A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism
Abstract
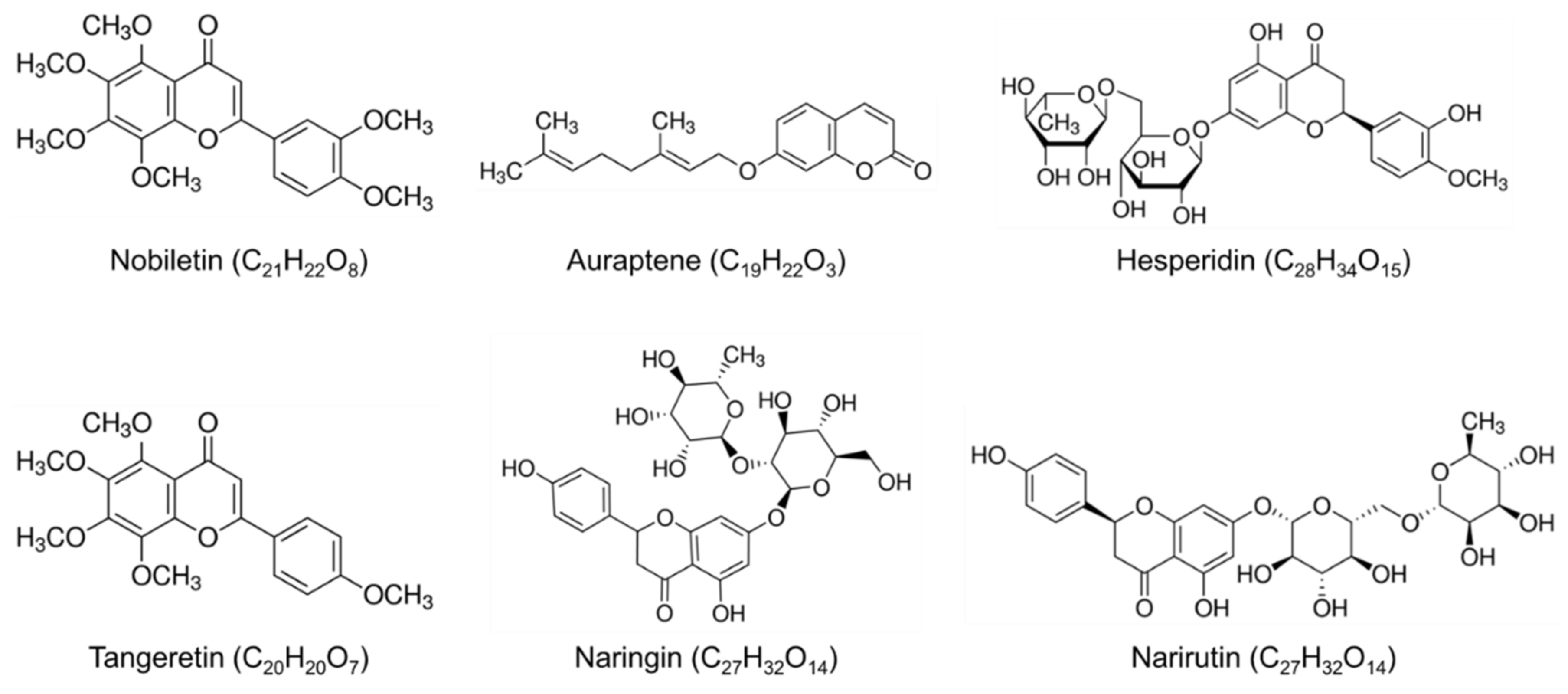
:1. Introduction
2. Citrus Compounds on Brain Health
2.1. Nobiletin-Rich Citrus Peel Extract Improves Cognitive Function
2.2. Effect of Flavanone-Rich Citrus Juices on Cognitive Function
2.3. Citrus Consumption and Cognitive Function: Evidence from Cohort Studies
2.4. Studies on Mental Health
3. Citrus Ingredients for Metabolic Function
3.1. Effects of Citrus Components on Body Weight, Body Composition, and Lipid Profiles
3.2. Liver Steatosis and Non-Alcoholic Fatty Liver Disease
3.3. Glycemia
3.4. Studies on Bone Metabolism
4. Stroke and Vascular Function
5. Other Functions and Future Perspectives
5.1. Circadian Rhythms
5.2. Gut Microbiota
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, L.; Tang, E.; Taylor, J.-P. Dementia: Timely diagnosis and early intervention. BMJ 2015, 350, h3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Price, D.L. New Perspectives on Alzheimer’s Disease. Annu. Rev. Neurosci. 1986, 9, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Oxidative Stress, Perturbed Calcium Homeostasis, and Immune Dysfunction in Alzheimer’s Disease. J. Neurovirol. 2002, 8, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Aisen, P.S. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol. 2002, 1, 279–284. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, Y.; Ihara, M. Mild Cognitive Impairment: At the Crossroad of Neurodegeneration and Vascular Dysfunction. Curr. Alzheimer Res. 2015, 12, 507–512. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 10031–10038. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Saito, S.; Inoue, T.; Satoh-Asahara, N.; Ihara, M. Potential Therapeutic Approaches for Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1992. [Google Scholar] [CrossRef] [Green Version]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Razay, G.; Vreugdenhil, A.; Wilcock, G. The Metabolic Syndrome and Alzheimer Disease. Arch. Neurol. 2007, 64, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittita, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef] [Green Version]
- Takeda, M.; Tanaka, T.; Okochi, M. New drugs for Alzheimer’s disease in Japan. Psychiatry Clin. Neurosci. 2011, 65, 399–404. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Felemban, S.G.; Alsubayiel, A.M.; et al. “Aducanumab” making a comeback in Alzheimer’s disease: An old wine in a new bottle. Biomed. Pharmacother. 2022, 148, 112746. [Google Scholar] [CrossRef]
- Silvestro, S.; Valeri, A.; Mazzon, E. Aducanumab and Its Effects on Tau Pathology: Is This the Turning Point of Amyloid Hypothesis? Int. J. Mol. Sci. 2022, 23, 2011. [Google Scholar] [CrossRef]
- Ashford, J.W.; Mahoney, L.; Burkett, T. A Role for Complementary and Integrative Medicine in Alzheimer’s Disease Prevention. J. Alzheimers Dis. 2015, 48, 13–14. [Google Scholar] [CrossRef]
- Khalsa, D.S. Stress, Meditation, and Alzheimer’s Disease Prevention: Where The Evidence Stands. J. Alzheimers Dis. 2015, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Steiner, G.Z.; Mathersul, D.C.; Macmillan, F.; Camfield, D.A.; Klupp, N.L.; Seto, S.W.; Huang, Y.; Hohenberg, M.I.; Chang, D.H. A Systematic Review of Intervention Studies Examining Nutritional and Herbal Therapies for Mild Cognitive Impairment and Dementia Using Neuroimaging Methods: Study Characteristics and Intervention Efficacy. Evid.-Based Complement. Altern. Med. 2017, 2017, 6083629. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, E.; Matsuzaki, K.; Sugimoto, N.; Tanabe, Y.; Hara, T.; Katakura, M.; Miyamoto, M.; Mishima, S.; Shido, O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients 2019, 11, 2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Sumiyoshi, E.; Yachie, A.; Shido, O. Chronic administration of theobromine inhibits mTOR signal in rats. Basic Clin. Pharmacol. Toxicol. 2019, 124, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Ohizumi, Y. A New Strategy for Preventive and Functional Therapeutic Methods for Dementia —Approach Using Natural Products. Yakugaku Zasshi 2015, 135, 449–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, K.; Yano, S.; Sumiyoshi, E.; Shido, O.; Katsube, T.; Tabata, M.; Okuda, M.; Sugimoto, H.; Yoshino, K.; Hashimoto, M. Long-Term Ultra-High Hydrostatic Pressurized Brown Rice Intake Prevents Bone Mineral Density Decline in Elderly Japanese Individuals. J. Nutr. Sci. Vitaminol. 2019, 65, S88–S92. [Google Scholar] [CrossRef] [Green Version]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Ohizumi, Y. Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders. Nutrients 2021, 13, 145. