathogenesis. J. Cell Mol. Med. 2009, 13, 4137–4145. [Google Scholar] [CrossRef]
- Yao, J.; Rettberg, J.R.; Klosinski, L.P.; Cadenas, E.; Brinton, R.D. Shift in Brain Metabolism in Late Onset Alzheimer’s Disease: Implications for Biomarkers and Therapeutic Interventions. Mol. Aspects Med. 2011, 32, 247–257. [Google Scholar] [CrossRef]
- Griffin, J.W.D.; Bradshaw, P.C. Amino Acid Catabolism in Alzheimer’s Disease Brain: Friend or Foe? Oxid. Med. Cell Longev. 2017, 2017, 5472792. [Google Scholar] [CrossRef] [PubMed]
- Lying-Tunell, U.; Lindblad, B.S.; Malmlund, H.O.; Persson, B. Cerebral Blood Flow and Metabolic Rate of Oxygen, Glucose, Lactate, Pyruvate, Ketone Bodies and Amino Acids. Acta Neurol. Scand. 1981, 63, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S.; Nitsch, R. Cerebral Excess Release of Neurotransmitter Amino Acids Subsequent to Reduced Cerebral Glucose Metabolism in Early-Onset Dementia of Alzheimer Type. J. Neural Transm. 1989, 75, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Norberg, K.; Siesiö, B.K. Oxidative Metabolism of the Cerebral Cortex of the Rat in Severe Insulin-Induced Hypoglycaemia. J. Neurochem. 1976, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tyce, G.M. Glucose and Amino Acid Metabolism in Rat Brain during Sustained Hypoglycemia. Neurochem. Res. 1983, 8, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Basun, H.; Forssell, L.G.; Almkvist, O.; Cowburn, R.F.; Eklöf, R.; Winblad, B.; Wetterberg, L. Amino Acid Concentrations in Cerebrospinal Fluid and Plasma in Alzheimer’s Disease and Healthy Control Subjects. J. Neural Transm. Park Dis. Dement. Sect. 1990, 2, 295–304. [Google Scholar] [CrossRef]
- Preston, J.E.; Segal, M.B. The Steady-State Amino Acid Fluxes across the Perfused Choroid Plexus of the Sheep. Brain Res. 1990, 525, 275–279. [Google Scholar] [CrossRef]
- Plum, F. The Physiology and Pathophysiology of the Cerebrospinal Fluidby Hugh Davson, Keasley Welch, and Malcolm B. Segal New York. Livingstone, I987 1013 Pp. Illustrated, $198.00. Ann. Neurol. 1988, 24, 106. [Google Scholar] [CrossRef]
- Serot, J.M.; Béné, M.C.; Faure, G.C. CSF Homocysteine, CSF Folates and Choroid Plexus. Neurobiol. Aging 2003, 24, 627–628, discussion 629. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Segal, M.B. The Structure of the Choroid Plexus and the Physiology of the Choroid Plexus Epithelium. Adv. Drug Deliv. Rev. 2004, 56, 1695–1716. [Google Scholar] [CrossRef]
- Segal, M.B.; Preston, J.E.; Collis, C.S.; Zlokovic, B.V. Kinetics and Na Independence of Amino Acid Uptake by Blood Side of Perfused Sheep Choroid Plexus. Am. J. Physiol. 1990, 258, F1288–F1294. [Google Scholar] [CrossRef] [PubMed]
- Samakashvili, S.; Ibáñez, C.; Simó, C.; Gil-Bea, F.J.; Winblad, B.; Cedazo-Mínguez, A.; Cifuentes, A. Analysis of Chiral Amino Acids in Cerebrospinal Fluid Samples Linked to Different Stages of Alzheimer Disease. Electrophoresis 2011, 32, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R.; Momma, S.; Aoyagi, M.; Rapoport, S.I. Kinetics of Neutral Amino Acid Transport across the Blood-Brain Barrier. J. Neurochem. 1987, 49, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Brain Metabolism: A Perspective from the Blood-Brain Barrier. Physiol. Rev. 1983, 63, 1481–1535. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Choi, T.B. Neutral Amino Acid Transport at the Human Blood-Brain Barrier. Fed. Proc. 1986, 45, 2073–2078. [Google Scholar]
- Aquilani, R.; Costa, A.; Maestri, R.; Cotta Ramusino, M.; Pierobon, A.; Dossena, M.; Solerte, S.B.; Condino, A.M.; Torlaschi, V.; Bini, P.; et al. Mini Nutritional Assessment May Identify a Dual Pattern of Perturbed Plasma Amino Acids in Patients with Alzheimer’s Disease: A Window to Metabolic and Physical Rehabilitation? Nutrients 2020, 12, 1845. [Google Scholar] [CrossRef]
- Aquilani, R.; Brugnatelli, S.; Dossena, M.; Maestri, R.; Delfanti, S.; Buonocore, D.; Boschi, F.; Simeti, E.; Condino, A.M.; Verri, M. Oxaliplatin-Fluoropyrimidine Combination (XELOX) Therapy Does Not Affect Plasma Amino Acid Levels and Plasma Markers of Oxidative Stress in Colorectal Cancer Surgery Patients: A Pilot Study. Nutrients 2019, 11, 2667. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Kokjohn, T.A.; Watson, M.D.; Woods, A.S.; Cotter, R.J.; Sue, L.I.; Kalback, W.M.; Emmerling, M.R.; Beach, T.G.; Roher, A.E. Elevated Abeta42 in Skeletal Muscle of Alzheimer Disease Patients Suggests Peripheral Alterations of AbetaPP Metabolism. Am. J. Pathol. 2000, 156, 797–805. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Zhou, L.; Li, Q.; Li, W. Plasma Metabolic Profiling of Mild Cognitive Impairment and Alzheimer’s Disease Using Liquid Chromatography/Mass Spectrometry. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 113–120. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Xu, J.; Begley, P.; Church, S.J.; Patassini, S.; Hollywood, K.A.; Jüllig, M.; Curtis, M.A.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; et al. Graded Perturbations of Metabolism in Multiple Regions of Human Brain in Alzheimer’s Disease: Snapshot of a Pervasive Metabolic Disorder. Biochim. Biophys. Acta 2016, 1862, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Schoenknecht, P.; Kassner, S.; Hildebrandt, W.; Kinscherf, R.; Schroeder, J. Cerebrospinal Fluid Concentrations of Functionally Important Amino Acids and Metabolic Compounds in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener Dis. 2010, 7, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gueli, M.C.; Taibi, G. Alzheimer’s Disease: Amino Acid Levels and Brain Metabolic Status. Neurol. Sci. 2013, 34, 1575–1579. [Google Scholar] [CrossRef]
- Burns, J.M.; Johnson, D.K.; Watts, A.; Swerdlow, R.H.; Brooks, W.M. Reduced Lean Mass in Early Alzheimer Disease and Its Association with Brain Atrophy. Arch. Neurol. 2010, 67, 428–433. [Google Scholar] [CrossRef]
- Bekkering, P.; Jafri, I.; van Overveld, F.J.; Rijkers, G.T. The Intricate Association between Gut Microbiota and Development of Type 1, Type 2 and Type 3 Diabetes. Expert Rev. Clin. Immunol. 2013, 9, 1031–1041. [Google Scholar] [CrossRef]
- Zhao, Y.; Dua, P.; Lukiw, W.J. Microbial Sources of Amyloid and Relevance to Amyloidogenesis and Alzheimer’s Disease (AD). J. Alzheimers Dis. Parkinsonism 2015, 5, 177. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lukiw, W.J. Alzheimer’s Disease and the Microbiome. Front. Cell Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimers Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef]
- Lazar, H.L. The Insulin Cardioplegia Trial. J. Thorac. Cardiovasc. Surg. 2002, 123, 842–844. [Google Scholar] [CrossRef]
