Effect of Catechins on Upper Respiratory Tract Infections in Winter: A Randomized, Placebo-Controlled, Double-Blinded Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Test Beverages
2.3. Study Design
2.4. Study Outcome
2.5. Measurements of Duration of URTIs and Severity of Symptoms
2.6. Physical Symptom Assessment
2.7. Statistical Analysis
3. Results
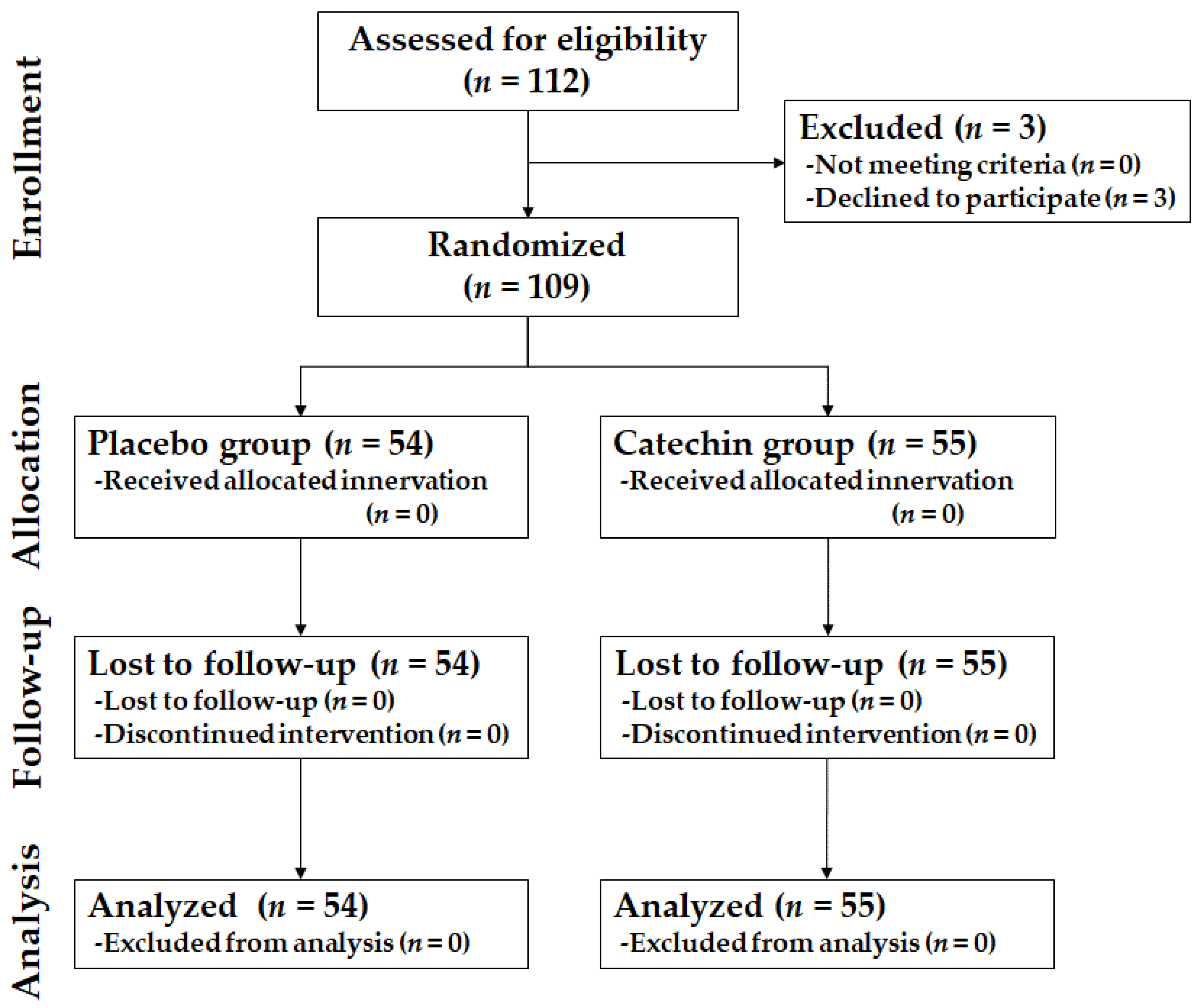
3.1. Participants and Baseline Characteristics
3.2. Effect of Catechins on Duration of URTIs and Severity of Symptoms
3.3. Effect of Catechins on Physical Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, D.L.; Finelli, L.; Bridges, C.B. 2009 H1N1 Influenza Pandemic: Field and Epidemiologic Investigations in the United States at the Start of the First Pandemic of the 21st Century. Clin. Infect. Dis. 2011, 52 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Writing Committee of the WHO CoCAoPI; Bautista, E.; Chotpitayasunondh, T.; Gao, Z.; Harper, S.A.; Shaw, M. Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. N. Engl. J. Med. 2010, 362, 1708–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, H.; Wang, X.; Yuan, X.; Xiao, G.; Wang, C.; Deng, T.; Yuan, Q.; Xiao, X. The Epidemiology and Clinical Information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1011–1019. [Google Scholar] [CrossRef]
- Talaat, M.; Afifi, S.; Dueger, E.; El-Ashry, N.; Marfin, A.; Kandeel, A.; Mohareb, E.; El-Sayed, N. Effects of Hand Hygiene Campaigns on Incidence of Laboratory-Confirmed Influenza and Absenteeism in Schoolchildren, Cairo, Egypt. Emerg. Infect. Dis. 2011, 17, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Yamada, H.; Matsushita, K.; Kaji, S.; Goto, T.; Okada, Y.; Kosuge, K.; Kitagawa, T. Green Tea Consumption Is Inversely Associated with the Incidence of Influenza Infection among Schoolchildren in a Tea Plantation Area of Japan. J. Nutr. 2011, 141, 1862–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.A.; Nantz, M.P.; Bukowski, J.F.; Percival, S.S. Specific Formulation of Camellia sinensis Prevents Cold and Flu Symptoms and Enhances Gamma, Delta T Cell Function: A Randomized, Double-Blind, Placebo-Controlled Study. J. Am. Coll. Nutr. 2007, 26, 445–452. [Google Scholar] [CrossRef]
- Furushima, D.; Nishimura, T.; Takuma, N.; Iketani, R.; Mizuno, T.; Matsui, Y.; Yamaguchi, T.; Nakashima, Y.; Yamamoto, S.; Hibi, M.; et al. Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeda, M.; Tominaga, T.; Kozuma, K.; Kitazawa, H.; Furushima, D.; Hibi, M.; Yamada, H. Preventive Effects of Tea and Tea Catechins against Influenza and Acute Upper Respiratory Tract Infections: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 4189–4202. [Google Scholar] [CrossRef]