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Citrus polyphenols and risk of type 2 diabetes: Evidence from mechanistic studies. Crit. Rev. Food Sci. Nutr. 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Saigusa, D.; Shibuya, M.; Jinno, D.; Yamakoshi, H.; Iwabuchi, Y.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; Ohizumi, Y.; Tomioka, Y.; et al. High-performance liquid chromatography with photodiode array detection for determination of nobiletin content in the brain and serum of mice administrated the natural compound. Anal. Bioanal. Chem. 2011, 400, 3635–3641. [Google Scholar] [CrossRef]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of Polymethoxyflavones into the Mouse Brain and Their Effect on MK-801-Induced Locomotive Hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef] [Green Version]
- Takiyama, M.; Matsumoto, T.; Watanabe, J. LC-MS/MS detection of citrus unshiu peel-derived flavonoids in the plasma and brain after oral administration of yokukansankachimpihange in rats. Xenobiotica 2019, 49, 1494–1503. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Matsuzaki, K.; Nakata, N.; Onozuka, H.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Ohizumi, Y. Nobiletin, a Citrus Flavonoid, Reverses Learning Impairment Associated withN-Methyl-D-aspartate Receptor Antagonism by Activation of Extracellular Signal-Regulated Kinase Signaling. J. Pharmacol. Exp. Ther. 2007, 321, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.-W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Memory Impairment and Abeta; Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchi, S.; Nakajima, A.; Yokosuka, A.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y.; et al. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009, 1295, 218–229. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.-T.L.; Shin, E.-J.; Kim, H.-C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef]
- Nagase, H.; Yamakuni, T.; Matsuzaki, K.; Maruyama, Y.; Kasahara, J.; Hinohara, Y.; Kondo, S.; Mimaki, Y.; Sashida, Y.; Tank, A.W.; et al. Mechanism of Neurotrophic Action of Nobiletin in PC12D Cells. Biochemistry 2005, 44, 13683–13691. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Shin, E.-J.; Nam, Y.; Kim, H.-C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Shimizu, K.; Kajima, K.; Yokosuka, A.; Mimaki, Y.; Oku, N.; Ohizumi, Y. Nobiletin Reduces Intracellular and Extracellular β-amyloid in iPS Cell-Derived Alzheimer’s Disease Model Neurons. Biol. Pharm. Bull. 2018, 41, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.S.; Goes, A.T.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrient 2014, 30, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Hemanth Kumar, B.; Dinesh Kumar, B.; Diwan, P.V. Hesperidin, a citrus flavonoid, protects against l-methionine-induced hyperhomocysteinemia by abrogation of oxidative stress, endothelial dysfunction and neurotoxicity in Wistar rats. Pharm. Biol. 2017, 55, 146–155. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Shirai, M.; Ono, K.; Teruya, T.; Yamano, A.; Tae Woo, J. Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study. Food Sci. Nutr. 2021, 9, 6844–6853. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Hossain, S.; Sumiyoshi, E.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: A possible supplement for brain health in the elderly. Food Funct. 2022, 13, 2768–2781. [Google Scholar] [CrossRef]
- Seki, T.; Kamiya, T.; Furukawa, K.; Azumi, M.; Ishizuka, S.; Takayama, S.; Nagase, S.; Arai, H.; Yamakuni, T.; Yaegashi, N. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: A case series. Geriatr. Gerontol. Int. 2013, 13, 236–238. [Google Scholar] [CrossRef]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.F. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.F.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef] [Green Version]
- Igase, M.; Okada, Y.; Ochi, M.; Igase, K.; Ochi, H.; Okuyama, S.; Furukawa, Y.; Ohyagi, Y. Auraptene in the Peels of Citrus Kawachiensis (Kawachibankan) Contributes to the Preservation of Cognitive Function: A Randomized, Placebo-Controlled, Double-Blind Study in Healthy Volunteers. J. Prev. Alzheimers Dis. 2017, 5, 197–201. [Google Scholar] [CrossRef]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus consumption and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Br. J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-C.; Cassidy, A.; Willett, W.C.; Rimm, E.B.; O’Reilly, E.J.; Okereke, O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016, 104, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Lehrner, J.; Eckersberger, C.; Walla, P.; Pötsch, G.; Deecke, L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol. Behav. 2000, 71, 83–86. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Azizzadeh Forouzi, M.; Rahiminezhad, E.; Ahmadinejad, M.; Dehghan, M. The Effects of Lavender and Citrus aurantium on Anxiety and Agitation of the Conscious Patients in Intensive Care Units: A Parallel Randomized Placebo-Controlled Trial. BioMed Res. Int. 2021, 2021, 5565956. [Google Scholar] [CrossRef]