- Li, X.; Alafuzoff, I.; Soininen, H.; Winblad, B.; Pei, J.-J. Levels of MTOR and Its Downstream Targets 4E-BP1, EEF2, and EEF2 Kinase in Relationships with Tau in Alzheimer’s Disease Brain. FEBS J. 2005, 272, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Maldonado, M.A.; Majumder, S.; Medina, D.X.; Holbein, W.; Magrí, A.; Oddo, S. Naturally Secreted Amyloid-Beta Increases Mammalian Target of Rapamycin (MTOR) Activity via a PRAS40-Mediated Mechanism. J. Biol. Chem. 2011, 286, 8924–8932. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, J.B.; Laberge, R.-M. Age-Related Neurodegeneration Prevention Through MTOR Inhibition: Potential Mechanisms and Remaining Questions. Curr. Top. Med. Chem. 2015, 15, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.M.; Phillips, D.; McCoy, J.P.; Aponte, A.M.; Shen, R.-F.; Balaban, R.S.; Finkel, T. The Mammalian Target of Rapamycin (MTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. J. Biol. Chem. 2006, 281, 27643–27652. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular Interplay between Mammalian Target of Rapamycin (MTOR), Amyloid-Beta, and Tau: Effects on Cognitive Impairments. J. Biol. Chem. 2010, 285, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of MTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-Beta Levels in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e9979. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Pankiewicz, J.; Scholtzova, H.; Li, Y.; Quartermain, D.; Duff, K.; Wisniewski, T. Links between the Pathology of Alzheimer’s Disease and Vascular Dementia. Neurochem. Res. 2004, 29, 1257–1266. [Google Scholar] [CrossRef]
- Clodfelder-Miller, B.J.; Zmijewska, A.A.; Johnson, G.V.W.; Jope, R.S. Tau Is Hyperphosphorylated at Multiple Sites in Mouse Brain in Vivo after Streptozotocin-Induced Insulin Deficiency. Diabetes 2006, 55, 3320–3325. [Google Scholar] [CrossRef]
- Planel, E.; Tatebayashi, Y.; Miyasaka, T.; Liu, L.; Wang, L.; Herman, M.; Yu, W.H.; Luchsinger, J.A.; Wadzinski, B.; Duff, K.E.; et al. Insulin Dysfunction Induces in Vivo Tau Hyperphosphorylation through Distinct Mechanisms. J. Neurosci. 2007, 27, 13635–13648. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Review of Insulin and Insulin-like Growth Factor Expression, Signaling, and Malfunction in the Central Nervous System: Relevance to Alzheimer’s Disease. J. Alzheimers Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Walsh, D.M.; Ye, Z.; Vekrellis, K.; Zhang, J.; Podlisny, M.B.; Rosner, M.R.; Safavi, A.; Hersh, L.B.; Selkoe, D.J. Insulin-Degrading Enzyme Regulates Extracellular Levels of Amyloid Beta-Protein by Degradation. J. Biol. Chem. 1998, 273, 32730–32738. [Google Scholar] [CrossRef] [PubMed]
- Biundo, F.; Del Prete, D.; Zhang, H.; Arancio, O.; D’Adamio, L. A Role for Tau in Learning, Memory and Synaptic Plasticity. Sci. Rep. 2018, 8, 3184. [Google Scholar] [CrossRef] [PubMed]
- Forner, S.; Baglietto-Vargas, D.; Martini, A.C.; Trujillo-Estrada, L.; LaFerla, F.M. Synaptic Impairment in Alzheimer’s Disease: A Dysregulated Symphony. Trends Neurosci. 2017, 40, 347–357. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired Insulin and Insulin-like Growth Factor Expression and Signaling Mechanisms in Alzheimer’s Disease--Is This Type 3 Diabetes? J. Alzheimers Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.J.; Goldin, A.; Fulmer, N.; Tavares, R.; Wands, J.R.; de la Monte, S.M. Insulin and Insulin-like Growth Factor Expression and Function Deteriorate with Progression of Alzheimer’s Disease: Link to Brain Reductions in Acetylcholine. J. Alzheimers Dis. 2005, 8, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.A.; Wells, D.G.; Fallon, J.R. The Insulin Receptor Tyrosine Kinase Substrate P58/53 and the Insulin Receptor Are Components of CNS Synapses. J. Neurosci. 1999, 19, 7300–7308. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Burgmans, S.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Hofman, P.A.M.; Verhey, F.R.J.; Backes, W.H. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular Unit Impairment in Early Alzheimer’s Disease Measured with Magnetic Resonance Imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Jansen, J.F.A.; Jeukens, C.R.L.P.N.; Burgmans, S.; van Buchem, M.A.; Muller, M.; Hofman, P.A.M.; Verhey, F.R.J.; van Osch, M.J.P.; Backes, W.H. Subtle Blood-Brain Barrier Leakage Rate and Spatial Extent: Considerations for Dynamic Contrast-Enhanced MRI. Med. Phys. 2017, 44, 4112–4125. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Neurovascular Dysfunction and Neurodegeneration in Dementia and Alzheimer’s Disease. Biochim. Biophys. Acta 2016, 1862, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Bensemain, F.; Hot, D.; Ferreira, S.; Dumont, J.; Bombois, S.; Maurage, C.-A.; Huot, L.; Hermant, X.; Levillain, E.; Hubans, C.; et al. Evidence for Induction of the Ornithine Transcarbamylase Expression in Alzheimer’s Disease. Mol. Psychiatry 2009, 14, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; Wartchow, K.M.; Flores, M.P.; Leite, M.C.; Quincozes-Santos, A.; Gonçalves, C.-A. Ammonia-Induced Oxidative Damage in Neurons Is Prevented by Resveratrol and Lipoic Acid with Participation of Heme Oxygenase 1. Neurotoxicology 2015, 49, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Murthy, C.R.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-Induced Production of Free Radicals in Primary Cultures of Rat Astrocytes. J. Neurosci. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef]
- Kosenko, E.; Felipo, V.; Montoliu, C.; Grisolía, S.; Kaminsky, Y. Effects of Acute Hyperammonemia in Vivo on Oxidative Metabolism in Nonsynaptic Rat Brain Mitochondria. Metab. Brain Dis. 1997, 12, 69–82. [Google Scholar] [CrossRef]
- Rao, K.V.; Mawal, Y.R.; Qureshi, I.A. Progressive Decrease of Cerebral Cytochrome C Oxidase Activity in Sparse-Fur Mice: Role of Acetyl-L-Carnitine in Restoring the Ammonia-Induced Cerebral Energy Depletion. Neurosci. Lett. 1997, 224, 83–86. [Google Scholar] [CrossRef]
- Qureshi, K.; Rao, K.V.; Qureshi, I.A. Differential Inhibition by Hyperammonemia of the Electron Transport Chain Enzymes in Synaptosomes and Non-Synaptic Mitochondria in Ornithine Transcarbamylase-Deficient Spf-Mice: Restoration by Acetyl-L-Carnitine. Neurochem. Res. 1998, 23, 855–861. [Google Scholar] [CrossRef]
- Le Prince, G.; Delaere, P.; Fages, C.; Lefrançois, T.; Touret, M.; Salanon, M.; Tardy, M. Glutamine Synthetase (GS) Expression Is Reduced in Senile Dementia of the Alzheimer Type. Neurochem. Res. 1995, 20, 859–862. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-Induced ROS Generation Causes Insulin Resistance in Mice with Chronic Renal Failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Ducker, G.S.; Billingham, L.K.; Martinez, C.A.; Mainolfi, N.; Suri, V.; Friedman, A.; Manfredi, M.G.; Weinberg, S.E.; Rabinowitz, J.D.; et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019, 29, 1003–1011.e4. [Google Scholar] [CrossRef]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.P.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A.; et al. D-Serine Levels in Alzheimer’s Disease: Implications for Novel Biomarker Development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef] [PubMed]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.-A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517.e8. [Google Scholar] [CrossRef] [PubMed]
- Mendonça Machado, N.; Torrinhas, R.S.; Sala, P.; Ishida, R.K.; Guarda, I.F.M.S.; de Moura, E.G.H.; Sakai, P.; Santo, M.A.; Linetzky Waitzberg, D. Type 2 Diabetes Metabolic Improvement After Roux-En-Y Gastric Bypass May Include a Compensatory Mechanism That Balances Fatty Acid β and ω Oxidation. JPEN J. Parenter Enter. Nutr. 2020, 44, 1417–1427. [Google Scholar] [CrossRef]