- Pasrija, D.; Anandharamakrishnan, C. Techniques for Extraction of Green Tea Polyphenols: A Review. Food Bioprocess Technol. 2015, 8, 935–950. [Google Scholar] [CrossRef]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a Tea Rich in Catechins Leads to a Reduction in Body Fat and Malondialdehyde-Modified LDL in Men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-Epimerized Tea Catechins Rich in Gallocatechin Gallate and Catechin Gallate Are More Effective to Inhibit Cholesterol Absorption than Tea Catechins Rich in Epigallocatechin Gallate and Epicatechin Gallate. J. Agric. Food Chem. 2003, 51, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin Gallate (GCG) Inhibits 3T3-L1 Differentiation and Lipopolysaccharide Induced Inflammation through MAPK and NF-κB Signaling. J. Funct. Foods. 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kobayashi, M.; Matsuda, Y.; Ojika, M.; Shigeoka, S.; Yamamoto, Y.; Tou, Y.; Inoue, T.; Katagiri, T.; Murai, A.; et al. Coffee and Caffeine Ameliorate Hyperglycemia, Fatty Liver, and Inflammatory Adipocytokine Expression in Spontaneously Diabetic KK-Ay Mice. J. Agric. Food Chem. 2010, 58, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kanayama, M.; Haida, M.; Fujimoto, S.; Oroguchi, T.; Sata, K.; Mita, N.; Kutsuzawa, T.; Ikeuchi, M.; Kondo, M.; et al. Lactococcus lactis JCM5805 Activates Anti-Viral Immunity and Reduces Symptoms of Common Cold and Influenza in Healthy Adults in a Randomized Controlled Trial. J. Funct. Foods. 2016, 24, 492–500. [Google Scholar] [CrossRef]
- Fujii, T.; Jounai, K.; Horie, A.; Takahashi, H.; Suzuki, H.; Ohshio, K.; Fujiwara, D.; Yamamoto, N. Effects of Heat-Killed Lactococcus lactis subsp. lactis JCM 5805 on Mucosal and Systemic Immune Parameters, and Antiviral Reactions to Influenza Virus in Healthy Adults; a Randomized Controlled Double-Blind Study. J. Funct. Foods. 2017, 35, 513–521. [Google Scholar] [CrossRef]
- Kostka, T.; Praczko, K. Interrelationship between Physical Activity, Symptomatology of Upper Respiratory Tract Infections, and Depression in Elderly People. Gerontology. 2007, 53, 187–193. [Google Scholar] [CrossRef]
- Miyachi, H.; Wake, H.; Tamaki, K.; Mitsuhashi, A.; Ikeda, T.; Inoue, K.; Tanaka, S.; Tanaka, K.; Miyaoka, H. Detecting Mental Disorders in Dental Patients with Occlusion-Related Problems. Psychiatry Clin. Neurosci. 2007, 61, 313–319. [Google Scholar] [CrossRef]
