Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Preparation of the Raw Powder and Ethanoic Extract
2.4. Experimental Design
2.5. Doses Selection
2.6. Animal Anesthesia and Collection of Blood Samples and Tissues
2.7. Biochemical Analysis
2.8. Light Microscope
2.9. Statistical Analysis
3. Results
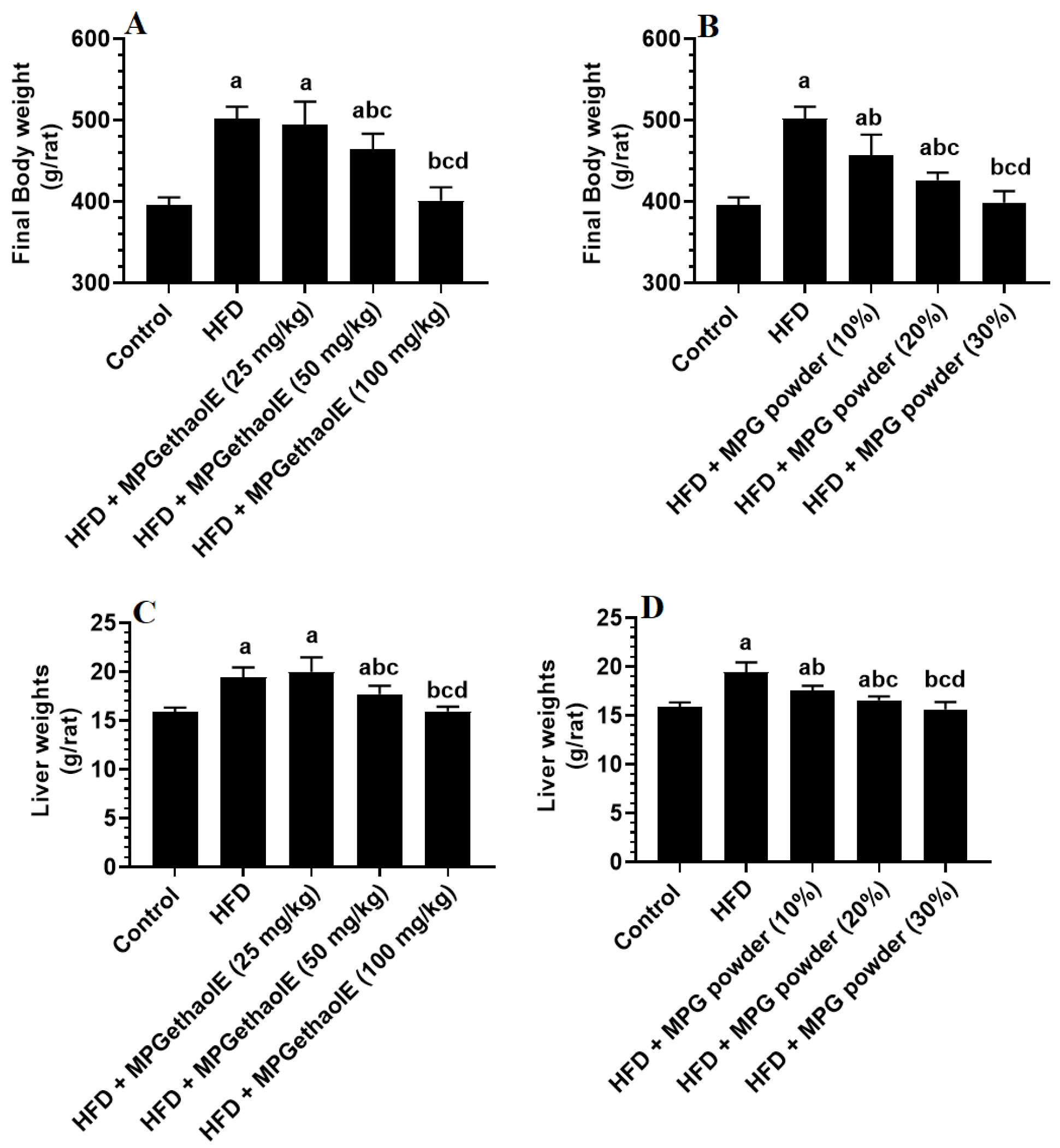
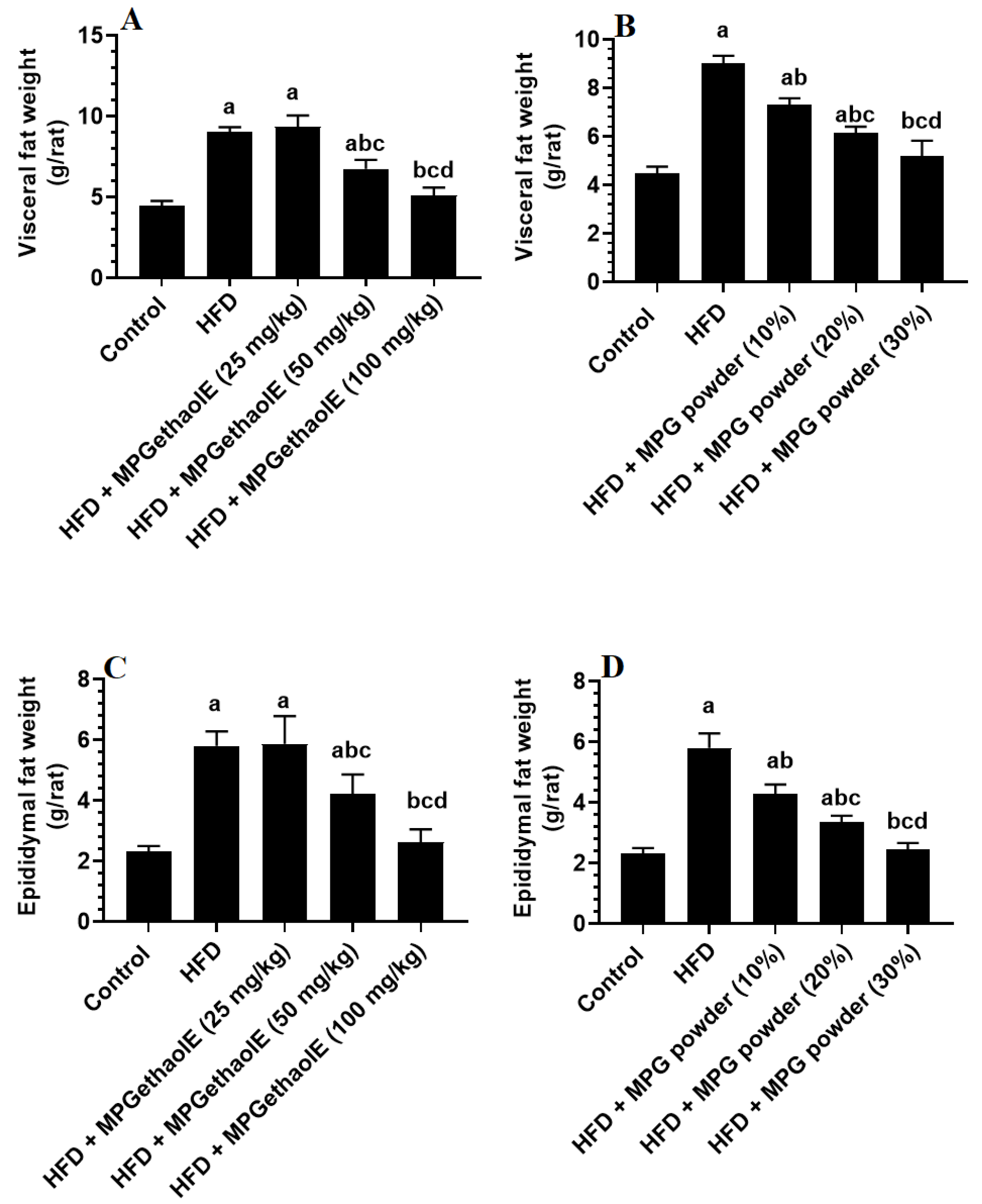
3.1. Changes in Body Weight and Adiposity Markers
3.2. Changes in Lipid Profile
3.3. Changes in Inflammatory Mediators
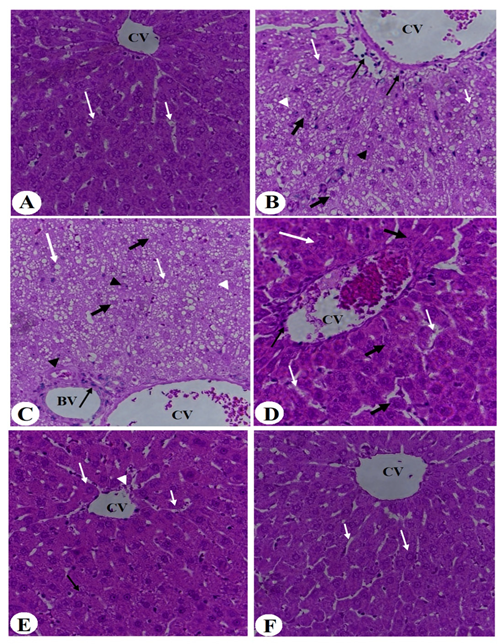
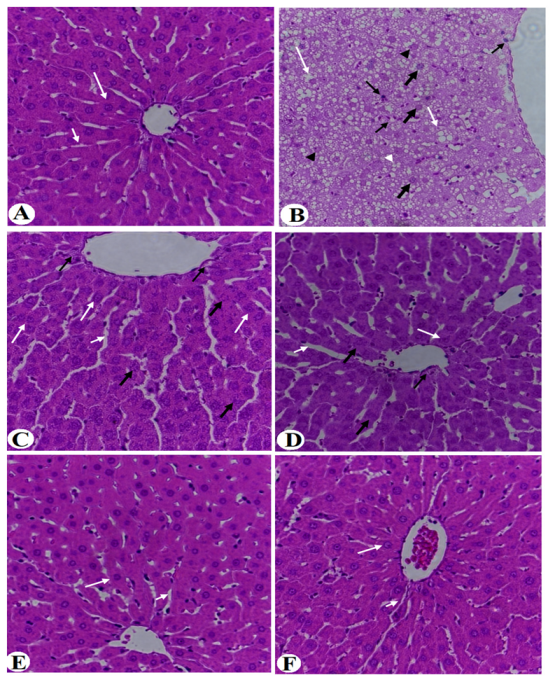
3.4. Improvement in Liver Histology
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [PubMed]
- Lu, Z.; Li, Y.; Yu, H.; Lopes-Virella, M.F.; Huang, Y. High-fat diet-induced metabolic syndrome increases ligature-induced alveolar bone loss in mice. Oral Dis. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 59–63. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Gao, B.; Tsukamoto, H. Inflammation in alcoholic and nonalcoholic fatty liver disease: Friend or foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Luci, C.; Bourinet, M.; Leclère, P.S.; Anty, R.; Gual, P. Chronic inflammation in non-alcoholic steatohepatitis: Molecular mechanisms and therapeutic strategies. Front. Endocrinol. 2020, 11, 597648. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes 2018, 67, 2485–2493. [Google Scholar] [CrossRef] [Green Version]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef] [PubMed]
- Aronis, A.; Madar, Z.; Tirosh, O. Mechanism underlying oxidative stress-mediated lipotoxicity: Exposure of J774.2 macrophages to triacylglycerols facilitates mitochondrial reactive oxygen species production and cellular necrosis. Free Radic. Biol. Med. 2005, 38, 1221–1230. [Google Scholar] [CrossRef]
- Zvenigorodskaia, L.A.; Samsonova, N.G.; Mel’nikova, N.V.; Cherkashova, E.A. Hypolipidemic therapy in patients with non-alcoholic fatty liver disease. Exp. Clin. Gastroenterol. 2010, 7, 25–33. [Google Scholar]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sugimoto, K.; Inui, H.; Fukusato, T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3777–37785. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Vázquez-Cabral, B.D.; González-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar]
- Xu, Y.; Guo, W.; Zhang, C.; Chen, F.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Herbal medicine in the treatment of non-alcoholic fatty liver diseases-efficacy, action mechanism, and clinical application. Front. Pharmacol. 2020, 11, 601. [Google Scholar] [CrossRef]