- McCormack, S.A.; Tague, L.L.; Gragoe, E.J.; Johnson, L.R. Regulation of Ornithine Decarboxylase Activity in LoVo Cells. Am. J. Physiol. 1990, 258, G934–G941. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic Profiling of Serum in the Progression of Alzheimer’s Disease by Capillary Electrophoresis-Mass Spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Procter, A.W.; Palmer, A.M.; Francis, P.T.; Lowe, S.L.; Neary, D.; Murphy, E.; Doshi, R.; Bowen, D.M. Evidence of Glutamatergic Denervation and Possible Abnormal Metabolism in Alzheimer’s Disease. J. Neurochem. 1988, 50, 790–802. [Google Scholar] [CrossRef]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients with Probable Alzheimer’s Disease and Depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef]
- Redjems-Bennani, N.; Jeandel, C.; Lefebvre, E.; Blain, H.; Vidailhet, M.; Guéant, J.L. Abnormal Substrate Levels That Depend upon Mitochondrial Function in Cerebrospinal Fluid from Alzheimer Patients. Gerontology 1998, 44, 300–304. [Google Scholar] [CrossRef]
- Rivett, A.J. Preferential Degradation of the Oxidatively Modified Form of Glutamine Synthetase by Intracellular Mammalian Proteases. J. Biol. Chem. 1985, 260, 300–305. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F.; St Clair, D.; Keller, J.N.; Pierce, W.M.; Klein, J.B.; Markesbery, W.R. Redox Proteomics Identification of Oxidatively Modified Hippocampal Proteins in Mild Cognitive Impairment: Insights into the Development of Alzheimer’s Disease. Neurobiol. Dis. 2006, 22, 223–232. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, T.; Ma, J.; Duan, W.; Xu, Q.; Li, X.; Han, L.; Li, W.; Wang, Z.; Zhang, D.; et al. Hypoxia-Inducible Factor-1α Mediates Hyperglycemia-Induced Pancreatic Cancer Glycolysis. ACAMC 2019, 19, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Sonnay, S.; Gruetter, R.; Duarte, J.M.N. How Energy Metabolism Supports Cerebral Function: Insights from 13C Magnetic Resonance Studies In Vivo. Front. Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Hudd, F.; Shiel, A.; Harris, M.; Bowdler, P.; McCann, B.; Tsivos, D.; Wearn, A.; Knight, M.; Kauppinen, R.; Coulthard, E.; et al. Novel Blood Biomarkers That Correlate with Cognitive Performance and Hippocampal Volumetry: Potential for Early Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 67, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Timmer, N.M.; Herbert, M.K.; Claassen, J.A.H.R.; Kuiperij, H.B.; Verbeek, M.M. Total Glutamine Synthetase Levels in Cerebrospinal Fluid of Alzheimer’s Disease Patients Are Unchanged. Neurobiol. Aging 2015, 36, 1271–1273. [Google Scholar] [CrossRef]
- Chalimoniuk, M.; Stolecka, A.; Cakała, M.; Hauptmann, S.; Schulz, K.; Lipka, U.; Leuner, K.; Eckert, A.; Muller, W.E.; Strosznajder, J.B. Amyloid Beta Enhances Cytosolic Phospholipase A2 Level and Arachidonic Acid Release via Nitric Oxide in APP-Transfected PC12 Cells. Acta Biochim. Pol. 2007, 54, 611–623. [Google Scholar] [CrossRef]
- Uehara, T.; Nakamura, T.; Yao, D.; Shi, Z.-Q.; Gu, Z.; Ma, Y.; Masliah, E.; Nomura, Y.; Lipton, S.A. S-Nitrosylated Protein-Disulphide Isomerase Links Protein Misfolding to Neurodegeneration. Nature 2006, 441, 513–517. [Google Scholar] [CrossRef]
- Cho, D.-H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 Mediates Beta-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Vural, H.; Sirin, B.; Yilmaz, N.; Eren, I.; Delibas, N. The Role of Arginine-Nitric Oxide Pathway in Patients with Alzheimer Disease. Biol. Trace Elem. Res. 2009, 129, 58–64. [Google Scholar] [CrossRef]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of Obesity-Induced Metabolic and Vascular Dysfunctions. Front. Biosci. 2019, 24, 890–934. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Williams, K.R.; Smith, D.G.; Smythe, G.A.; Croitoru-Lamoury, J.; Brew, B.J. Quinolinic Acid in the Pathogenesis of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2003, 527, 167–176. [Google Scholar] [CrossRef]
- Bonda, D.J.; Mailankot, M.; Stone, J.G.; Garrett, M.R.; Staniszewska, M.; Castellani, R.J.; Siedlak, S.L.; Zhu, X.; Lee, H.; Perry, G.; et al. Indoleamine 2,3-Dioxygenase and 3-Hydroxykynurenine Modifications Are Found in the Neuropathology of Alzheimer’s Disease. Redox Rep. 2010, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Severiano, F.; Escalante, B.; Ríos, C. Nitric Oxide Synthase Inhibition Prevents Acute Quinolinate-Induced Striatal Neurotoxicity. Neurochem. Res. 1998, 23, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Metabolite Profiling for the Identification of Altered Metabolic Pathways in Alzheimer’s Disease. J. Pharm. Biomed Anal. 2015, 107, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J. Brain Serotonin Content: Physiological Regulation by Plasma Neutral Amino Acids. Science 1972, 178, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; McTavish, S.F.; Clark, L.; Goodwin, G.M.; Cowen, P.J. Tyrosine Depletion Attenuates Dopamine Function in Healthy Volunteers. Psychopharmacology 2001, 154, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, H.J.; Scarnà, A.; Harmer, C.J.; McTavish, S.B.; Odontiadis, J.; Cowen, P.J.; Goodwin, G.M. A Dose-Finding Study on the Effects of Branch Chain Amino Acids on Surrogate Markers of Brain Dopamine Function. Psychopharmacology 2002, 160, 192–197. [Google Scholar] [CrossRef]
- Scarnà, A.; McTavish, S.F.B.; Cowen, P.J.; Goodwin, G.M.; Rogers, R.D. The Effects of a Branched Chain Amino Acid Mixture Supplemented with Tryptophan on Biochemical Indices of Neurotransmitter Function and Decision-Making. Psychopharmacology 2005, 179, 761–768. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Bianchi, G.; Martelli, M.; Talerico, T.; Servadei, L.; Zoli, M.; Mariani, E. Plasma Amino Acid Concentrations in Patients with Amnestic Mild Cognitive Impairment or Alzheimer Disease. Am. J. Clin. Nutr. 2004, 80, 483–488. [Google Scholar] [CrossRef]
- Corso, G.; Cristofano, A.; Sapere, N.; la Marca, G.; Angiolillo, A.; Vitale, M.; Fratangelo, R.; Lombardi, T.; Porcile, C.; Intrieri, M.; et al. Serum Amino Acid Profiles in Normal Subjects and in Patients with or at Risk of Alzheimer Dementia. Dement Geriatr. Cogn. Dis. Extra 2017, 7, 143–159. [Google Scholar] [CrossRef]
- Xu, G.; Kwon, G.; Marshall, C.A.; Lin, T.A.; Lawrence, J.C.; McDaniel, M.L. Branched-Chain Amino Acids Are Essential in the Regulation of PHAS-I and P70 S6 Kinase by Pancreatic Beta-Cells. A Possible Role in Protein Translation and Mitogenic Signaling. J. Biol. Chem. 1998, 273, 28178–28184. [Google Scholar] [CrossRef]
- Scaini, G.; Mello-Santos, L.M.; Furlanetto, C.B.; Jeremias, I.C.; Mina, F.; Schuck, P.F.; Ferreira, G.C.; Kist, L.W.; Pereira, T.C.B.; Bogo, M.R.; et al. Acute and Chronic Administration of the Branched-Chain Amino Acids Decreases Nerve Growth Factor in Rat Hippocampus. Mol. Neurobiol. 2013, 48, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.S.W.; Carvalho-Silva, M.; Gomes, L.M.; Zapelini, H.G.; Schuck, P.F.; Ferreira, G.C.; Scaini, G.; Streck, E.L. Intracerebroventricular Administration of α-Ketoisocaproic Acid Decreases Brain-Derived Neurotrophic Factor and Nerve Growth Factor Levels in Brain of Young Rats. Metab. Brain Dis. 2016, 31, 377–383. [Google Scholar] [CrossRef]