- Iketani, R.; Furushima, D.; Nishimura, T.; Hirama, Y.; Onishi, S.; Kanbara, H.; Mori, T.; Ota, N.; Ohno, Y.; Yamada, H. The Effect of Tea Catechins on Natural Killer Cell Activity in the Elderly: A Pilot Study. Rinsho Yakuri/Jpn. J. Clin. Pharmacol. Ther. 2019, 50, 139–145. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chen, T.S.; Liou, S.Y.; Hsieh, C.C. Immunomodulatory Effects of EGCG Fraction of Green Tea Extract in Innate and Adaptive Immunity via T Regulatory Cells in Murine Model. Immunopharmacol. Immunotoxicol. 2014, 36, 364–370. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.J.; Yang, I.; Buckley, B.; Yang, C.S. Human Urinary Metabolite Profile of Tea Polyphenols Analyzed by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry with Data-Dependent Acquisition. Rapid Commun. Mass Spectrom. 2008, 22, 1567–1578. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4(+) T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- van der Hooft, J.J.J.; de Vos, R.C.H.; Mihaleva, V.; Bino, R.J.; Ridder, L.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.P.; Vervoort, J. Structural Elucidation and Quantification of Phenolic Conjugates Present in Human Urine after Tea Intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Biotransformation of (−)-Epigallocatechin and (−)-Gallocatechin by Intestinal Bacteria Involved in Isoflavone Metabolism. Biol. Pharm. Bull. 2015, 38, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monobe, M.; Ema, K.; Tokuda, Y.; Maeda-Yamamoto, M. Effect on the Epigallocatechin Gallate/Epigallocatechin Ratio in a Green Tea (Camellia sinensis L.) Extract of Different Extraction Temperatures and Its Effect on IgA Production in Mice. Biosci. Biotechnol. Biochem. 2010, 74, 2501–2503. [Google Scholar] [CrossRef] [Green Version]
- Colpitts, C.C.; Schang, L.M. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; Okamoto, M.; Dapat, I.; Katumi, M.; Oshitani, H. Inactivation of SARS-CoV-2 by Catechins from Green Tea. Jpn. J. Infect. Dis. 2021, 74, 421–423. [Google Scholar] [CrossRef]
- Yamauchi, T.; Takeuchi, S.; Uchida, M.; Saito, M.; Kokaze, A. The Association between the Dynamics of COVID-19, Related Measures, and Daytime Population in Tokyo. Sci. Rep. 2022, 12, 3063. [Google Scholar] [CrossRef]
- Arai, Y.; Oguma, Y.; Abe, Y.; Takayama, M.; Hara, A.; Urushihara, H.; Takebayashi, T. Behavioral Changes and Hygiene Practices of Older Adults in Japan During the First Wave of COVID-19 Emergency. BMC Geriatr. 2021, 21, 137. [Google Scholar] [CrossRef]