- Bruno, A.; Pandolfo, G.; Crucitti, M.; Cedro, C.; Zoccali, R.A.; Muscatello, M.R.A. Bergamot Polyphenolic Fraction Supplementation Improves Cognitive Functioning in Schizophrenia: Data from an 8-week, open-label pilot study. J. Clin. Psychopharmacol. 2017, 37, 468–471. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Kato, S.; Hossain, S.; Ohno, M.; Shido, O. Twelve-Month Studies on Perilla Oil Intake in Japanese Adults—Possible Supplement for Mental Health. Foods 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Matsuzaki, K.; Hossain, S.; Ito, T.; Wakatsuki, H.; Tanabe, Y.; Ohno, M.; Kato, S.; Yamashita, K.; Shido, O. Perilla Seed Oil Enhances Cognitive Function and Mental Health in Healthy Elderly Japanese Individuals by Enhancing the Biological Antioxidant Potential. Foods 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Yoshida, M.; Sun, W.; Nakajima, A.; Lai, Y.; Osaka, N.; Matsuzaki, K.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; et al. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: Identification of the substances responsible for the pharmacological action. J. Neural Transm. 2013, 120, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Okuyama, S.; Semba, T.; Toyoda, N.; Epifano, F.; Genovese, S.; Fiorito, S.; Taddeo, V.A.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Auraptene and Other Prenyloxyphenylpropanoids Suppress Microglial Activation and Dopaminergic Neuronal Cell Death in a Lipopolysaccharide-Induced Model of Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 1716. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Minami, S.; Shimada, N.; Makihata, N.; Nakajima, M.; Furukawa, Y. Anti-inflammatory and neuroprotective effects of auraptene, a citrus coumarin, following cerebral global ischemia in mice. Eur. J. Pharmacol. 2013, 699, 118–123. [Google Scholar] [CrossRef]
- Furukawa, Y.; Washimi, Y.-S.; Hara, R.-I.; Yamaoka, M.; Okuyama, S.; Sawamoto, A.; Nakajima, M. Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells. Molecules 2020, 25, 1117. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.-S.; Yuan, C.; Ascherio, A.; Rosner, B.A.; Willett, W.C.; Blacker, D. Long-term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women. Neurology 2021, 97, e1041–e1056. [Google Scholar] [CrossRef]
- Katon, W.J. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol. Psychiatry 2003, 54, 216–226. [Google Scholar] [CrossRef]
- Harvey, P.D.; Reichenberg, A.; Bowie, C.R. Cognition and Aging in Psychopathology: Focus on Schizophrenia and Depression. Annu. Rev. Clin. Psychol. 2006, 2, 389–409. [Google Scholar] [CrossRef]
- Morozova, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Pavlov, K.; Soloveva, K.; Volkova, M.; Alekseeva, P.; Andryshchenko, A.; Kostyuk, G.; et al. Neurobiological Highlights of Cognitive Impairment in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 1217. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.-Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2010, 32, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Kola, L.; Kohrt, B.A.; Hanlon, C.; Naslund, J.A.; Sikander, S.; Balaji, M.; Benjet, C.; Cheung, E.Y.L.; Eaton, J.; Gonsalves, P.; et al. COVID-19 mental health impact and responses in low-income and middle-income countries: Reimagining global mental health. Lancet Psychiatry 2021, 8, 535–550. [Google Scholar] [CrossRef]
- Hasheminia, D.; Kalantar Motamedi, M.R.; Karimi Ahmadabadi, F.; Hashemzehi, H.; Haghighat, A. Can Ambient Orange Fragrance Reduce Patient Anxiety During Surgical Removal of Impacted Mandibular Third Molars? J. Oral Maxillofac. Surg. 2014, 72, 1671–1676. [Google Scholar] [CrossRef]
- Aleman, A.; Hijman, R.; De Haan, E.H.; Kahn, R.S. Memory impairment in schizophrenia: A meta-analysis. Am. J. Psychiatry 1999, 156, 1358–1366. [Google Scholar] [CrossRef]
- Starc, M.; Murray, J.D.; Santamauro, N.; Savic, A.; Diehl, C.; Cho, Y.T.; Srihari, V.; Morgan, P.T.; Krystal, J.H.; Wang, X.-J.; et al. Schizophrenia is associated with a pattern of spatial working memory deficits consistent with cortical disinhibition. Schizophr. Res. 2017, 181, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Onitsuka, T.; Hirano, Y.; Nakazawa, T.; Ichihash, K.; Miura, K.; Inada, K.; Mitoma, R.; Yasui-Furukori, N.; Hashimoto, R. Toward recovery in schizophrenia: Current concepts, findings, and future research directions. Psychiatry Clin. Neurosci. 2022. [Google Scholar] [CrossRef]