- Parrella, E.; Maxim, T.; Maialetti, F.; Zhang, L.; Wan, J.; Wei, M.; Cohen, P.; Fontana, L.; Longo, V.D. Protein Restriction Cycles Reduce IGF-1 and Phosphorylated Tau, and Improve Behavioral Performance in an Alzheimer’s Disease Mouse Model. Aging Cell 2013, 12, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Horst, D.; Grace, N.D.; Conn, H.O.; Schiff, E.; Schenker, S.; Viteri, A.; Law, D.; Atterbury, C.E. Comparison of Dietary Protein with an Oral, Branched Chain-Enriched Amino Acid Supplement in Chronic Portal-Systemic Encephalopathy: A Randomized Controlled Trial. Hepatology 1984, 4, 279–287. [Google Scholar] [CrossRef]
- Cerra, F.B.; Cheung, N.K.; Fischer, J.E.; Kaplowitz, N.; Schiff, E.R.; Dienstag, J.L.; Bower, R.H.; Mabry, C.D.; Leevy, C.M.; Kiernan, T. Disease-Specific Amino Acid Infusion (F080) in Hepatic Encephalopathy: A Prospective, Randomized, Double-Blind, Controlled Trial. JPEN J. Parenter Enter. Nutr. 1985, 9, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Egberts, E.H.; Schomerus, H.; Hamster, W.; Jürgens, P. Branched Chain Amino Acids in the Treatment of Latent Portosystemic Encephalopathy. A Double-Blind Placebo-Controlled Crossover Study. Gastroenterology 1985, 88, 887–895. [Google Scholar] [CrossRef]
- Jordan, M.K.; Brunner, R.L.; Hunt, M.M.; Berry, H.K. Preliminary Support for the Oral Administration of Valine, Isoleucine and Leucine for Phenylketonuria. Dev. Med. Child Neurol. 1985, 27, 33–39. [Google Scholar] [CrossRef]
- Berry, H.K.; Brunner, R.L.; Hunt, M.M.; White, P.P. Valine, Isoleucine, and Leucine. A New Treatment for Phenylketonuria. Am. J. Dis. Child 1990, 144, 539–543. [Google Scholar] [CrossRef]
- Aquilani, R.; Iadarola, P.; Contardi, A.; Boselli, M.; Verri, M.; Pastoris, O.; Boschi, F.; Arcidiaco, P.; Viglio, S. Branched-Chain Amino Acids Enhance the Cognitive Recovery of Patients with Severe Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2005, 86, 1729–1735. [Google Scholar] [CrossRef]
- Aquilani, R.; Boselli, M.; Boschi, F.; Viglio, S.; Iadarola, P.; Dossena, M.; Pastoris, O.; Verri, M. Branched-Chain Amino Acids May Improve Recovery from a Vegetative or Minimally Conscious State in Patients with Traumatic Brain Injury: A Pilot Study. Arch. Phys. Med. Rehabil. 2008, 89, 1642–1647. [Google Scholar] [CrossRef]
- Cole, J.T.; Mitala, C.M.; Kundu, S.; Verma, A.; Elkind, J.A.; Nissim, I.; Cohen, A.S. Dietary Branched Chain Amino Acids Ameliorate Injury-Induced Cognitive Impairment. Proc. Natl. Acad. Sci. USA 2010, 107, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hassmén, P.; Blomstrand, E.; Ekblom, B.; Newsholme, E.A. Branched-Chain Amino Acid Supplementation during 30-km Competitive Run: Mood and Cognitive Performance. Nutrition 1994, 10, 405–410. [Google Scholar] [PubMed]
- Mittleman, K.D.; Ricci, M.R.; Bailey, S.P. Branched-Chain Amino Acids Prolong Exercise during Heat Stress in Men and Women. Med. Sci. Sports Exerc. 1998, 30, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tynkkynen, J.; Chouraki, V.; van der Lee, S.J.; Hernesniemi, J.; Yang, Q.; Li, S.; Beiser, A.; Larson, M.G.; Sääksjärvi, K.; Shipley, M.J.; et al. Association of Branched-Chain Amino Acids and Other Circulating Metabolites with Risk of Incident Dementia and Alzheimer’s Disease: A Prospective Study in Eight Cohorts. Alzheimers Dement. 2018, 14, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S.M.; Islam, M.M.; Zaganas, I. Interaction between Glutamate Dehydrogenase (GDH) and L-Leucine Catabolic Enzymes: Intersecting Metabolic Pathways. Neurochem. Int. 2011, 59, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.E.; Hutson, S.M. BCAA Metabolism and NH3 Homeostasis. Adv. Neurobiol. 2016, 13, 99–132. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Grunstein, L.; Nissim, I.; Stern, J.; Pleasure, D.; Nissim, I. Astrocyte Leucine Metabolism: Significance of Branched-Chain Amino Acid Transamination. J. Neurochem. 1996, 66, 378–385. [Google Scholar] [CrossRef]
- Bixel, M.G.; Hutson, S.M.; Hamprecht, B. Cellular Distribution of Branched-Chain Amino Acid Aminotransferase Isoenzymes among Rat Brain Glial Cells in Culture. J. Histochem. Cytochem. 1997, 45, 685–694. [Google Scholar] [CrossRef]
- Salcedo, C.; Andersen, J.V.; Vinten, K.T.; Pinborg, L.H.; Waagepetersen, H.S.; Freude, K.K.; Aldana, B.I. Functional Metabolic Mapping Reveals Highly Active Branched-Chain Amino Acid Metabolism in Human Astrocytes, Which Is Impaired in IPSC-Derived Astrocytes in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 736580. [Google Scholar] [CrossRef]
- García-Espinosa, M.A.; Wallin, R.; Hutson, S.M.; Sweatt, A.J. Widespread Neuronal Expression of Branched-Chain Aminotransferase in the CNS: Implications for Leucine/Glutamate Metabolism and for Signaling by Amino Acids. J. Neurochem. 2007, 100, 1458–1468. [Google Scholar] [CrossRef]
- Cotman, C.W.; Nieto-Sampedro, M.; Harris, E.W. Synapse Replacement in the Nervous System of Adult Vertebrates. Physiol. Rev. 1981, 61, 684–784. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Marchini, J.S. Mechanisms and Nutritional Significance of Metabolic Responses to Altered Intakes of Protein and Amino Acids, with Reference to Nutritional Adaptation in Humans. Am. J. Clin. Nutr. 1990, 51, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.C.; Rafii, M.; Ball, R.O.; Pencharz, P.B. Threonine Requirement of Young Men Determined by Indicator Amino Acid Oxidation with Use of L-[1-(13)C]Phenylalanine. Am. J. Clin. Nutr. 2000, 71, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nedachi, T.; Nagasawa, T. Lysine Suppresses Protein Degradation through Autophagic-Lysosomal System in C2C12 Myotubes. Mol. Cell Biochem. 2014, 391, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Dietary L-Lysine Suppresses Autophagic Proteolysis and Stimulates Akt/MTOR Signaling in the Skeletal Muscle of Rats Fed a Low-Protein Diet. J. Agric. Food Chem. 2015, 63, 8192–8198. [Google Scholar] [CrossRef]
- Basic Neurochemistry: Molecular, Cellular, and Medical Aspects, 6th ed.; Siegel, G.J., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; Chapter 24; ISBN 978-0-397-51820-3. [Google Scholar]
- Hu, Q.; Teng, W.; Li, J.; Hao, F.; Wang, N. Homocysteine and Alzheimer’s Disease: Evidence for a Causal Link from Mendelian Randomization. J. Alzheimers Dis. 2016, 52, 747–756. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Hinrichs, H.R.; Petersen, R.O.; Baserga, R. Incorporation of thymidine into DNA of mouse organs. Arch. Pathol. 1964, 78, 245–253. [Google Scholar]
- Ballou, J.E.; Thompson, R.C. Studies of Metabolic Turnover with Tritium as a Tracer. V. The Predominantly Non-Dynamic State of Body Constituents in the Rat. J. Biol. Chem. 1956, 223, 795–809. [Google Scholar]
- Gan, J.C.; Jeffay, H. Origins and Metabolism of the Intracellular Amino Acid Pools in Rat Liver and Muscle. Biochim. Biophys. Acta 1967, 148, 448–459. [Google Scholar] [CrossRef]