- Bastidas, E.G.; Roura, R.; Rizzolo DA, D.; Massanés, T.; Gomis, R. Quinoa (chenopodium quinoa willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2016, 25, 148–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyami, J.; Whitehouse, E.; Yakubov, G.E.; Pritchard, S.E.; Hoad, C.L.; Blackshaw, E.; Heissam, K.; Cordon, S.M.; Bligh, H.F.J.; Spiller, R.C.; et al. Glycaemic, gastrointestinal, hormonal and appetitive responses to pearl millet or oats porridge breakfasts: A randomised, crossover trial in healthy humans. Br. J. Nutr. 2019, 122, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, V.; Nepolean, T.; Senthilvel, S.; Varshney, R.K.; Vadez, V.; Srivastava, R.K.; Shah, T.M.; Supriya, A.; Kumar, S.; Ramana Kumari, B.; et al. Pearl Millet [Pennisetum glaucum (L.) R. br.] consensus linkage map constructed using four RIL mapping populations and newly developed est-SSRS. BMC Genom. 2013, 14, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanga, S.; Singh, A.; Orsat, V.; Raghavan, V. Nutritional Comparison of Millets with Other Superfoods; Mcgill University: Montreal, QC, Canada, 2018; pp. 1–17. [Google Scholar]
- Osman, M.A. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J. Saudi Soc. Agric. Sci. 2011, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.R.N. Millets: Their unique nutritional and health-promoting attributes. In Gluten-Free Ancient Grains; Woodhead Publishing: Sawston, UK, 2017; pp. 55–103. [Google Scholar]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential use of pearl millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food security, processing, health benefits and nutritional products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, J.; Pranisha, J.; Trueman, P.; Ramachandran, S.; Saigopal, S.; Viswanathan, V. Regular exercise with an active lifestyle improves the lipid profile of individuals with diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2016, 37, 262–266. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Tsusaka, T.W.; Botha, R.; Rajendran, A.; Givens, D.I.; Parasannanavar, D.J.; Subramaniam, K.; Prasad, K.D.V.; Vetriventhan, M.; et al. A systematic review and meta-analysis of the potential of millets for managing and reducing the risk of developing diabetes mellitus. Front Nutr. 2021, 8, 687428. [Google Scholar] [CrossRef]