- Misiak, B.; Leszek, J.; Kiejna, A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease—The emerging role of systemic low-grade inflammation and adiposity. Brain Res. Bull. 2012, 89, 144–149. [Google Scholar] [CrossRef]
- Tahmi, M.; Palta, P.; Luchsinger, J.A. Metabolic Syndrome and Cognitive Function. Curr. Cardiol. Rep. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Frisardi, V.; Solfrizzi, V.; Seripa, D.; Capurso, C.; Santamato, A.; Sancarlo, D.; Vendemiale, G.; Pilotto, A.; Panza, F. Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.-X.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, C.; Gerbi, A.; Tenca, G.; Juchaux, F.; Bernard, F.-X. Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes. Mechanism of action by inhibition of cAMP-phosphodiesterase (PDE). Phytomedicine 2008, 15, 783–792. [Google Scholar] [CrossRef]
- Dallas, C.; Gerbi, A.; Elbez, Y.; Caillard, P.; Zamaria, N.; Cloarec, M. Clinical Study to Assess the Efficacy and Safety of a Citrus Polyphenolic Extract of Red Orange, Grapefruit, and Orange (Sinetrol-XPur) on Weight Management and Metabolic Parameters in Healthy Overweight Individuals. Phytother. Res. 2014, 28, 212–218. [Google Scholar] [CrossRef]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Rouanet, J.M. A 12-week randomized double-blind parallel pilot trial of Sinetrol XPur on body weight, abdominal fat, waist circumference, and muscle metabolism in overweight men. Int. J. Food Sci. Nutr. 2015, 66, 471–477. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Bae, M.H.; Sung, H.C.; Kim, N.K.; Sung, E.; Lee, H.-J. Efficacy and Safety of Sinetrol-XPur on Weight and Body Fat Reduction in Overweight or Obese Adults: A 12-Week, Randomized, Double-Blind, Parallel, Placebo-Controlled Trial. J. Med. Food 2020, 23, 335–342. [Google Scholar] [CrossRef]
- Aptekmann, N.P.; Cesar, T.B. Orange juice improved lipid profile and blood lactate of overweight middle-aged women subjected to aerobic training. Maturitas 2010, 67, 343–347. [Google Scholar] [CrossRef]
- Hancke, J.; Srivastava, S.; Caceres, D.D.; Burgos, R.A.; Alarcon, P. An exploratory double-blind, randomized, placebo-controlled study to assess the efficacy of CitruSlim on body composition and lipid parameters in obese individuals. Phytother. Res. 2021, 35, 7039–7049. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, G.; Shen, T.; Zhang, S.; Rao, J.; Shi, R. Effects of 12-week supplementation of Citrus bergamia extracts-based formulation CitriCholess on cholesterol and body weight in older adults with dyslipidemia: A randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2017, 16, 251. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Martin, M.V.; Soto, M.J.; Rico, M.C.; Vallejo, F.; Tomas-Barberan, F.A.; Perez-de-la-Cruz, A.J.; Gil, A.; Mesa, M.D. Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults. J. Nutr. 2015, 145, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Shikishima, Y.; Tsutsumi, R.; Kawakami, A.; Miura, H.; Nii, Y.; Sakaue, H. Sudachi peel extract powder including the polymethoxylated flavone sudachitin improves visceral fat content in individuals at risk for developing diabetes. Food Sci. Nutr. 2021, 9, 4076–4084. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Yang, H.J.; Ha, K.-C.; So, B.-O.; Choi, E.-K.; Chae, S.-W. A randomized, double-blind, placebo-controlled clinical trial to investigate the anti-diabetic effect of Citrus junos Tanaka peel. J. Funct. Foods 2015, 18, 532–537. [Google Scholar] [CrossRef]
- Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Horcajada, M.N.; Offord-Cavin, E.; Peacock, M.; Weaver, C.M. Effect of Hesperidin With and Without a Calcium (Calcilock) Supplement on Bone Health in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2016, 101, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Jeong, H.; Hur, S.; Lee, J.; Kwon, O. Efficacy and Safety of Kudzu Flower–Mandarin Peel on Hot Flashes and Bone Markers in Women during the Menopausal Transition: A Randomized Controlled Trial. Nutrients 2020, 12, 3237. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. The Polymethoxy Flavonoid Sudachitin Inhibits Interleukin-1β-Induced Inflammatory Mediator Production in Human Periodontal Ligament Cells. BioMed Res. Int. 2021, 2021, 6586. [Google Scholar] [CrossRef]
- Dow, C.A.; Going, S.B.; Chow, H.-H.S.; Patil, B.S.; Thomson, C.A. The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metabolism 2012, 61, 1026–1035. [Google Scholar] [CrossRef]