- Smeets, J.S.J.; Horstman, A.M.H.; Schijns, O.E.M.G.; Dings, J.T.A.; Hoogland, G.; Gijsen, A.P.; Goessens, J.P.B.; Bouwman, F.G.; Wodzig, W.K.W.H.; Mariman, E.C.; et al. Brain Tissue Plasticity: Protein Synthesis Rates of the Human Brain. Brain 2018, 141, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.C.; Cook, M.P.; Qin, M.; Kang, J.; Burlin, T.V.; Smith, C.B. Measurement of Regional Rates of Cerebral Protein Synthesis with L-[1-11C]Leucine and PET with Correction for Recycling of Tissue Amino Acids: I. Kinetic Modeling Approach. J. Cereb. Blood Flow Metab. 2005, 25, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.B.; Schmidt, K.C.; Qin, M.; Burlin, T.V.; Cook, M.P.; Kang, J.; Saunders, R.C.; Bacher, J.D.; Carson, R.E.; Channing, M.A.; et al. Measurement of Regional Rates of Cerebral Protein Synthesis with L-[1-11C]Leucine and PET with Correction for Recycling of Tissue Amino Acids: II. Validation in Rhesus Monkeys. J. Cereb. Blood Flow Metab. 2005, 25, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Dallman, P.R.; Spirito, R.A. Brain Response to Protein Undernutrition. Mechanism of Preferential Protein Retention. J. Clin. Investig. 1972, 51, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yin, Y.; Wu, G. Dietary Essentiality of “Nutritionally Non-Essential Amino Acids” for Animals and Humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Lindsay, C.B.; Montecinos-Oliva, C.; Arrazola, M.S.; Retamales, R.M.; Bunout, D.; Hirsch, S.; Inestrosa, N.C. Is L-Methionine a Trigger Factor for Alzheimer’s-like Neurodegeneration? Changes in Aβ Oligomers, Tau Phosphorylation, Synaptic Proteins, Wnt Signaling and Behavioral Impairment in Wild-Type Mice. Mol. Neurodegener 2015, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roman, I.; Barja, G. Regulation of Longevity and Oxidative Stress by Nutritional Interventions: Role of Methionine Restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of Inflammation Is Altered in Alzheimer’s Disease. Alzheimers Dement. 2015, 11, 40–50.e1-2. [Google Scholar] [CrossRef]
- Kepka, A.; Ochocinska, A.; Borzym-Kluczyk, M.; Skorupa, E.; Stasiewicz-Jarocka, B.; Chojnowska, S.; Waszkiewicz, N. Preventive Role of L-Carnitine and Balanced Diet in Alzheimer’s Disease. Nutrients 2020, 12, 1987. [Google Scholar] [CrossRef]
- Muñoz Fernández, S.S.; Lima Ribeiro, S.M. Nutrition and Alzheimer Disease. Clin. Geriatr. Med. 2018, 34, 677–697. [Google Scholar] [CrossRef]
 increase
increase  decrease. BBB: Blood-Brain-Barrier.
decrease. BBB: Blood-Brain-Barrier.
 increase
increase  decrease. BBB: Blood-Brain-Barrier.
decrease. BBB: Blood-Brain-Barrier.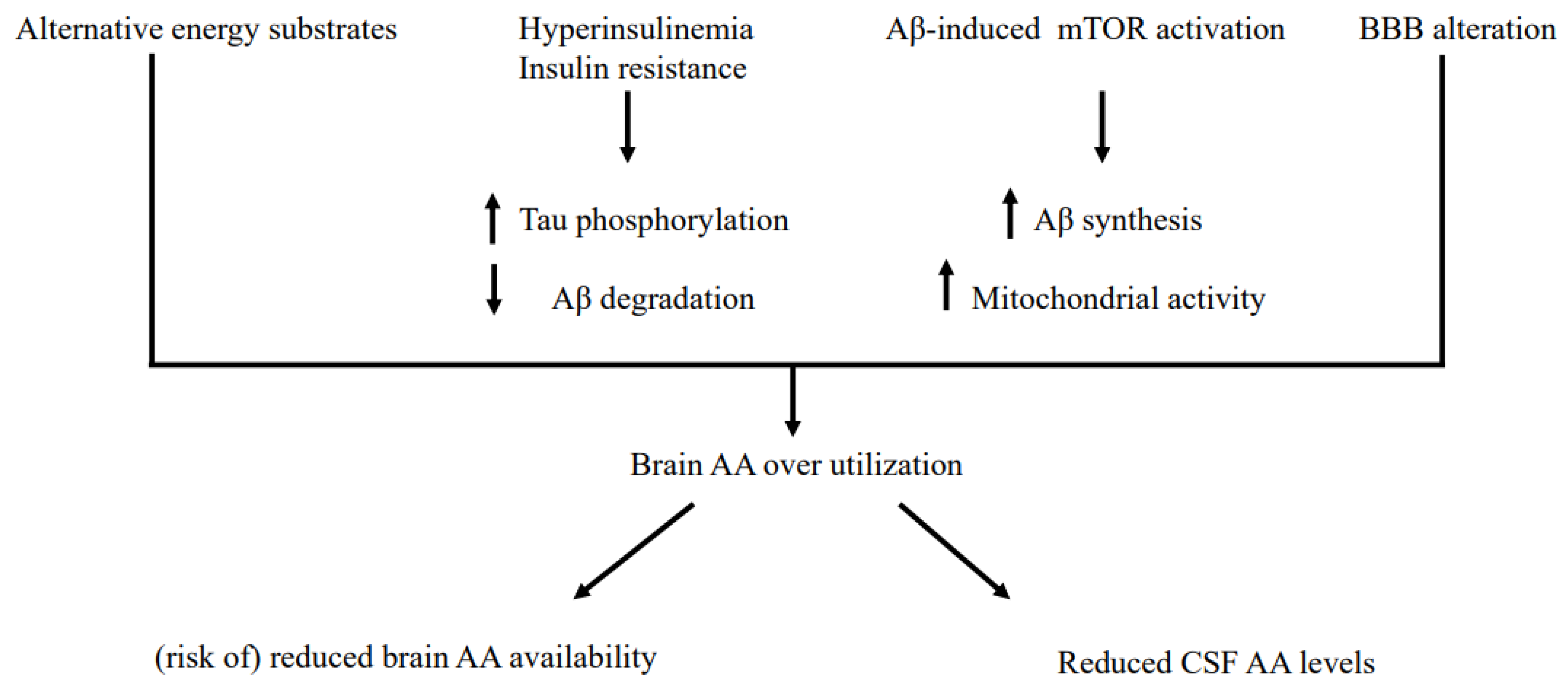
| Variables | AD Patients |
|---|---|
| Demographic variables | |
| Age (years) | 74.41 ± 8.17 |
| Male gender | 46% |
| Disease duration (years) | 3.4 ± 3.2 |
| Anthropometric variables | |
| Education (years) | 6.57 ± 8.17 |
| Body weight (kg) | 63.30 ± 13.24 |
| Height (cm) | 160.63 ± 9.97 |
| Body mass index (kg/m2) | 24.61 ± 4.87 |
| Mid-arm circumference (cm) | 26.15 ± 3.29 |
| Biohumoral variables | |
| Glucose (NV 70–115 mg/dL) | 88.88 ± 13.78 |
| Insulin (NV 4–24 µU/mL) | 14.10 ± 5.72 |
| HOMA-IR (NV < 2.4) | 4.44 ± 2.19 |
| Glycosylated hemoglobin (NV 4.8–5.9%) | 5.65 ± 0.85 |
| Total cholesterol (NV < 200 mg/dL) | 192.94 ± 32.90 |
| HDL cholesterol (NV: M > 55 mg/dL; F > 65 mg/dL) | 57.66 ± 14.77 |
| LDL cholesterol (NV < 100 mg/dL) | 116.03 ± 26.95 |
| Transferrin (NV 200–360 mg/dL) | 227.29 ± 40.90 |
| Iron (NV 59–158 µg/dL) | 86.41 ± 25.80 |
| Triglycerides (NV 0–200 mg/dL) | 97.42 ± 38.34 |
| Vitamin B12 (NV 191–663 pg/mL) | 302.87 ± 114.49 |
| Folate (NV 3.1–17.5 ng/mL) | 6.84 ± 3.75 |
| Creatinine (NV: M 0.73–1.18 mg/dL; F 0.55–1.02 mg/dL) | 0.86 ± 0.22 |
| Albumin (NV 55.8-66.1%) | 57.65 ± 5.02 |
| Total protein (NV 6.2–7.5 g/dL) | 6.24 ± 0.41 |
| White blood cell count (NV 4.00–10.00 × 103 /µL) | 6.35 ± 1.64 |
| Red blood cell count (NV: M 4.30–5.70 × 106 /µL; F 3.80–5.20 × 106 /µL) | 4.29 ± 0.45 |
| Hemoglobin (NV: M 13.2–17.3 g/dL; F 11.7–15.5 g/dL) | 13.06 ± 1.28 |
| Erythrosedimentation rate (NV < 15 mm/1st h) | 19.90 ± 21.92 |
| AD biomarker concentrations in CSF | |
| tau protein (NV < 404 pg/mL) | 488.25 ± 458.38 |
| p-tau (NV < 56.5 pg/mL) | 78.48 ± 34.13 |
| β-amyloid (NV > 599 pg/mL) | 511.48 ± 379.68 |
| β-amyloid/tau (NV > 1.6) | 2.02 ± 3.13 |
| Neurocognitive tests Mini Mental State Examination (MMSE < 24 denotes cognitive impairment) | 16.28 ± 6.53 |
| CTRL | AD Patients | p Value * | |
|---|---|---|---|
| Aspartic Acid | 1.38 ± 0.52 | 1.46 ± 0.95 | 0.38 |
| Glutamic Acid | 57.10 ± 18.66 | 57.41 ± 18.01 | 0.97 |
| Asparagine | 15.41 ± 3.46 | 3.20 ± 2.83 * | <0.0001 |
| Serine | 15.50 ± 5.18 | 8.00 ± 4.39 * | 0.0006 |
| Glutamine | 617.50 ± 100.74 | 248.15 ± 116.49 * | <0.0001 |
| Histidine | 9.85 ± 2.51 | 8.24 ± 3.02 | 0.13 |
| Glycine | 9.36 ± 5.01 | 4.05 ± 2.35 * | <0.0001 |
| Threonine | 38.23 ± 8.98 | 27.26 ± 15.46 * | 0.001 |
| Citrulline | 1.56 ± 1.30 | 1.53 ± 0.94 | 0.82 |
| Alanine | 59.02 ± 19.49 | 37.45 ± 15.92 * | 0.0008 |
| Arginine | 34.31 ± 11.09 | 17.84 ± 5.83 * | <0.0001 |
| Tyrosine | 13.02 ± 7.10 | 9.14 ± 3.68 * | 0.023 |
| Cysteine | 10.36 ± 4.23 | 8.23 ± 3.17 | 0.14 |
| Valine | 34.24 ± 14.33 | 19.84 ± 10.74 * | 0.0004 |
| Methionine | 4.55 ± 1.86 | 2.45 ± 1.43 * | 0.0004 |
| Tryptophan | 4.51 ± 1.88 | 2.06 ± 1.45 * | <0.0001 |
| Phenylalanine | 17.04 ± 4.16 | 10.33 ± 3.75 * | <0.0001 |
| Isoleucine | 11.93 ± 3.56 | 6.63 ± 3.66 * | <0.0001 |
| Leucine | 24.95 ± 8.17 | 14.16 ± 7.02 * | 0.0002 |
| Lysine | 32.23 ± 11.10 | 20.53 ± 6.73 * | 0.001 |
| Ornithine | 5.21 ± 3.70 | 4.40 ± 4.94 | 0.20 |
| Total AAs | 922.38 ± 307.78 | 504.71 ± 191.35 * | 0.0003 |
| EAAs | 184.78 ± 52.85 | 111.88 ± 46.25 * | <0.0001 |
| BCAAs | 63.21 ± 20.25 | 40.63 ± 21.25 * | 0.0005 |
| BCAAs/TAAs | 0.08 ± 0.04 | 0.08 ± 0.03 | 0.17 |
| EAAs/TAAs | 0.22 ± 0.09 | 0.23 ± 0.06 | 0.07 |
| BCAAs/EAAs | 0.34 ± 0.04 | 0.36 ± 0.06 | 0.48 |
| Trp ratio (%) | 0.05 ± 0.01 | 0.04 ± 0.03 * | 0.0003 |
| Controls | AD Patients | p Value * | |
|---|---|---|---|
| Aspartic Acid | 7.33 ± 2.40 | 7.47 ± 3.91 | 0.53 |
| Glutamic Acid | 89.89 ± 46.50 | 63.56 ± 18.81 * | 0.033 |
| Asparagine | 38.11 ± 6.74 | 39.84 ± 5.09 | 0.59 |
| Serine | 90.67 ± 22.63 | 99.31 ± 22.39 | 0.27 |
| Glutamine | 514.44 ± 136.72 | 514.09 ± 82.96 | 1.00 |
| Histidine | 47.89 ± 7.49 | 67.16 ± 11.99 * | 0.00019 |
| Glycine | 205.22 ± 66.65 | 226.47 ± 63.13 | 0.20 |
| Threonine | 101.33 ± 17.33 | 116.59 ± 25.06 | 0.19 |
| Citrulline | 30.11 ± 14.71 | 35.50 ± 12.10 | 0.31 |
| Alanine | 456.67 ± 113.67 | 340.22 ± 71.92 * | 0.004 |
| Arginine | 49.00 ± 16.17 | 64.75 ± 22.36 * | 0.042 |
| Tyrosine | 53.44 ± 12.84 | 51.81 ± 9.83 | 0.71 |
| Cysteine | na | na | na |
| Valine | 180.13 ± 43.68 | 203.23 ± 54.19 | 0.25 |
| Methionine | na | na | na |
| Tryptophan | 40.89 ± 6.45 | 43.59 ± 8.62 | 0.26 |
| Phenylalanine | 57.67 ± 9.70 | 51.16 ± 9.54 | 0.08 |
| Isoleucine | 64.44 ± 17.22 | 54.44 ± 15.34 * | 0.058 |
| Leucine | 128.67 ± 31.20 | 104.22 ± 27.79 * | 0.025 |
| Lysine | 208.22 ± 42.67 | 196.75 ± 33.61 | 0.61 |
| Ornithine | 87.11 ± 32.15 | 82.84 ± 23.78 | 0.73 |
| Total AAs | 2839.81 ± 412.48 | 2366.48 ± 315.21 * | 0.004 |