- Álvarez, P.; Alvarado, C.; Mathieu, F.; Jiménez, L.; De la Fuente, M. Diet Supplementation for 5 Weeks with Polyphenol-Rich Cereals Improves Several Functions and the Redox State of Mouse Leucocytes. Eur. J. Nutr. 2006, 45, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Pae, M.; Ren, Z.; Meydani, M.; Shang, F.; Smith, D.; Meydani, S.N.; Wu, D. Dietary Supplementation with High Dose of Epigallocatechin-3-Gallate Promotes Inflammatory Response in Mice. J. Nutr. Biochem. 2012, 23, 526–531. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Kumari, A.; Kulurkar, P.M.; Raj, R.; Gulati, A.; Padwad, Y.S. Consumption of Green Tea Epigallocatechin-3-Gallate Enhances Systemic Immune Response, Antioxidative Capacity and HPA Axis Functions in Aged Male Swiss Albino Mice. Biogerontology 2017, 18, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kinouchi Shimizu, N.; Hakamata, W.; Unno, K.; Asai, T.; Oku, N. Preventive Effect of Green Tea Catechins on Experimental Tumor Metastasis in Senescence-Accelerated Mice. Biol. Pharm. Bull. 2010, 33, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Catechin Beverage | Placebo Beverage | |
|---|---|---|
| Epigallocatechin gallate (mg) | 112 | 0 |
| Epicatechin gallate (mg) | 36 | 0 |
| Epigallocatechin (mg) | 107 | 0 |
| Epicatechin (mg) | 33 | 0 |
| Gallocatechin gallate (mg) | 71 | 0 |
| Catechin gallate (mg) | 15 | 0 |
| Gallocatechin (mg) | 94 | 0 |
| Catechin (mg) | 22 | 0 |
| Total catechins (mg) | 490 | 0 |
| Caffeine (mg) | 22 | 21 |
| Variables | Catechin | Placebo | p-Value | |
|---|---|---|---|---|
| Participants | n | 55 | 54 | |
| Women proportion *,a | % | 49.1 | 50.0 | 0.9244 |
| Age *,b | 44.8 ± 13.3 | 44.2 ± 13.7 | 0.8438 | |
| Smoking rate *,a | yes, % | 5.5 | 9.3 | 0.6934 |
| Exercise habits “yes” *,a | yes, % | 45.5 | 55.6 | 0.3881 |
| Green tea intake “more than twice per week” *,a | yes, % | 47.3 | 57.4 | 0.3857 |
| History of influenza over the past 3–4 years *,a | yes, % | 21.8 | 16.7 | 0.6607 |
| Proportion of those living alone *,a | % | 32.7 | 25.9 | 0.5692 |
| Proportion of those under the age of 15 years *,a | % | 32.7 | 25.9 | 0.5692 |
| Item | Group | n | Score * | p-Value # |
|---|---|---|---|---|
| Somatic symptoms | Catechin | 55 | 1.1 ± 1.4 | 0.8047 |
| Placebo | 54 | 1.1 ± 1.6 | ||
| Anxiety/insomnia | Catechin | 55 | 1.7 ± 1.9 | 0.1772 |
| Placebo | 54 | 1.3 ± 1.7 | ||
| Social dysfunction | Catechin | 55 | 0.4 ± 1.2 | 0.5915 |
| Placebo | 54 | 0.5 ± 1.2 | ||
| Severe depression | Catechin | 55 | 0.3 ± 0.8 | 0.3475 |
| Placebo | 54 | 0.1 ± 0.4 | ||
| Total score | Catechin | 55 | 8.7 ± 8.7 | 0.1362 |
| Placebo | 54 | 7.1 ± 8.9 |
| Symptoms | Group | n | Normal, Slight, Mild | Moderate, Severe | p-Value | |
|---|---|---|---|---|---|---|
| Chill | Catechin | 55 | 4606 | 14 | 0.1047 | |
| Placebo | 54 | 4512 | 24 | |||
| Feverishness | Catechin | 55 | 4614 | 6 | 0.2890 | |
| Placebo | 54 | 4534 | 2 | |||
| Running nose | Catechin | 55 | 4611 | 9 | 0.0127 | # |
| Placebo | 54 | 4513 | 23 | |||
| Articular pain | Catechin | 55 | 4619 | 1 | 0.0678 | |