- Habauzit, V.; Verny, M.-A.; Milenkovic, D.; Barber-Chamoux, N.; Mazur, A.; DuBray, C.; Morand, C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2015, 102, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.J.; Mendis, B.; Macdonald, I.A. Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food Funct. 2016, 7, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, G.; O’Donnell, A.; Davis-Plourde, K.; Zelber-Sagi, S.; Ghosh, S.; DeCarli, C.S.; Thibault, E.G.; Sperling, R.A.; Johnson, K.A.; Beiser, A.S.; et al. Non-alcoholic fatty liver disease, liver fibrosis, and regional amyloid-β and tau pathology in middle-aged adults: The Framingham study. J. Alzheimers Dis. 2022, 86, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Hadjihambi, A. Cerebrovascular alterations in NAFLD: Is it increasing our risk of Alzheimer’s disease? Anal. Biochem. 2021, 636, 114387. [Google Scholar] [CrossRef] [PubMed]
- Lampignano, L.; Donghia, R.; Griseta, C.; Lagravinese, G.; Sciarra, S.; Zupo, R.; Castellana, F.; Bortone, I.; Guerra, V.; Tirelli, S.; et al. Liver Health and Dementia in an Italian Older Population: Findings From the Salus in Apulia Study. Front. Aging Neurosci. 2021, 13, 748888. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Hu, F.; Deng, X.; Zhang, Y. Non-alcoholic Fatty Liver Disease and Longitudinal Cognitive Changes in Middle-Aged and Elderly Adults. Front. Med. 2022, 8, 738835. [Google Scholar] [CrossRef]
- Shang, Y.; Nasr, P.; Ekstedt, M.; Widman, L.; Stål, P.; Hultcrantz, R.; Kechagias, S.; Hagström, H. Non-alcoholic fatty liver disease does not increase dementia risk although histology data might improve risk prediction. JHEP Rep. 2021, 3, 100218. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, K.W.; Park, J.B.; Lee, A.H.J.; Hwang, I.K. Variation in Major Antioxidants and Total Antioxidant Activity of Yuzu (Citrus junos Sieb ex Tanaka) during Maturation and between Cultivars. J. Agric. Food Chem. 2004, 52, 5907–5913. [Google Scholar] [CrossRef]
- Shim, J.-H.; Chae, J.-I.; Cho, S.-S. Identification and Extraction Optimization of Active Constituents in Citrus junos Seib ex TANAKA Peel and Its Biological Evaluation. Molecules 2019, 24, 680. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Lee, M.-K.; Jeong, K.-S.; Choi, M.-S. The Hypoglycemic Effects of Hesperidin and Naringin Are Partly Mediated by Hepatic Glucose-Regulating Enzymes in C57BL/KsJ-db/db Mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Hur, H.J.; Yang, H.J.; Kim, H.J.; Kim, M.J.; Park, J.H.; Sung, M.J.; Kim, M.S.; Kwon, D.Y.; Hwang, J.-T. Citrus junos Tanaka Peel Extract Exerts Antidiabetic Effects via AMPK and PPAR-both In Vitro and In Vivo in Mice Fed a High-Fat Diet. Evid.-Based Complement. Altern. Med. 2013, 2013, 921012. [Google Scholar] [CrossRef] [Green Version]
- Exton-Smith, A.N.; Millard, P.H.; Erica, P.R.P.; Wheeler, F. Pattern of development and loss of bone with age. Lancet 1969, 294, 1154–1157. [Google Scholar] [CrossRef]
- Firooznia, H.; Golimbu, C.; Rafii, M.; Schwartz, M.S.; Alterman, E.R. Quantitative computed tomography assessment of spinal trabecular bone. II. In osteoporotic women with and without vertebral fractures. J. Comput. Tomogr. 1984, 8, 99–103. [Google Scholar] [CrossRef]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef]
- Roberts, S.; Colombier, P.; Sowman, A.; Mennan, C.; Rölfing, J.H.D.; Guicheux, J.; Edwards, J.R. Ageing in the musculoskeletal system. Acta Orthop. 2016, 87 (Suppl. S363), 15–25. [Google Scholar] [CrossRef] [Green Version]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J.; Kassem, M. Extrinsic Mechanisms Involved in Age-Related Defective Bone Formation. J. Clin. Endocrinol. Metab. 2011, 96, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Hossain, S.; Matsuzaki, K.; Shido, O.; Yoshino, K. The journey from white rice to ultra-high hydrostatic pressurized brown rice: An excellent endeavor for ideal nutrition from staple food. Crit. Rev. Food Sci. Nutr. 2020, 62, 1502–1520. [Google Scholar] [CrossRef]
- Downey, C.L.; Young, A.; Burton, E.F.; Graham, S.M.; Macfarlane, R.J.; Tsapakis, E.M.; Tsiridis, E. Dementia and osteoporosis in a geriatric population: Is there a common link? World J. Orthop. 2017, 8, 412–423. [Google Scholar] [CrossRef]