| EAAs | 1115.67 ± 175.91 | 876.98 ± 159.22 * | 0.002 |
| BCAAs | 373.24 ± 91.60 | 361.88 ± 96.20 | 0.74 |
| BCAAs/TAAs | 0.13 ± 0.04 | 0.15 ± 0.03 * | 0.036 |
| EAAs/TAAs | 0.39 ± 0.03 | 0.37 ± 0.03 | 0.057 |
| BCAAs/EAAs | 0.34 ± 0.09 | 0.41 ± 0.04 * | 0.001 |
| Trp ratio (%) | 0.09 ± 0.02 | 0.10 ± 0.02 | 0.39 |
| Liquor | CTRL | MNA Grp 1 | MNA Grp 2 | MNA Grp 3 | p Global | CTRL vs. MNA1 p * | CTRL vs. MNA2 p * | CTRL vs. MNA3 p * |
|---|---|---|---|---|---|---|---|---|
| Aspartic Acid | 1.38 ± 0.52 | 0.82 ± 0.11 * | 1.44 ± 1.17 | 1.76 ± 0.85 | 0.006 | 0.026 | 0.93 | 0.98 |
| Glutamic Acid | 57.10 ± 18.66 | 45.83 ± 15.08 | 49.61 ± 20.74 | 68.51 ± 9.16 | 0.011 | 0.68 | 0.85 | 0.38 |
| Asparagine | 15.41 ± 3.46 | 2.51 ± 1.42 * | 3.43 ± 3.90 * | 3.33 ± 2.34 * | <0.0001 | 0.002 | 0.00021 | 0.0006 |
| Serine | 15.50 ± 5.18 | 6.02 ± 1.97 * | 8.31 ± 5.46 * | 8.66 ± 4.19 | 0.005 | 0.013 | 0.043 | 0.053 |
| Glutamine | 617.50 ± 100.74 | 192.37 ± 106.76 * | 261.70 ± 116.83 * | 262.44 ± 121.35 * | <0.0001 | 0.00035 | 0.001 | 0.00042 |
| Hystidine | 9.85 ± 2.51 | 8.21 ± 4.14 | 7.89 ± 3.38 | 8.55 ± 2.25 | 0.39 | |||
| Glycine | 9.36 ± 5.01 | 3.13 ± 2.27 * | 4.42 ± 3.10 * | 4.17 ± 1.63 * | 0.0007 | 0.004 | 0.011 | 0.007 |
| Threonine | 38.23 ± 8.98 | 21.35 ± 11.25 * | 29.41 ± 21.68 * | 28.10 ± 10.97 | 0.008 | 0.023 | 0.049 | 0.11 |
| Citrulline | 1.56 ± 1.30 | 1.03 ± 0.56 | 1.40 ± 0.94 | 1.85 ± 1.01 | 0.18 | |||
| Alanine | 59.02 ± 19.49 | 36.33 ± 18.79 | 33.44 ± 17.63 * | 41.07 ± 13.49 | 0.002 | 0.13 | 0.002 | 0.24 |
| Arginine | 34.31 ± 11.09 | 15.36 ± 6.55 * | 17.83 ± 5.21 * | 18.91 ± 6.09 * | 0.0005 | 0.007 | 0.007 | 0.006 |
| Tyrosine | 13.02 ± 7.10 | 8.13 ± 4.07 | 9.68 ± 4.93 | 9.15 ± 2.33 | 0.12 | |||
| Cysteine | 10.36 ± 4.23 | 6.65 ± 2.49 | 8.25 ± 3.95 | 8.89 ± 2.68 | 0.19 | |||
| Valine | 34.24 ± 14.33 | 13.99 ± 7.67 * | 18.00 ± 11.62 * | 23.78 ± 10.22 | 0.00035 | 0.002 | 0.003 | 0.35 |
| Methionine | 4.55 ± 1.86 | 1.73 ± 0.99 * | 2.35 ± 1.66 * | 2.84 ± 1.34 | 0.002 | 0.006 | 0.011 | 0.12 |
| Tryptophan | 4.51 ± 1.88 | 1.44 ± 0.63 * | 1.94 ± 1.01 * | 2.42 ± 1.89 * | <0.0001 | 0.0008 | 0.002 | 0.005 |
| Phenylalanine | 17.04 ± 4.16 | 9.21 ± 4.82 * | 9.82 ± 4.33 * | 11.21 ± 2.73 * | 0.0006 | 0.010 | 0.002 | 0.042 |
| Isoleucine | 11.93 ± 3.56 | 4.96 ± 2.42 * | 5.52 ± 3.36 * | 8.21 ± 3.86 | <0.0001 | 0.002 | 0.00030 | 0.15 |
| Leucine | 24.95 ± 8.17 | 10.75 ±5.85 * | 12.77 ± 6.88 * | 16.73 ± 7.07 | 0.00024 | 0.002 | 0.002 | 0.18 |
| Lysine | 32.2 ±11.10 | 17.04 ±5.46 * | 19.37 ± 8.35 * | 22.93 ± 5.13 | 0.001 | 0.013 | 0.004 | 0.48 |
| Ornithine | 5.21 ± 3.70 | 3.89 ± 2.73 | 5.42 ± 7.92 | 3.77 ± 1.46 | 0.49 | |||
| Total AAs | 922.38 ± 307.78 | 411.28 ± 187.85 * | 513.10 ± 224.29 * | 538.16 ± 164.10 * | 0.002 | 0.006 | 0.017 | 0.07 |
| EAAs | 184.78 ± 52.85 | 89.47 ± 41.21 * | 108.15 ± 62.05 * | 124.42 ± 30.13 | 0.00018 | 0.001 | 0.001 | 0.11 |
| BCAAs | 63.21 ± 20.25 | 29.70 ± 15.79 * | 36.29 ± 21.75 * | 48.72 ± 20.99 | 0.00040 | 0.003 | 0.003 | 0.39 |
| BCAAs/TAAs | 0.08 ± 0.04 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.10 ± 0.04 | 0.17 | |||
| EAAs/TAAs | 0.22 ± 0.09 | 0.22 ± 0.02 | 0.21 ± 0.04 | 0.24 ± 0.07 | 0.16 | |||
| BCAAs/EAAs | 0.34 ± 0.04 | 0.33 ± 0.05 | 0.34 ± 0.04 | 0.38 ± 0.08 | 0.23 | |||
| Trp ratio (%) | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 * | 0.04 ± 0.05 * | 0.002 | 0.10 | 0.14 | 0.001 |
| Group 1 Malnourished | Group 2 At Risk | Group 3 Normonourished | Group 1 + 2 Combined | |
|---|---|---|---|---|
| CSF | 70 | 70 | 35 | 70 |
| Plasma | 0 | 0 | 5.2 | 26.3 |
| Group 1 Malnourished | Group 2 At risk | Group 3 Normonurished | Group 1 + 2 Combined | |
|---|---|---|---|---|
| CSF | - | Alanine | - | Alanine |
| Aspartate | - | - | - | |
| * Asparagine | * Asparagine | * Asparagine | Asparagine | |
| * Serine | * Serine | * Serine | Serine | |
| * Glutamine | * Glutamine | * Glutamine | Glutamine | |
| * Glycine | * Glycine | * Glycine | - | |
| Threonine | Threonine | - | Threonine | |
| * Arginine | * Arginine | * Arginine | Arginine | |
| Valine | Valine | - | Valine | |
| Methionine | Methionine | - | Methionine | |
| * Tryptophane | * Tryptophane | * Tryptophane | Tryptophane | |
| * Phenylalanine | * Phenylalanine | * Phenylalanine | Phenylalanine | |
| Isoleucine | Isoleucine | - | Isoleucine | |
| Leucine | Leucine | - | Leucine | |
| Lysine | Lysine | - | Lysine | |
| Total amino acids | Total amino acids | - | Total amino acids | |
| Essential amino acids | Essential amino acids | - | Essential amino acids | |
| Brain chain amino acids | Brain chain amino acids | - | Brain chain amino acids | |
| - | - | Trp/ratio |
| Plasma | CTRL | MNA Grp 1–2 | MNA Grp 3 | p Global | CTRL vs. MNA 1 + 2 p * | CTRL vs. MNA 3 p * | MNA1 + 2 vs. MNA 3 p ^ |
|---|---|---|---|---|---|---|---|
| Aspartic Acid | 7.33 ± 2.40 | 7.44 ± 4.43 | 7.50 ± 3.46 | 0.76 | |||
| Glutamic Acid | 89.89 ± 46.50 | 60.38 ± 18.28 * | 66.75 ± 19.38 | 0.051 | 0.044 | 0.38 | 0.57 |
| Asparagine | 38.11 ± 6.74 | 38.56 ± 5.16 | 41.13 ± 4.83 | 0.41 | |||
| Serine | 90.67 ± 22.63 | 99.31 ± 25.00 | 99.31 ± 20.27 | 0.53 | |||
| Glutamine | 514.44 ± 136.72 | 504.75 ± 79.54 | 523.44 ± 87.82 | 0.96 | |||
| Histidine | 47.89 ± 7.49 | 64.44 ± 10.78 * | 69.88 ± 12.85 * | 0.00047 | 0.010 | 0.00032 | 0.60 |
| Glycine | 205.22 ± 66.65 | 238.06 ± 79.17 | 214.88 ± 41.02 | 0.38 | |||
| Threonine | 101.33 ± 17.33 | 119.06 ± 25.61 | 114.13 ± 25.09 | 0.31 | |||
| Citrulline | 30.11 ± 14.71 | 33.25 ± 10.13 | 37.75 ± 13.76 | 0.49 | |||
| Alanine | 456.67 ± 113.67 | 345.13 ± 84.88 * | 335.31 ± 58.61 * | 0.016 | 0.026 | 0.030 | 1.00 |
| Arginine | 49.00 ± 16.17 | 63.94 ± 19.13 | 65.56 ± 25.80 | 0.12 | |||
| Tyrosine | 53.44 ± 12.84 | 54.50 ± 9.61 | 49.13 ± 9.58 | 0.38 | |||
| Cysteine | na | na | na | na | |||
| Valine | 180.13 ± 43.68 | 181.72 ± 49.84 | 224.74 ± 50.97 * | 0.025 | 1.00 | 0.10 | 0.042 |
| Methionine | na | na | na | na | |||
| Tryptophan | 40.89 ± 6.45 | 42.38 ± 10.42 | 44.81 ± 6.48 | 0.34 | |||
| Phenylalanine | 57.67 ± 9.70 | 51.44 ± 9.04 | 50.88 ± 10.31 | 0.15 | |||
| Isoleucine | 64.44 ± 17.22 | 48.88 ± 12.40 * | 60.00 ± 16.32 | 0.044 | 0.047 | 0.65 | 0.28 |
| Leucine | 128.67 ± 31.20 | 93.19 ± 25.56 * | 115.25 ± 26.14 * | 0.006 | 0.008 | 0.62 | 0.07 |
| Lysine | 208.22 ± 42.67 | 190.88 ± 38.90 | 202.63 ± 27.35 | 0.54 | |||
| Ornithine | 87.11 ± 32.15 | 81.56 ± 24.84 | 84.13 ±23.41 | 0.93 | |||
| Total AA | 2839.81 ± 412.48 | 2322.02 ± 363.07 * | 2410.94 ± 263.25 | 0.010 | 0.008 | 0.062 | 0.80 |
| EAAs | 1115.67 ± 175.91 | 830.53 ± 162.84 * | 923.42 ± 145.85 | 0.002 | 0.002 | 0.08 | 0.36 |
| BCAAs | 373.24 ± 91.60 | 323.78 ± 86.86 | 399.99 ±92.11 | 0.060 | |||
| BCAAs/TAAs | 0.13 ± 0.04 | 0.14 ± 0.02 | 0.16 ± 0.03 *^ | 0.004 | 0.81 | 0.008 | 0.027 |
| EAAs/TAAs | 0.39 ± 0.03 | 0.36 ± 0.03 * | 0.38 ± 0.03 | 0.017 | 0.025 | 0.78 | 0.10 |
| BCAAs/EAAs | 0.34 ± 0.09 | 0.39 ± 0.04 | 0.43 ± 0.04 *^ | 0.00027 | 0.15 | 0.00021 | 0.050 |
| Trp ratio (%) | 0.09 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.28 |
| Liquor | CTRL | MNA Grp1 + 2 | MNA Grp 3 | p Global | CTRL vs. MNA 1 + 2 p * | CTRL vs. MNA 3 p * | MNA 1 + 2 vs. MNA 3 p ^ |