| Placebo | 54 | 4530 | 6 | |||
| Nasal congestion | Catechin | 55 | 4616 | 4 | 0.0181 | # |
| Placebo | 54 | 4522 | 14 | |||
| Sore throat | Catechin | 55 | 4614 | 6 | 0.1250 | |
| Placebo | 54 | 4535 | 1 | |||
| Cough | Catechin | 55 | 4619 | 1 | 1.0000 | |
| Placebo | 54 | 4536 | 0 | |||
| Headache | Catechin | 55 | 4613 | 7 | <0.0001 | ### |
| Placebo | 54 | 4504 | 31 | |||
| Variables | Criteria |
|---|---|
| Nasopharyngeal symptoms | For the symptoms “running nose” and “nasal congestion”, the total number of “normal”, “slight”, or “mild” days and the total number of “moderate” or “severe” days were tabulated. |
| Hypo-pharyngeal symptoms | For the symptoms “sore throat” and “cough”, the total number of “normal”, “slight”, or “mild” days, and the total number of “moderate” or “severe” days were tabulated. |
| Systemic symptoms | For the symptoms “headache”, “chill”, “general malaise”, “feverishness”, and “articular pain”, the total number of “normal”, “slight”, or “mild” days and the total number of “moderate” or “severe” days were tabulated. |
| Symptoms | Group | n | Normal, Slight, Mild | Moderate, Severe | p-Value | |
|---|---|---|---|---|---|---|
| Nasopharyngeal symptoms | Catechin | 55 | 9227 | 13 | 0.0006 | ### |
| Placebo | 54 | 9035 | 37 | |||
| Hypo-pharyngeal symptoms | Catechin | 55 | 9233 | 7 | 0.0703 | |
| Placebo | 54 | 9071 | 1 | |||
| Systemic symptoms | Catechin | 55 | 23,043 | 57 | 0.1158 | |
| Placebo | 54 | 22,605 | 74 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozato, N.; Yamaguchi, T.; Kusaura, T.; Kitazawa, H.; Hibi, M.; Osaki, N.; Ono, T. Effect of Catechins on Upper Respiratory Tract Infections in Winter: A Randomized, Placebo-Controlled, Double-Blinded Trial. Nutrients 2022, 14, 1856. https://doi.org/10.3390/nu14091856
Ozato N, Yamaguchi T, Kusaura T, Kitazawa H, Hibi M, Osaki N, Ono T. Effect of Catechins on Upper Respiratory Tract Infections in Winter: A Randomized, Placebo-Controlled, Double-Blinded Trial. Nutrients. 2022; 14(9):1856. https://doi.org/10.3390/nu14091856
Chicago/Turabian StyleOzato, Naoki, Tohru Yamaguchi, Tatsuya Kusaura, Hidefumi Kitazawa, Masanobu Hibi, Noriko Osaki, and Takahiro Ono. 2022. "Effect of Catechins on Upper Respiratory Tract Infections in Winter: A Randomized, Placebo-Controlled, Double-Blinded Trial" Nutrients 14, no. 9: 1856. https://doi.org/10.3390/nu14091856
APA StyleOzato, N., Yamaguchi, T., Kusaura, T., Kitazawa, H., Hibi, M., Osaki, N., & Ono, T. (2022). Effect of Catechins on Upper Respiratory Tract Infections in Winter: A Randomized, Placebo-Controlled, Double-Blinded Trial. Nutrients, 14(9), 1856. https://doi.org/10.3390/nu14091856