- Ichinose, T.; Matsuzaki, K.; Kato, M.; Tanabe, Y.; Tachibana, N.; Morikawa, M.; Kato, S.; Ohata, S.; Ohno, M.; Wakatsuki, H.; et al. Intake of Docosahexaenoic Acid-Enriched Milk Beverage Prevents Age-Related Cognitive Decline and Decreases Serum Bone Resorption Marker Levels. J. Oleo Sci. 2021, 70, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Habauzit, V.; Trzeciakiewicz, A.; Morand, C.; Gil-Izquierdo, A.; Mardon, J.; Lebecque, P.; Davicco, M.J.; Chee, W.S.; Coxam, V.; et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J. Appl. Physiol. 2008, 104, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, S.M.; Horcajada, M.-N.; Offord, E. Phytonutrients for bone health during ageing. Br. J. Clin. Pharmacol. 2013, 75, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.A. Psychological sequelae in stroke patients. Aust. Fam. Physician 1991, 20, 1605–1607. [Google Scholar]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. Potential Biomarkers for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 602. [Google Scholar] [CrossRef]
- Bachman, D.L.; Wolf, P.A.; Linn, R.T.; Knoefel, J.E.; Cobb, J.L.; Belanger, A.J.; White, L.R.; D’Agostino, R.B. Incidence of dementia and probable Alzheimer’s disease in a general population: The Framingham Study. Neurology 1993, 43, 515–519. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Rimm, E.B.; O’Reilly, É.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary Flavonoids and Risk of Stroke in Women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef]
- Goetz, M.E.; Judd, S.E.; Hartman, T.J.; McClellan, W.; Anderson, A.; Vaccarino, V. Flavanone Intake Is Inversely Associated with Risk of Incident Ischemic Stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J. Nutr. 2016, 146, 2233–2243. [Google Scholar] [CrossRef] [Green Version]
- Victoria-Montesinos, D.; Abellán Ruiz, M.S.; Luque Rubia, A.J.; Guillén Martínez, D.; Pérez-Piñero, S.; Sánchez Macarro, M.; García-Muñoz, A.M.; Cánovas García, F.; Castillo Sánchez, J.; López-Román, F.J. Effectiveness of Consumption of a Combination of Citrus Fruit Flavonoids and Olive Leaf Polyphenols to Reduce Oxidation of Low-Density Lipoprotein in Treatment-Naïve Cardiovascular Risk Subjects: A Randomized Double-Blind Controlled Study. Antioxidants 2021, 10, 589. [Google Scholar] [CrossRef]
- Macarro, M.S.; Rodríguez, J.P.M.; Morell, E.B.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; García, F.C.; Sánchez, J.C.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef]
- Oben, J.; Enonchong, E.; Kothari, S.; Chambliss, W.; Garrison, R.; Dolnick, D. Phellodendron and Citrusextracts benefit cardiovascular health in osteoarthritis patients: A double-blind, placebo-controlled pilot study. Nutr. J. 2008, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Oben, J.; Enonchong, E.; Kothari, S.; Chambliss, W.; Garrison, R.; Dolnick, D. Phellodendron and Citrus extracts benefit joint health in osteoarthritis patients: A pilot, double-blind, placebo-controlled study. Nutr. J. 2009, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother. Res. 2018, 32, 1073–1079. [Google Scholar] [CrossRef]
- Morand, C.; DuBray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: A randomized controlled trial (Citrus study). Eur. J. Nutr. 2021, 60, 1277–1288. [Google Scholar] [CrossRef]
- Li, L.; Lyall, G.K.; Martinez-Blazquez, J.A.; Vallejo, F.; Tomas-Barberan, F.A.; Birch, K.M.; Boesch, C. Blood Orange Juice Consumption Increases Flow-Mediated Dilation in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020, 150, 2287–2294. [Google Scholar] [CrossRef]
- Scheffers, F.R.; Boer, J.M.A.; Verschuren, W.M.M.; Verheus, M.; Van Der Schouw, Y.T.; Sluijs, I.; Smit, H.A.; Wijga, A.H. Pure fruit juice and fruit consumption and the risk of CVD: The European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) study. Br. J. Nutr. 2019, 121, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurbau, A.; Au-Yeung, F.; Mejia, S.B.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Different Fruit and Vegetable Sources With Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef] [Green Version]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Tanabe, Y.; Hossain, S.; Matsuzaki, K.; Ohno, M.; Kato, S.; Katakura, M.; Shido, O. Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults. Molecules 2020, 25, 2099. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Kress, G.J.; Liao, F.; Dimitry, J.; Cedeno, M.R.; Fitzgerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068. [Google Scholar] [CrossRef]
- Nohara, K.; Shin, Y.; Park, N.; Jeong, K.; He, B.; Koike, N.; Yoo, S.-H.; Chen, Z. Ammonia-lowering activities and carbamoyl phosphate synthetase 1 (Cps1) induction mechanism of a natural flavonoid. Nutr. Metab. 2015, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Nohara, K.; Park, N.; Park, Y.-S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::luciferase Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.-H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef] [Green Version]