|---|---|---|---|---|---|---|---|
| Aspartic Acid | 1.38 ± 0.52 | 1.22 ± 0.98 | 1.76 ± 0.85 ^ | 0.015 | 0.12 | 0.87 | 0.018 |
| Glutamic Acid | 57.10 ± 18.66 | 48.28 ± 18.53 | 68.51 ± 9.16 ^ | 0.004 | 0.38 | 0.21 | 0.003 |
| Asparagine | 15.41 ± 3.46 | 3.11 ± 3.22 * | 3.33 ± 2.34 * | <0.0001 | <0.0001 | 0.00029 | 0.95 |
| Serine | 15.50 ± 5.18 | 7.50 ± 4.60 * | 8.66 ± 4.19 * | 0.002 | 0.002 | 0.027 | 0.91 |
| Glutamine | 617.50 ± 100.74 | 237.23 ± 115.15 * | 262.44 ± 121.35 * | <0.0001 | <0.0001 | 0.00021 | 0.99 |
| Histidine | 9.85 ± 2.51 | 8.01 ± 3.54 | 8.55 ± 2.25 | 0.24 | |||
| Glycine | 9.36 ± 5.01 | 3.97 ± 2.83 * | 4.17 ± 1.63 * | 0.00027 | 0.00041 | 0.003 | 0.98 |
| Threonine | 38.23 ± 8.98 | 26.56 ± 18.68 * | 28.10 ± 10.97 | 0.003 | 0.003 | 0.056 | 0.79 |
| Citrulline | 1.56 ± 1.30 | 1.27 ± 0.83 | 1.85 ± 1.01 | 0.14 | |||
| Alanine | 59.02 ± 19.49 | 34.46 ± 17.51 * | 41.07 ± 13.49 | 0.0010 | 0.0006 | 0.13 | 0.28 |
| Arginine | 34.31 ± 11.09 | 16.96 ± 5.64 * | 18.91 ± 6.09 * | 0.00017 | 0.00027 | 0.003 | 0.95 |
| Tyrosine | 13.02 ± 7.10 | 9.14 ± 4.58 | 9.15 ± 2.33 | 0.07 | |||
| Cysteine | 10.36 ± 4.23 | 7.68 ± 3.51 | 8.89 ± 2.68 | 0.11 | |||
| Valine | 34.24 ± 14.33 | 16.59 ± 10.33 * | 23.78 ± 10.22 | 0.00012 | <0.0001 | 0.20 | 0.060 |
| Methionine | 4.55 ± 1.86 | 2.13 ± 1.45 * | 2.84 ± 1.34 | 0.0006 | 0.00039 | 0.060 | 0.41 |
| Tryptophan | 4.51 ± 1.88 | 1.76 ± 0.90 * | 2.42 ± 1.89 * | <0.0001 | <0.0001 | 0.002 | 0.74 |
| Phenylalanine | 17.04 ± 4.16 | 9.61 ± 4.37 * | 11.21 ± 2.73 * | 0.00018 | 0.00012 | 0.021 | 0.49 |
| Isoleucine | 11.93 ± 3.56 | 5.33 ± 3.00 * | 8.21 ± 3.86 | <0.0001 | <0.0001 | 0.08 | 0.061 |
| Leucine | 24.95 ± 8.17 | 12.05 ± 6.43 * | 16.73 ± 7.07 | <0.0001 | <0.0001 | 0.09 | 0.11 |
| Lysine | 32.23 ± 11.10 | 18.55 ± 7.36 * | 22.93 ± 5.13 | 0.00045 | 0.00029 | 0.28 | 0.08 |
| Ornithine | 5.21 ± 3.70 | 4.88 ± 6.49 | 3.77 ± 1.46 | 0.30 | |||
| Total AAs | 922.38 ± 307.78 | 477.16 ± 212.10 * | 538.16 ± 164.10 * | 0.0008 | 0.0006 | 0.038 | 0.61 |
| EAAs | 184.78 ± 52.85 | 101.56 ± 54.97 * | 124.42 ± 30.13 | <0.0001 | <0.0001 | 0.058 | 0.14 |
| BCAAs | 63.21 ± 20.25 | 33.97 ± 19.60 * | 48.72 ± 20.99 ^ | 0.00013 | <0.0001 | 0.22 | 0.055 |
| BCAAs/TAAs | 0.08 ± 0.04 | 0.07 ± 0.02 | 0.10 ± 0.04 | 0.09 | |||
| EAAs/TAAs | 0.22 ± 0.09 | 0.21 ± 0.03 | 0.24 ± 0.07 | 0.09 | |||
| BCAAs/EAAs | 0.34 ± 0.04 | 0.33 ± 0.04 | 0.38 ± 0.08 | 0.12 | |||
| Trp ratio (%) | 0.05 ± 0.01 | 0.03 ± 0.01 * | 0.04 ± 0.05 * | 0.0007 | 0.018 | 0.0007 | 0.60 |
| Variable | CTRL | AD | p * |
|---|---|---|---|
| Aspartic Acid | 0.17 ± 0.05 | 0.24 ± 0.16 | 0.27 |
| Glutamic Acid | 0.93 ± 0.81 | 0.98 ± 0.43 | 0.27 |
| Asparagine | 0.43 ± 0.09 | 0.08 ± 0.07 * | <0.0001 |
| Serine | 0.19 ± 0.05 | 0.08 ± 0.04 * | 0.00022 |
| Glutamine | 1.34 ± 0.36 | 0.50 ± 0.27 * | <0.0001 |
| Histidine | 0.21 ± 0.05 | 0.13 ± 0.06 * | 0.002 |
| Glycine | 0.04 ± 0.02 | 0.02 ± 0.01 * | <0.0001 |
| Threonine | 0.42 ± 0.11 | 0.23 ± 0.11 * | 0.0006 |
| Citrulline | 0.05 ± 0.03 | 0.05 ± 0.03 | 0.80 |
| Alanine | 0.16 ± 0.05 | 0.11 ± 0.04 * | 0.015 |
| Arginine | 0.76 ± 0.28 | 0.30 ± 0.14 * | <0.0001 |
| Tyrosine | 0.25 ± 0.08 | 0.17 ± 0.06 * | 0.029 |
| Cysteine | na | na | na |
| Valine | 0.19 ± 0.06 | 0.10 ± 0.04 * | 0.00011 |
| Methionine | na | na | na |
| Tryptophan | 0.10 ± 0.03 | 0.05 ± 0.03 * | 0.00023 |
| Phenylalanine | 0.32 ± 0.08 | 0.20 ± 0.08 * | 0.001 |
| Isoleucine | 0.22 ± 0.11 | 0.12 ± 0.05 * | 0.001 |
| Leucine | 0.22 ± 0.09 | 0.14 ± 0.06 * | 0.003 |
| Lysine | 0.18 ± 0.07 | 0.11 ± 0.04 * | 0.003 |
| Ornithine | 0.05 ± 0.05 | 0.05 ± 0.05 | 0.88 |
| Total AAs | 0.38 ± 0.08 | 0.22 ± 0.08 * | 0.00017 |
| EAAs | 0.18 ± 0.05 | 0.13 ± 0.05 * | 0.007 |
| BCAAs | 0.18 ± 0.05 | 0.11 ± 0.05 * | 0.0010 |
| BCAAs/TAAs | 0.48 ± 0.09 | 0.54 ± 0.17 | 0.64 |
| EAAs/TAAs | 0.48 ± 0.05 | 0.61 ± 0.14 * | 0.002 |
| BCAAs/EAAs | 1.02 ± 0.18 | 0.87 ± 0.11 * | 0.006 |
| Trp ratio (%) | 0.51 ± 0.16 | 0.40 ± 0.36 * | 0.008 |
| Variable | CTRL | MNA Grp 1 | MNA Grp 2 | MNA Grp 3 | p Global | CTRL vs. MNA 1 p * | CTRL vs. MNA 2 p * | CTRL vs. MNA 3 p * |
|---|---|---|---|---|---|---|---|---|
| Aspartic Acid | 0.17 ± 0.05 | 0.14 ± 0.08 | 0.23 ± 0.20 | 0.28 ± 0.13 | 0.055 | 0.99 | 1.00 | 0.24 |
| Glutamic Acid | 0.93 ± 0.81 | 0.80 ± 0.41 | 0.88 ± 0.40 | 1.13 ± 0.43 | 0.21 | |||
| Asparagine | 0.43 ± 0.09 | 0.06 ± 0.03 * | 0.09 ± 0.10 * | 0.08 ± 0.06 * | 0.00042 | 0.004 | 0.002 | 0.002 |
| Serine | 0.19 ± 0.05 | 0.06 ± 0.03 * | 0.09 ± 0.05 * | 0.09 ± 0.03 * | 0.002 | 0.006 | 0.013 | 0.015 |
| Glutamine | 1.34 ± 0.36 | 0.32 ± 0.13 * | 0.54 ± 0.28 * | 0.54 ± 0.28 * | 0.0005 | 0.002 | 0.005 | 0.005 |
| Histidine | 0.21 ± 0.05 | 0.11 ± 0.06 | 0.14 ± 0.08 * | 0.13 ± 0.04 * | 0.015 | 0.052 | 0.041 | 0.047 |
| Glycine | 0.04 ± 0.02 | 0.01 ± 0.01 * | 0.02 ± 0.01 * | 0.02 ± 0.01 * | 0.0010 | 0.005 | 0.005 | 0.007 |
| Threonine | 0.42 ± 0.11 | 0.16 ± 0.08 * | 0.24 ± 0.13 * | 0.25 ± 0.09 * | 0.003 | 0.004 | 0.028 | 0.038 |
| Citrulline | 0.05 ± 0.03 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.04 | 0.18 | |||
| Alanine | 0.16 ± 0.05 | 0.09 ± 0.04 | 0.10 ± 0.04 * | 0.13 ± 0.04 | 0.019 | 0.11 | 0.030 | 0.72 |
| Arginine | 0.76 ± 0.28 | 0.22 ± 0.12 * | 0.30 ± 0.09 * | 0.33 ± 0.17 * | 0.00043 | 0.002 | 0.003 | 0.004 |
| Tyrosine | 0.25 ± 0.08 | 0.13 ± 0.05 * | 0.18 ± 0.08 | 0.18 ± 0.04 | 0.043 | 0.028 | 0.47 | 0.54 |
| Cysteine | na | na | na | na | na | |||
| Valine | 0.19 ± 0.06 | 0.07 ± 0.03 * | 0.10 ± 0.06 * | 0.11 ± 0.03 * | 0.0007 | 0.001 | 0.007 | 0.013 |
| Methionine | na | na | na | na | na | |||
| Tryptophan | 0.10 ± 0.03 | 0.03 ± 0.01 * | 0.05 ± 0.03 * | 0.05 ± 0.04 * | 0.002 | 0.004 | 0.017 | 0.012 |
| Phenylalanine | 0.32 ± 0.08 | 0.14 ± 0.06 * | 0.21 ± 0.09 * | 0.22 ± 0.06 | 0.003 | 0.002 | 0.042 | 0.13 |
| Isoleucine | 0.22 ± 0.11 | 0.09 ± 0.04 * | 0.12 ± 0.07 * | 0.14 ± 0.04 | 0.002 | 0.005 | 0.010 | 0.21 |
| Leucine | 0.22 ± 0.09 | 0.10 ± 0.05 * | 0.14 ± 0.07 | 0.14 ± 0.04 | 0.017 | 0.026 | 0.064 | 0.12 |
| Lysine | 0.18 ± 0.07 | 0.09 ± 0.04 * | 0.11 ± 0.05 * | 0.11 ± 0.02 | 0.017 | 0.028 | 0.050 | 0.14 |
| Ornithine | 0.05 ± 0.05 | 0.04 ± 0.03 | 0.06 ± 0.07 | 0.05 ± 0.02 | 0.98 | |||
| Total AAs | 0.38 ± 0.08 | 0.16 ± 0.07 * | 0.23 ± 0.10 * | 0.23 ± 0.08 * | 0.001 | 0.003 | 0.017 | 0.009 |
| EAAs | 0.18 ± 0.05 | 0.10 ± 0.05 * | 0.13 ± 0.07 | 0.14 ± 0.03 | 0.023 | 0.024 | 0.11 | 0.25 |
| BCAAs | 0.18 ± 0.05 | 0.08 ± 0.04 * | 0.12 ± 0.06 * | 0.12 ± 0.04 | 0.005 | 0.007 | 0.024 | 0.08 |
| BCAAs/TAAs | 0.48 ± 0.09 | 0.50 ± 0.06 | 0.53 ± 0.14 | 0.57 ± 0.22 | 0.85 | |||
| EAAs/TAAs | 0.48 ± 0.05 | 0.60 ± 0.07 | 0.60 ± 0.11 | 0.63 ± 0.18 * | 0.023 | 0.13 | 0.070 | 0.030 |
| BCAAs/EAAs | 1.02 ± 0.18 | 0.83 ± 0.03 | 0.88 ± 0.10 | 0.89 ± 0.14 | 0.044 | 0.11 | 0.09 | 0.15 |
| Trp ratio (%) | 0.51 ± 0.16 | 0.35 ± 0.09 | 0.35 ± 0.13 | 0.45 ± 0.52 * | 0.059 | 0.45 | 0.23 | 0.046 |
| Variable | Spearman r | Pearson r |
|---|---|---|
| Aspartic Acid | 0.19 (0.33) | 0.07 (0.73) |
| Glutamic Acid | 0.20 (0.30) | 0.09 (0.63) |
| Asparagine | −0.16 (0.41) | −0.16 (0.42) |
| Serine | −0.24 (0.23) | −0.19 (0.33) |
| Glutamine | −0.26 (0.19) | −0.24 (0.22) |
| Histidine | −0.28 (0.15) | −0.31 (0.11) |