- Tiwari, N.; Shere, D.M. Effect of finger millet (eleusine coracana) buns supplementation on serum glucose and serum lipids level in type 2 diabetics. Asian J. Dairy Food Res. 2017, 36, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.; Rizvi, S. Current understanding of dietary polyphenols and their role in health and disease. Curr. Nutr. Food Sci. 2009, 5, 249–263. [Google Scholar] [CrossRef]
- Koli, R.; Köhler, K.; Tonteri, E.; Peltonen, J.; Tikkanen, H.; Fogelholm, M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: An intervention study. Nutr. J. 2015, 14, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2014, 2, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Musayeib, N.M.A.; Amina, M.; Al-Hamoud, G.A.; Mohamed, G.A.; Ibrahim, S.R.M.; Shabana, S. Plectrabarbene, a New Abietane Diterpene from Plectranthus barbatus Aerial Parts. Molecules 2020, 25, 2365. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental Assessment of moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid.-Based Complement Altern. Med. 2012, 2012, 519084. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Amir, R.M.; Ameer, K.; Rakha, A.; Faiz, F.; Hayat, I.; Nadeem, M.; Ahmed, Z.; Riaz, A.; Ashraf, I. Characterization of oat bran β-glucan with special reference to efficacy study to elucidate its health claims for diabetic patients. Food Sci. Technol. 2021, 41, 105–112. [Google Scholar] [CrossRef]
- Kim, H.C.; Song, J.M.; Kim, C.J.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Shin, S.H. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 16. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.L.; Carvalho Ld Oliveira, A.C.; Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gan, J.Q.; Wang, H. Neurocognitive mechanisms of mathematical giftedness: A literature review. Appl. Neuropsychol. Child 2016, 6, 79–94. [Google Scholar] [CrossRef]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why do mice overeat high-fat diets? How high-fat diet alters the regulation of daily caloric intake in mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtaza, N.; Baboota, R.K.; Jagtap, S.; Singh, D.P.; Khare, P.; Sarma, S.M.; Podili, K.; Alagesan, S.; Chandra, T.S.; Bhutani, K.K.; et al. Finger millet bran supplementation alleviates obesity-induced oxidative stress, inflammation and gut microbial derangements in high-fat diet-fed mice. Br. J. Nutr. 2014, 112, 1447–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichiah, P.B.; Moon, H.J.; Park, J.E.; Moon, Y.J.; Cha, Y.S. Ethanolic extract of seabuckthorn (Hippophae rhamnoides L.) prevents high-fat diet-induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutr. Res. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Collier, G.R.; Ocana, A.; Rao, A.V.; Buckley, G.; Lam, Y.; Mayer, A.; Thompson, L.U. Metabolic effects of a low-glycemic-index diet. Am. J. Clin. Nutr. 1987, 46, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Pasman, W.J.; Saris, W.H.; Wauters, M.A.; Westerterp-Plantenga, M.S. Effect of one week of fibre supplementation on hunger and satiety ratings and energy intake. Appetite 1997, 29, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, P.; Anderson, C.M.; Valentine, C.; Nguyen, P.; Marimuthu, A.; West, B.L.; Baxter, J.D.; Kushner, P.J. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol. Endocrinol. 2000, 14, 1976–1985. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Guan, X.; Huang, K.; Liu, J.; Liu, D.; Duan, R. Effects of millet whole grain supplementation on the lipid profile and gut bacteria in rats fed with high-fat diet. J. Funct. Foods 2019, 59, 49–59. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, R.; Liu, Z.; Niu, Y.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Hypoglycemic effect of prolamin from cooked foxtail millet (Setaria italic) on streptozotocin-induced diabetic mice. Nutrients 2020, 12, 3452. [Google Scholar] [CrossRef]
- Joshi, S.; Srivastava, S. Hypoglycemic and hypolipidemic effect of barnyard millet consumption in type 2 diabetic subjects. Int. J. Curr. Microbiol. Appl. Sci. (IJCMAS) 2021, 10, 467–477. [Google Scholar]
- Nishizawa, N.; Togawa, T.; Park, K.O.; Sato, D.; Miyakoshi, Y.; Inagaki, K.; Ohmori, N.; Ito, Y.; Nagasawa, T. Dietary Japanese millet protein ameliorates plasma levels of adiponectin, glucose, and lipids in type 2 diabetic mice. Biosci. Biotechnol. Biochem. 2009, 73, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.N.; Kable, M.E.; Marco, M.L.; De Leon, A.; Rust, B.; Baker, J.E.; Horn, W.; Burnett, D.; Keim, N.L. The Effects of Moderate Whole Grain Consumption on Fasting Glucose and Lipids, Gastrointestinal Symptoms, and Microbiota. Nutrients 2017, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2019, 26, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreckley, E. The L-cell in nutritional sensing and the regulation of appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, Y.-J.; Doo, H.-K.; Ahn, S.-Y.; Kim, Y.-S.; Seong, J.-K.; Park, I.-S.; Min, B.-H. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J. Ethnopharmacol. 2003, 85, 283–287. [Google Scholar] [CrossRef]