- Wirianto, M.; Wang, C.; Kim, E.; Koike, N.; Gomez-Gutierrez, R.; Nohara, K.; Escobedo, G.; Choi, J.M.; Han, C.; Yagita, K.; et al. The clock modulator Nobiletin mitigates astrogliosis-associated neuroinflammation and disease hallmarks in an Alzheimer’s disease model. FASEB J. 2022, 36, e22186. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The Potential Role of Gut Microbiota in Alzheimer’s Disease: From Diagnosis to Treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef]
- Toledo, A.R.L.; Monroy, G.R.; Salazar, F.E.; Lee, J.-Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut–Brain Axis as a Pathological and Therapeutic Target for Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhu, J.; Zhao, D.-G.; Ma, Y.-Y.; Li, D.; Ho, C.-T.; Huang, Q. Bidirectional interaction of nobiletin and gut microbiota in mice fed with a high-fat diet. Food Funct. 2021, 12, 3516–3526. [Google Scholar] [CrossRef]
- Kou, G.; Li, P.; Hu, Y.; Chen, H.; Amoah, A.N.; Traore, S.S.; Cui, Z.; Lyu, Q. Nobiletin activates thermogenesis of brown and white adipose tissue in high-fat diet-fed C57BL/6 mice by shaping the gut microbiota. FASEB J. 2021, 35, e21267. [Google Scholar] [CrossRef] [PubMed]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nemoto, K.; Ohizumi, Y. An evaluation of the genotoxicity and subchronic toxicity of the peel extract of Ponkan cultivar ‘Ohta ponkan’ (Citrus reticulata Blanco) that is rich in nobiletin and tangeretin with anti-dementia activity. Regul. Toxicol. Pharmacol. 2020, 114, 104670. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107. [Google Scholar] [PubMed]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
| Intervention or Cohort Analysis | Dosage or Frequency | Study Design | Subjects | Duration | Reference |
|---|---|---|---|---|---|
| Cognitive health | |||||
| Nobiletin-containing test food (Nobilex®) | 3 capsules (containing 30 mg nobiletin and 17.4 mg tangeretin) | Randomized, double-blind, placebo-controlled trial | Healthy elderly individuals (n = 108), aged over 65 | 16 weeks | [48] |
| Nobiletin-rich ponkan peel powder and perilla seed oil | 1.12 g ponkan peel powder (containing 2.91 mg nobiletin) and 1.47 mL perilla seed oil | Randomized, double-blind, parallel-armed trial | Healthy elderly individuals (n = 49), aged 60–85 | 12 months | [49] |
| Nobiletin-rich citrus peel extract | 30 g of citrus peels boiled in 500 mL of water and concentrated to 300 mL | Randomized, double-blind, placebo-controlled trial | Alzheimer's disease patients taking donepezil (n = 11) | 12 months | [50] |
| High flavanone orange juice | 500 mL (flavanone 305 mg) | Randomized, double-blind, placebo-controlled crossover trial | Healthy elderly people (n = 37), aged 60–81 | 8 weeks | [51] |
| Flavonoid-rich orange juice | 240 mL (flavonoid 272 mg) | Randomized, double-blind, placebo-controlled, crossover trial | Healthy middle-aged adults (n = 24), aged 30–65 | Acute | [52] |
| Flavanone-rich orange juice | 500 mL (flavanone 70.5 mg) | Randomized, single-blind, cross over trial | Healthy young adults (n = 40), aged 18–30 | Acute | [53] |
| Auraptene-rich Kawachi Bankan extract | 125 mL (auraptene 6 mg) | Randomized double-blind, placebo-controlled trial | Healthy elderly people (n = 82), aged 62–80 | 24 weeks | [54] |
| Daily citrus intake | 3–4 or more times per week | Retrospective cohort study | 13,373 adults (over 65 years) | 5.7 years follow up | [55] |
| Mental health | |||||
| Daily citrus intake | Total dietary flavonoid intake | Prospective cohort study | 82,643 women, aged 36–55 and 53–80 | 10 years follow up | [56] |
| Flavonoid rich orange juice | 380 mL (flavonoid 600 mg) | Randomized single-blind trial | Depressive symptoms in young individuals (n = 40), aged 20–30 | 8 weeks | [57] |
| Citrus sinensis essential oils | Diffused through electric dispenser | Randomized trial | Patients undergoing treatment at a dental (n = 72), aged 22–57 | Acute | [58] |
| Citrus aurantium or lavender essential oils | Inhalation of 5 drops of lavender or Citrus aurantium essential oils for 30 min | Randomized, parallel group placebo-controlled trial | Subjects admitted to intensive care units (n = 150), aged 18–60 | Acute | [59] |
| Flavanone-rich bergamot polyphenol fraction | 1000 mg | Open-label pilot study | Patients diagnosed with schizophrenia (n = 20), aged 20–58 | 8 weeks | [60] |
| Intervention or Cohort Analysis | Dosage or Frequency | Study Design | Subjects | Duration | Reference |
|---|---|---|---|---|---|
| Body weight, lipid profiles, and fat content | |||||
| Citrus-based polyphenolic dietary supplement, SINETROL® | 4 capsules (1400 mg) | Randomized, double blind, placebo-controlled trial | Overweight subjects (n = 20), aged 25–55 (BMI 27–33) | 12 weeks | [84] |