| Glycine | −0.19 (0.33) | −0.26 (0.18) |
| Threonine | −0.14 (0.45) | −0.22 (0.26) |
| Citrulline | −0.15 (0.44) | −0.11 (0.58) |
| Alanine | −0.11 (0.56) | −0.15 (0.45) |
| Arginine | −0.40 (0.031) | −0.52 (0.004) |
| Tyrosine | −0.02 (0.93) | −0.22 (0.24) |
| Cysteine | −0.17 (0.37) | −0.22 (0.25) |
| Valine | 0.09 (0.65) | −0.05 (0.79) |
| Methionine | 0.02 (0.93) | −0.01 (0.96) |
| Tryptophan | −0.08 (0.67) | 0.25 (0.19) |
| Phenylalanine | −0.04 (0.83) | −0.17 (0.38) |
| Isoleucine | 0.09 (0.64) | −0.01 (0.94) |
| Leucine | 0.03 (0.87) | −0.08 (0.67) |
| Lysine | −0.07 (0.71) | −0.22 (0.25) |
| Ornithine | −0.24 (0.22) | −0.21 (0.28) |
| Total AAs | −0.21 (0.27) | −0.27 (0.16) |
| EAAs | −0.00 (1.00) | −0.17 (0.37) |
| BCAAs | 0.07 (0.73) | −0.06 (0.78) |
| BCAAs/TAAs | 0.32 (0.09) | 0.28 (0.14) |
| EAAs/TAAs | 0.22 (0.25) | 0.24 (0.21) |
| BCAAs/EAAs | 0.38 (0.045) | 0.26 (0.17) |
| Trp ratio (%) | −0.33 (0.09) | 0.33 (0.08) |
| tau protein (NV < 404 pg/mL) | 0.19 (0.37) | 0.00 (1.00) |
| p-tau (NV < 56.5 pg/mL) | 0.22 (0.32) | 0.08 (0.72) |
| β-amyloid (NV > 599 pg/mL) | 0.28 (0.18) | 0.11 (0.62) |
| β-amyloid/tau (NV > 1.6) | 0.05 (0.83) | 0.06 (0.76) |
| Variable | Spearman r | Pearson r |
|---|---|---|
| Aspartic Acid | 0.07 (0.73) | 0.00 (0.99) |
| Glutamic Acid | 0.18 (0.34) | 0.13 (0.50) |
| Asparagine | 0.09 (0.65) | 0.10 (0.61) |
| Serine | 0.12 (0.53) | 0.22 (0.26) |
| Glutamine | −0.37 (0.049) | −0.31 (0.10) |
| Histidine | −0.30 (0.11) | −0.33 (0.08) |
| Glycine | 0.13 (0.49) | 0.18 (0.34) |
| Threonine | 0.14 (0.46) | 0.29 (0.13) |
| Citrulline | 0.09 (0.62) | 0.27 (0.16) |
| Alanine | 0.25 (0.18) | 0.21 (0.27) |
| Arginine | 0.14 (0.46) | 0.21 (0.27) |
| Tyrosine | 0.21 (0.26) | 0.24 (0.20) |
| Cysteine | na | na |
| Valine | 0.35 (0.055) | 0.37 (0.044) |
| Methionine | na | na |
| Tryptophan | −0.07 (0.72) | 0.02 (0.92) |
| Phenylalanine | −0.05 (0.81) | −0.04 (0.82) |
| Isoleucine | 0.34 (0.067) | 0.46 (0.011) |
| Leucine | 0.32 (0.09) | 0.40 (0.027) |
| Lysine | 0.10 (0.59) | 0.04 (0.83) |
| Ornithine | −0.18 (0.36) | 0.18 (0.36) |
| Total AAs | −0.12 (0.53) | −0.12 (0.51) |
| EAAs | 0.17 (0.36) | 0.10 (0.59) |
| BCAAs | 0.36 (0.054) | 0.40 (0.030) |
| BCAAs/TAAs | 0.49 (0.006) | 0.56 (0.001) |
| EAAs/TAAs | 0.40 (0.028) | 0.37 (0.047) |
| BCAAs/EAAs | 0.58 (0.001) | 0.72 (<0.0001) |
| Trp ratio (%) | 0.03 (0.90) | −0.01 (0.95) |
| Variable | Spearman r | Pearson r |
|---|---|---|
| Aspartic Acid | −0.01 (0.99) | −0.13 (0.76) |
| Glutamic Acid | −0.23 (0.59) | −0.23 (0.59) |
| Asparagine | 0.45 (0.27) | 0.36 (0.39) |
| Serine | 0.96 (0.0007) | 0.80 (0.018) |
| Glutamine | 0.13 (0.76) | 0.41 (0.32) |
| Histidine | −0.24 (0.58) | −0.19 (0.65) |
| Glycine | 0.14 (0.75) | 0.56 (0.15) |
| Threonine | 0.17 (0.70) | 0.14 (0.75) |
| Citrulline | 0.31 (0.46) | 0.30 (0.47) |
| Alanine | −0.21 (0.62) | −0.00 (0.99) |
| Arginine | 0.57 (0.15) | 0.62 (0.10) |
| Tyrosine | 0.00 (1.00) | 0.15 (0.72) |
| Cysteine | na | na |
| Valine | 0.74 (0.046) | 0.61 (0.10) |
| Methionine | na | na |
| Tryptophan | −0.10 (0.84) | 0.00 (1.00) |
| Phenylalanine | −0.29 (0.50) | −0.08 (0.86) |
| Isoleucine | 0.17 (0.70) | 0.01 (0.98) |
| Leucine | 0.10 (0.84) | 0.24 (0.57) |
| Lysine | −0.24 (0.58) | 0.07 (0.88) |
| Ornithine | 0.14 (0.75) | 0.06 (0.90) |
| Total AA | 0.29 (0.50) | 0.24 (0.56) |
| EAAs | −0.05 (0.93) | 0.08 (0.85) |
| BCAAs | 0.69 (0.069) | 0.48 (0.23) |
| BCAAs/TAAs | 0.90 (0.005) | 0.50 (0.21) |
| EAAs/TAAs | 0.38 (0.36) | 0.51 (0.20) |
| BCAAs/EAAs | 0.60 (0.13) | 0.32 (0.44) |
| Trp ratio (%) | 0.62 (0.11) | 0.53 (0.18) |
| Reduced CSF AAs | Effects on Brain Metabolism | Effects on Brain Activities |
|---|---|---|
| Serine | Reduced NMDA glutamate receptor | Reduced formation of new synapses |
| Reduced protein–serine kinase | Reduced enzyme synthesis of neurotransmitter biosynthesis and degradation | |
| Glycine | Reduced activity inhibition | Contribution to synaptic and brain toxicity |
| Asparagine | Reduced stimulation of ornithine decarboxylase | Reduced urea cycle activity |
| Glutamine | Reduced astrocyte detoxification of ammonia Aβ reduced autophagy Difficulties in maintaining synaptic transmission | Hyperammonia Maintenance of altered mitochondria Contribution to reducing synaptic transmission |
| Arginine | Disruption of urea cycle Elevated production of NO, nitrosative stress | Hyperammonia Mitochondrial dysfunction Degeneration of synapses and neurons Endothelial dysfunction Reduced synthesis of creatine Increased energy deficit |
| Tryptophan | Reduced serotonin formation | Reduced serotoninergic neurotransmission Contribution to AD pathogenesis, severity, cognitive impairments |
| Phenylalanine | Reduced synthesis of dopamine, norepinephrine, epinephrine, tyramine | Reduced catecholaminergic neurotransmission Impairments in behavioural and cognitive functioning Increased perception of fatigue |
| BCAAs | Impairment in glutamate turnover Impairment in regulation of glutamate, glutamine, GABA Reduced protein synthesis | Increased neurotoxicity Unbalanced excitatory/inhibitory ratio Reduced processes of repairing, sprouting, circuity remodelling |
| Lysine | Reduced protein synthesis Reduced autoproteolytic activity Reduced formation of pipecolic acid | Increased catabolic activity Reduced modulation of GABAergic transmission |
| Threonine | Reduced protein synthesis Reduced protein threonine kinase | Increased catabolic activity Reduced synthesis of enzymes for neurotransmitter biosynthesis and degradation, neurotransmitter receptors and transporters, ion channels Reduced neural plasticity |
| Methionine | Reduced methyl production (methylation) | Reduced initiation of protein synthesis Reduced protein protection (e.g. myelin) Reduced formation of creatine, carnitine, melatonin, and polyamines, reduced metabolism of serotonin Reduced formation of cysteine and hence glutathione formation |
| Alanine | Reduced transamination process | Reduced energy generation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquilani, R.; Costa, A.; Maestri, R.; Cotta Ramusino, M.; Perini, G.; Boselli, M.; Iadarola, P.; Buonocore, D.; Verri, M.; Dossena, M.; et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients 2022, 14, 1872. https://doi.org/10.3390/nu14091872
Aquilani R, Costa A, Maestri R, Cotta Ramusino M, Perini G, Boselli M, Iadarola P, Buonocore D, Verri M, Dossena M, et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients. 2022; 14(9):1872. https://doi.org/10.3390/nu14091872
Chicago/Turabian StyleAquilani, Roberto, Alfredo Costa, Roberto Maestri, Matteo Cotta Ramusino, Giulia Perini, Mirella Boselli, Paolo Iadarola, Daniela Buonocore, Manuela Verri, Maurizia Dossena, and et al. 2022. "Is the Brain Undernourished in Alzheimer’s Disease?" Nutrients 14, no. 9: 1872. https://doi.org/10.3390/nu14091872
APA StyleAquilani, R., Costa, A., Maestri, R., Cotta Ramusino, M., Perini, G., Boselli, M., Iadarola, P., Buonocore, D., Verri, M., Dossena, M., & Boschi, F. (2022). Is the Brain Undernourished in Alzheimer’s Disease? Nutrients, 14(9), 1872. https://doi.org/10.3390/nu14091872