- Feng, K.; Zhu, X.; Chen, T.; Peng, B.; Lu, M.; Zheng, H.; Huang, Q.; Ho, C.T.; Chen, Y.; Cao, Y. Prevention of obesity and hyperlipidemia by heptamethoxyflavone in high-fat diet-induced rats. J. Agric. Food Chem. 2019, 67, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.R.; Sciarrillo, C.M.; Kurti, S.P.; Emerson, E.M.; Rosenkranz, S.K. High-fat meal–induced changes in markers of inflammation and angiogenesis in healthy adults who differ by age and physical activity level. Curr. Dev. Nutr. 2018, 3, nzy098. [Google Scholar] [CrossRef]
- van der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Salinas, C.A.; García, E.G.; Robles, L.; Riano, D.; Ruiz-Gomez, D.G.; García-Ulloa, A.C.; Melgarejo, M.A.; Zamora, M.; Guillen-Pineda, L.E.; Mehta, R. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J. Clin. Endocrinol. Metab. 2008, 93, 4075–4079. [Google Scholar] [CrossRef]
- Cahill, F.; Amini, P.; Wadden, D.; Khalili, S.; Randell, E.; Vasdev, S.; Gulliver, W.; Sun, G. Short-term overfeeding increases circulating adiponectin independent of obesity status. PLoS ONE 2013, 8, e74215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Martins, A.M.D.; Dias, D.M.; Carvalho, C.W.P.; Martino, H.S.D. Germinated millet flour (Pennisetum glaucum (L.) R. Br.) reduces inflammation, oxidative stress, and liver steatosis in rats fed with high-fat high-fructose diet. J. Cereal Sci. 2021, 99, 103207. [Google Scholar] [CrossRef]
- Shi, J.; Shan, S.; Li, H.; Song, G.; Li, Z. Anti-inflammatory effects of millet bran derived-bound polyphenols in LPS-induced HT-29 cell via ROS/miR-149/Akt/NF-κB signaling pathway. Oncotarget 2017, 8, 74582–74594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Liu, M.; Zou, Z.; Wang, M.; Wang, Z.; Ju, X.; Hao, G. Anti-inflammatory activity of peptides derived from millet bran in vitro and in vivo. Food Funct. 2022, 13, 1881–1889. [Google Scholar] [CrossRef]
- Raeder, J.; Larson, D.; Li, W.; Kepko, E.L.; Fuller-Rowell, T. OpenGGCM simulations for the THEMIS mission. Space Sci. Rev. 2008, 141, 535–555. [Google Scholar] [CrossRef]
- Calcaterra, V.; De Amici, M.; Klersy, C.; Torre, C.; Brizzi, V.; Scaglia, F.; Albanesi, M.; Albertini, R.; Allais, B.; Larizza, D. Adiponectin, IL-10 and metabolic syndrome in obese children and adolescents. Acta Bio-Med. Atenei Parm. 2009, 80, 117–123. [Google Scholar]
- Başaranoğlu, M.; Kayaçetin, S.; Yılmaz, N.; Kayaçetin, E.; Tarçın, O.; Sonsuz, A. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2223. [Google Scholar] [CrossRef]