| Citrus-based polyphenol extract, Sinetrol®-Xpur | 2 tablets (900 mg) | Randomized, double-blinded, controlled study | Overweight subjects (n = 95) or overweight/obese participants (n = 100) | 12 weeks | [85,86,87] |
| Orange juice with aerobic training | 500 mL of orange juice and 1 h aerobic training 3 times a week | Randomized, controlled study | Overweight (weighing 75.5 ± 14.2 kg) women (n = 26), aged 30–48 | 3 months | [88] |
| Citrus flavanone-O-glycosides and eurypeptides, CitruSlim | 200 mg or 400 mg | Randomized, double-blind, placebo-controlled trial | 97 participants (ages 18–60) | 112 days | [89] |
| Bergamot extract-based formulation, CitriCholess | 2 capsules (containing 500 mg Citrus bergamia Risso extract and others) | Randomized, double-blind, placebo-controlled trial | 98 participants (mean age 65) | 12 weeks | [90] |
| Normal or high polyphenol concentration in orange juice | 500 mL (containing 299 or 741.5 mg polyphenols) | Randomized, double-blind crossover study | Non-smoking obese subjects (n = 100), aged 18–65 | 12 weeks | [91] |
| Sudachi peel extract powder | 1050 mg purified sudachi extract (including 4.9 mg sudachitin) | Randomized, double-blind, placebo-controlled trial | Mild overweight (BMI 23–30 kg/m2) subjects (n = 41), aged 30–65 | 12 weeks | [92] |
| Citrus junos Tanaka peel extract | 4250 mg | Randomized, double-blind, crossover, placebo-controlled clinical trial | Subjects with impaired fasting glucose (n = 40), average 52.75 years | 8 weeks | [93] |
| Bone health | |||||
| Hesperidin and calcium supplement | 500 mg hesperidin with or without calcium supplement | Randomized, double-blind crossover design | Healthy postmenopausal women (n = 12), mean age 66.3 years | 350 days | [94] |
| Extract mixture of kudzu flower and mandarin (Citrus unshiu Markovich) peel | 1150 mg | Randomized controlled parallel-armed design | Peri- or post-menopausal women (n = 84), aged 45–60 | 12 weeks | [95] |
| Intervention or Cohort Analysis | Dosage or Frequency | Study Design | Subjects | Duration | Reference |
|---|---|---|---|---|---|
| Daily flavanone intake | >62.95 mg/day | Prospective cohort study | 69,622 women, aged 30–55 | 14 years follow up | [131] |
| Daily flavonoid intake | >48 mg/day | Prospective cohort study | 20,024 subjects, aged 45 years or older | 6.5 years follow up | [132] |
| Flavonoid-rich hydroethanolic extract Citrolive™ | 2 capsules (1000 mg) | Randomized, double-blind, controlled study | 23 participants (mean age 41.9) with cardiovascular risk (cholesterol level > 200 mg/dL and LDL > 130 mg/dL) | 3 months | [133] |
| Flavonoid-rich hydroethanolic extract Citrolive™ | 2 capsules (1000 mg) | Randomized, double-blind, placebo-controlled study | Healthy individuals (n = 96), aged 40–75 | 8 weeks | [134] |
| Extracts of Phellodendron amurense bark and Citrus sinensis peel, NP06-1 | 4 capsules (1480 mg) | Randomized, double-blind, placebo-controlled pilot study | Normal weight (BMI 18.9–24.9) or overweight (BMI 25–40) subjects (n = 80), aged 25–60 | 8 weeks | [135,136] |
| Hesperidin supplementation | 500 mg | Randomized double-blind controlled clinical trial | Patients with type 2 diabetes mellitus (n = 64), aged 30–65 | 6 weeks | [137] |
| Orange juice or hesperidin | 500 mL orange juice (292 mg hesperidin and 47.5 mg narirutin) or pure hesperidin 292 mg | Randomized, controlled, crossover study | Healthy overweight men (n = 24), aged 50–65 | 4 weeks | [138] |
| Orange juice or hesperidin-enriched orange juice | 500 mL (containing 345 mg or 600 mg hesperidin) | Randomized, parallel, double-blind, placebo-controlled trial | Pre- or stage-1 hypertensive individuals (n = 159), aged 18–65 | 12 weeks | [139] |
| Blood orange juice | 400 mL (hesperidin and narirutin concentration were 80.2 and 9.5 mg/dL) | Randomized, controlled, single-blind, crossover trial | Overweight or obese subjects (n = 15) (BMI: 28.3 ± 3.1 kg/m2), aged 20–45 | 2 weeks | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, K.; Nakajima, A.; Guo, Y.; Ohizumi, Y. A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients 2022, 14, 1847. https://doi.org/10.3390/nu14091847
Matsuzaki K, Nakajima A, Guo Y, Ohizumi Y. A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients. 2022; 14(9):1847. https://doi.org/10.3390/nu14091847
Chicago/Turabian StyleMatsuzaki, Kentaro, Akira Nakajima, Yuanqiang Guo, and Yasushi Ohizumi. 2022. "A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism" Nutrients 14, no. 9: 1847. https://doi.org/10.3390/nu14091847
APA StyleMatsuzaki, K., Nakajima, A., Guo, Y., & Ohizumi, Y. (2022). A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients, 14(9), 1847. https://doi.org/10.3390/nu14091847







