Cyclo(His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides
Abstract
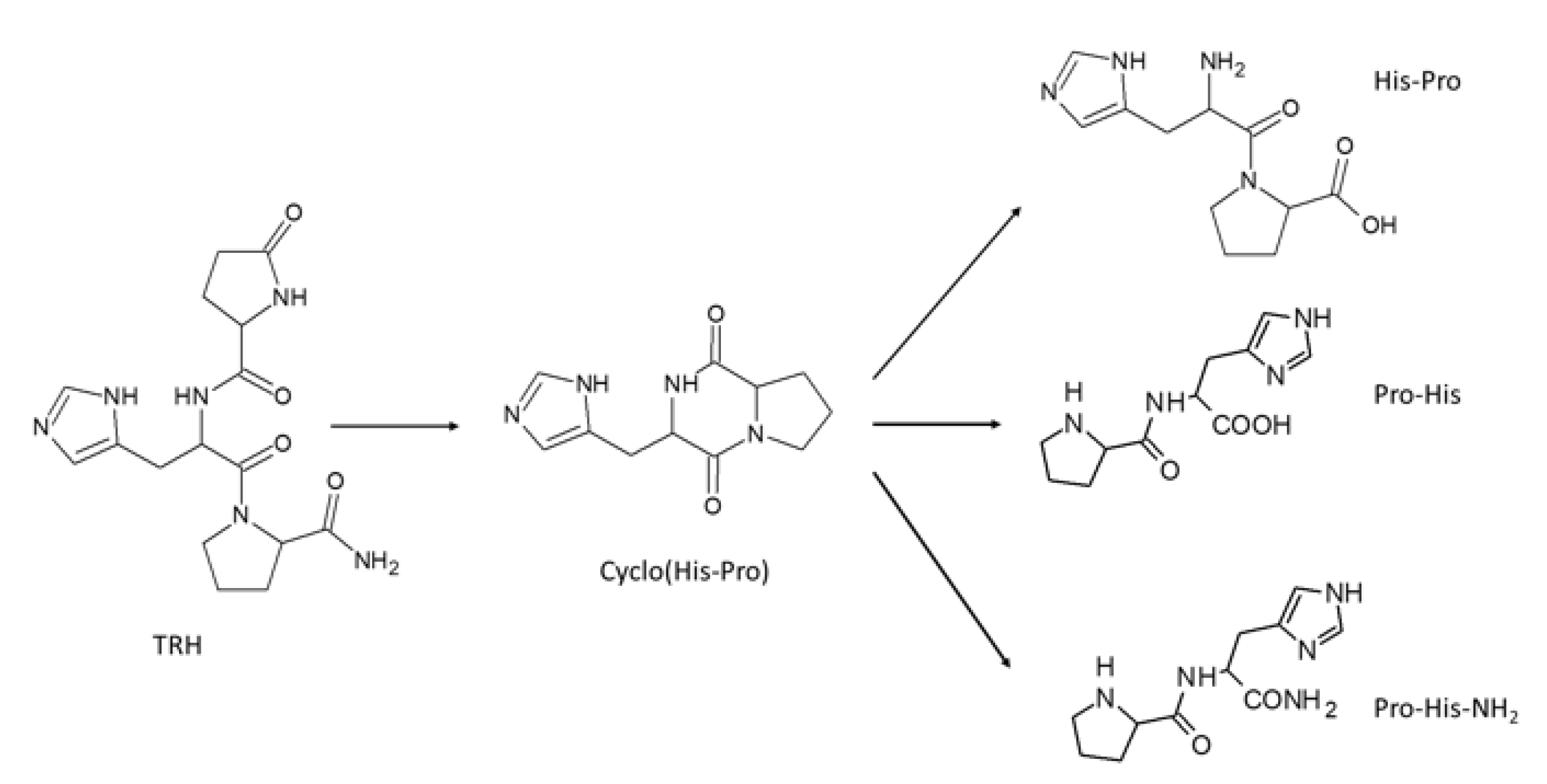
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
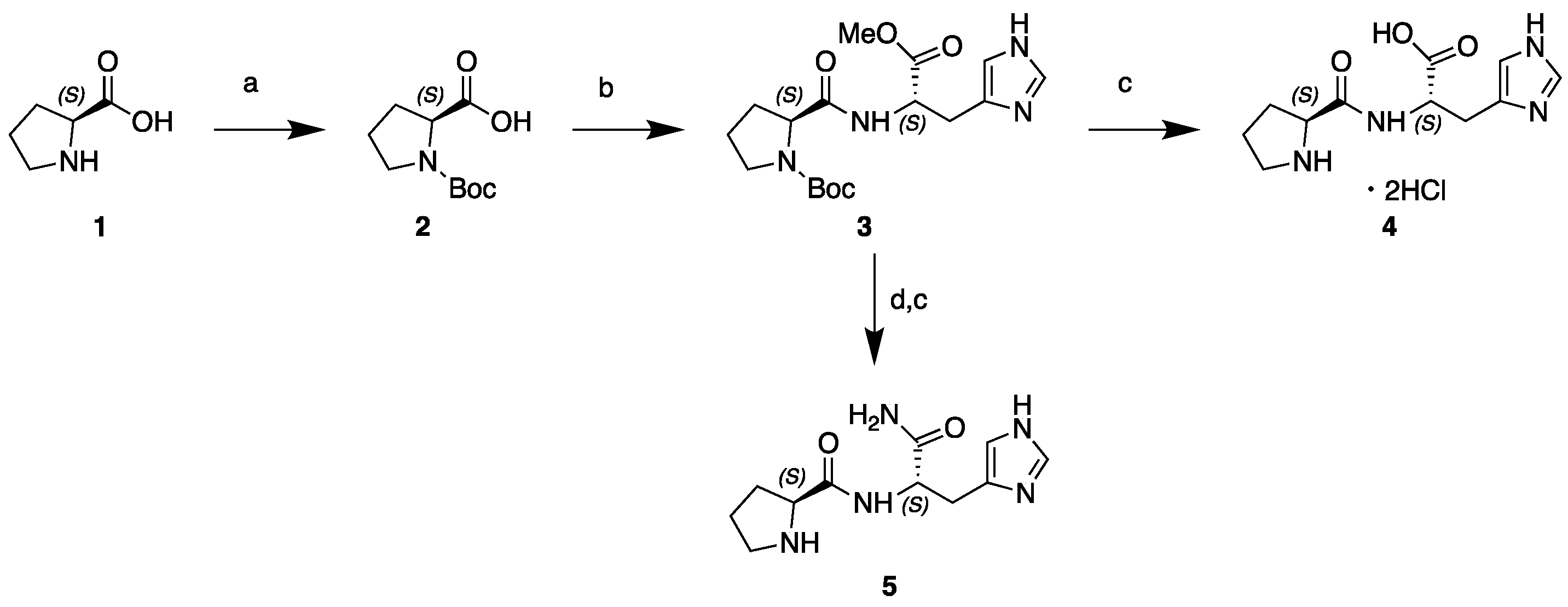