- Tell, G.; Vascotto, C.; Tiribelli, C. Alterations in the redox state and liver damage: Hints from the EASL Basic School of Hepatology. J. Hepatol. 2013, 58, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Green, R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019, 3, 55–64. [Google Scholar] [CrossRef]
- Lian, C.-Y.; Zhai, Z.-Z.; Li, Z.-F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem.-Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.G. Endoplasmic reticulum stress and autophagy dysregulation in alcoholic and non-alcoholic liver diseases. Clin. Mol. Hepatol. 2020, 26, 715. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, A.M.; Al-Oweisi, F.A.; Edwards, S.G.; Al-Nadabi, H.; Al-Fahdi, A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothny-Wilkes, G.; Kulms, D.; Poppelmann, B.; Luger, T.A.; Kubin, M.; Schwarz, T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 1998, 273, 29247–29253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R. The role of adipose tissue in Fatty liver diseases. Liver Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Régnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C.; et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 6489. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, R.; Mao, J. Separation and identification of antioxidant peptides from foxtail millet (Setaria italica) prolamins enzymatic hydrolysate. Cereal Chem. 2019, 96, 981–993. [Google Scholar] [CrossRef]
- Kalaivanisailaja, J.; Manju, V.; Nalini, N. Lipid profile in mice fed a high-fat diet after exogenous leptin administration. Pol. J. Pharmacol. 2003, 55, 763–770. [Google Scholar]
- Veerapur, V.; Prabhakar, K.; Kandadi, M.; Srinivasan, K.; Unnikrishnan, M. Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: A mechanistic approach. Pharm. Biol. 2010, 48, 1137–1148. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Wang, N.; Zhou, Q.; Lu, Y.; Qu, Z.; Wang, H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021, 9, 4266–4277. [Google Scholar] [CrossRef]
- Xia, X.; Li, G.; Song, J.; Zheng, J.; Kan, J. Hypocholesterolaemic effect of whole-grain highland hull-less barley in rats fed a high-fat diet. Br. J. Nutr. 2018, 119, 1102–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-M.; Yuan, R.-S.; Zhuang, W.-Y.; Sun, J.-H.; Wu, J.-Y.; Li, H.; Chen, J.-G. Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis. 2016, 15, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, F.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Gnoni, A. Quercetin inhibition of SREBPs and ChREBP expression results in reduced cholesterol and fatty acid synthesis in C6 glioma cells. Int. J. Biochem. Cell Biol. 2019, 117, 105618. [Google Scholar] [CrossRef]
- Hodson, L.; Rosqvist, F.; Parry, S.A. The influence of dietary fatty acids on liver fat content and metabolism. Proc. Nutr. Soc. 2020, 79, 30–41. [Google Scholar] [CrossRef]
- Saritha, K.; Mani, A.E.; Priyalaxmi, M.; Patterson, J. Antibacterial activity and biochemical constituents of seaweed Ulva lactuca. Glob. J. Pharmacol. 2013, 7, 276–282. [Google Scholar]
- Tall, A.R. Plasma cholesteryl ester transfer protein. J. Lipid Res. 1993, 34, 1255–1274. [Google Scholar] [CrossRef]
- Mabuchi, H.; Nohara, A.; Inazu, A. Cholesteryl ester transfer protein (CETP) deficiency and CETP inhibitors. Mol. Cells 2014, 37, 777. [Google Scholar] [CrossRef] [Green Version]
- Berlian, G.; Tandrasasmita, O.M.; Suciptan, D.A.; Tjandrawinata, R.R. Forhidrol, a bioactive fraction of Phaleria macrocarpa (Scheff.) Boerl., increases reverse cholesterol transport pathway by down-regulation of cholesteryl ester transfer protein activity. J. Biol. Res. Boll. Della Soc. Ital. Di Biol. Sper. 2018, 91, 1. [Google Scholar] [CrossRef] [Green Version]
- Basu, A. Role of berry bioactive compounds on lipids and lipoproteins in diabetes and metabolic syndrome. Nutrients 2019, 11, 1983. [Google Scholar] [CrossRef] [Green Version]
- Rizzetto, S.; Koppstein, D.N.P.; Samir, J.; Singh, M.; Reed, J.H.; Cai, C.H.; Lloyd, A.R.; Eltahla, A.A.; Goodnow, C.C.; Luciani, F. B-cell receptor reconstruction from single-cell RNA-seq with VDJPuzzle. Bioinformatics 2018, 34, 2846–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Hikita, N. Cord blood-based approach to assess candidate vaccine adjuvants designed for neonates and infants. Vaccines 2021, 9, 95. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Liu, J.; Kang, R.; Tang, D. Mitophagy receptors in tumor biology. Front. Cell Dev. Biol. 2020, 8, 594203. [Google Scholar] [CrossRef]
- Mana, M.D.; Hussey, A.M.; Tzouanas, C.N.; Imada, S.; Barrera Millan, Y.; Bahceci, D.; Saiz, D.R.; Webb, A.T.; Lewis, C.A.; Carmeliet, P.; et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 2021, 35, 109212. [Google Scholar] [CrossRef]
- Fan-Jiang, P.Y.; Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Pterostilbene attenuates high-fat diet and dextran sulfate sodium-induced colitis via suppressing inflammation and intestinal fibrosis in mice. J. Agric. Food Chem. 2021, 69, 7093–7103. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Zhou, X.E.; Melcher, K.; Xu, H.E. Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 2017, 45, 150–159. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yu, E.; Lu, M.; Xie, J. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 527, 735428. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Sinha, R.A.; Singh, B.K.; Yen, P.M. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Sakamoto, S. Variation, geographical distribution and genetical analysis of esterase isozymes in foxtail millet, Setaria italica (L.) P. Beauv. Theor. Appl. Genet. 1984, 67, 529–533. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Shen, R.; Ma, Y.; Jiang, L.; Dong, J.; Zhu, Y.; Ren, G. Chemical composition, antioxidant, and antiproliferative activities of nine Chinese proso millet varieties. Food Agric. Immunol. 2018, 29, 625–637. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Sha, X.; Rahman, E.; Wang, Y.; Ji, B.; Wu, W.; Zhou, F. Antioxidant capacity and amino acid profile of millet bran wine and the synergistic interaction between major polyphenols. J. Food Sci. Technol. 2018, 55, 1010–1020. [Google Scholar] [CrossRef]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. Assessment of the phenolic compounds of pearl and finger millets obtained from South Africa and Zimbabwe. Food Sci. Nutr. 2020, 8, 4888–4896. [Google Scholar] [CrossRef]





| STD (D12450H) | HFD (D12451) | |||
|---|---|---|---|---|
| Gm % | Kcal % | Gm % | Kcal % | |
| Carbogydrate | 67.3 | 70% | 41 | 35% |
| Proteins | 19.2 | 20% | 24 | 20% |
| Fat | 4.3 | 10% | 24 | 45% |
| Total (Kcal/gm) | 100 (3.85 Kcal/g) | 100 (4.73 Kcal/g) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022, 14, 1791. https://doi.org/10.3390/nu14091791
Alzahrani NS, Alshammari GM, El-Ansary A, Yagoub AEA, Amina M, Saleh A, Yahya MA. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients. 2022; 14(9):1791. https://doi.org/10.3390/nu14091791
Chicago/Turabian StyleAlzahrani, Nadiah S., Ghedeir M. Alshammari, Afaf El-Ansary, Abu ElGasim A. Yagoub, Musarat Amina, Ali Saleh, and Mohammed Abdo Yahya. 2022. "Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet" Nutrients 14, no. 9: 1791. https://doi.org/10.3390/nu14091791
APA StyleAlzahrani, N. S., Alshammari, G. M., El-Ansary, A., Yagoub, A. E. A., Amina, M., Saleh, A., & Yahya, M. A. (2022). Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients, 14(9), 1791. https://doi.org/10.3390/nu14091791