2.2. Synthesis of L-Pro-l-His and l-Pro-l-His-NH2
2.2.1. N-Boc-l-Pro-OH (2)
2.2.2. N-Boc-l-Pro-l-His-OMe (3)
2.2.3. l-Pro-l-His-OH dihydrochloride (4)
2.2.4. l-Pro-l-His-NH2 (5)
2.3. Quenching Activity towards HNE
2.4. Serum Stability
2.5. Computational Studies
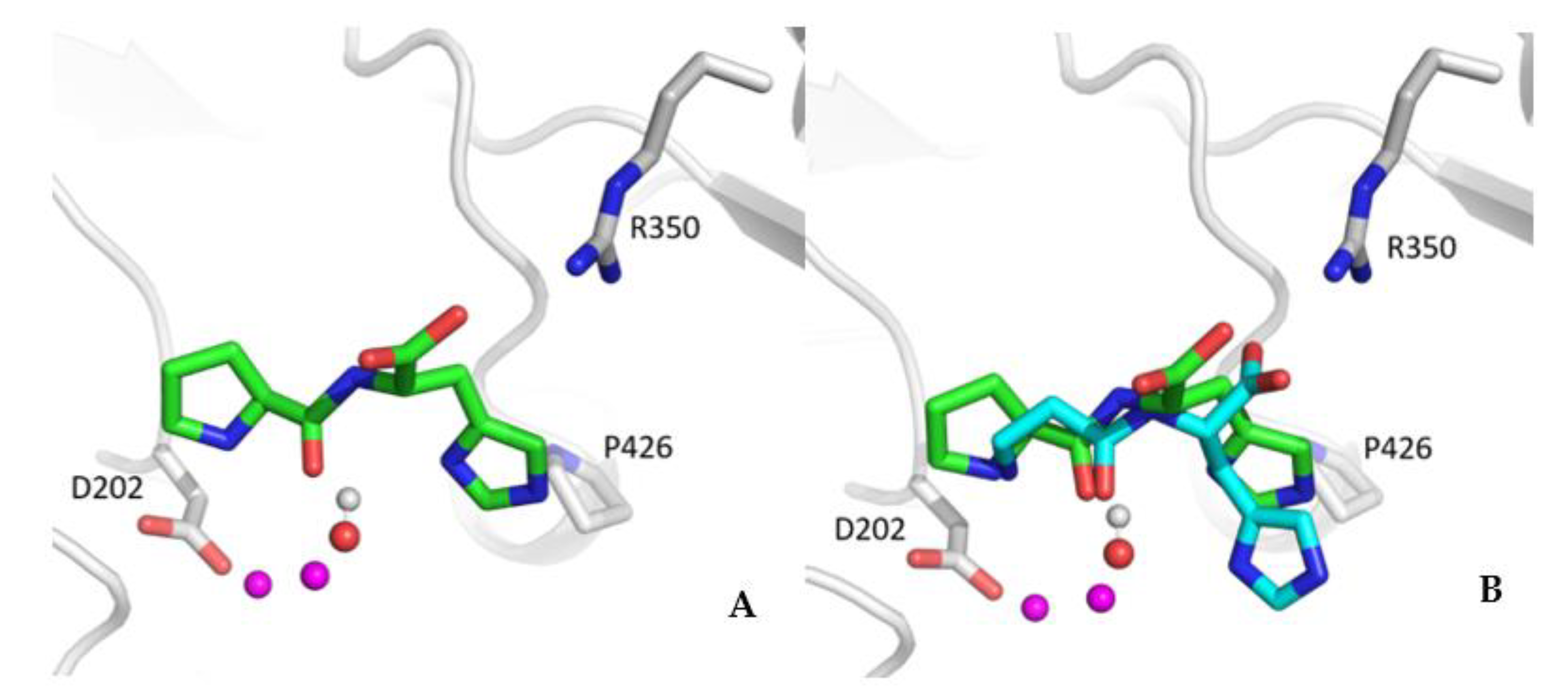
3. Results
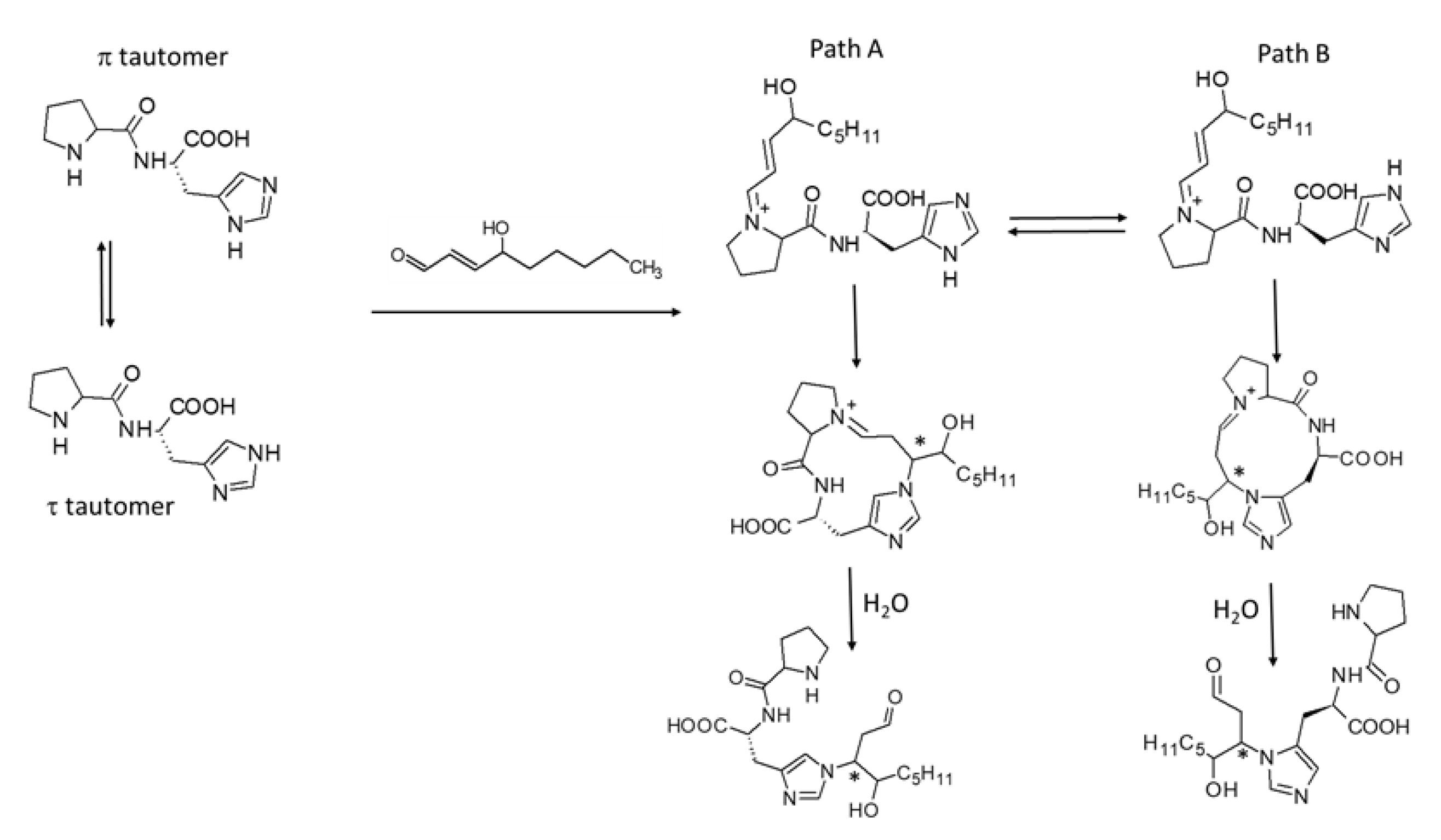
3.1. Quenching Activity towards HNE of the Tested Peptides
3.2. Serum Stability of the Studied Peptides
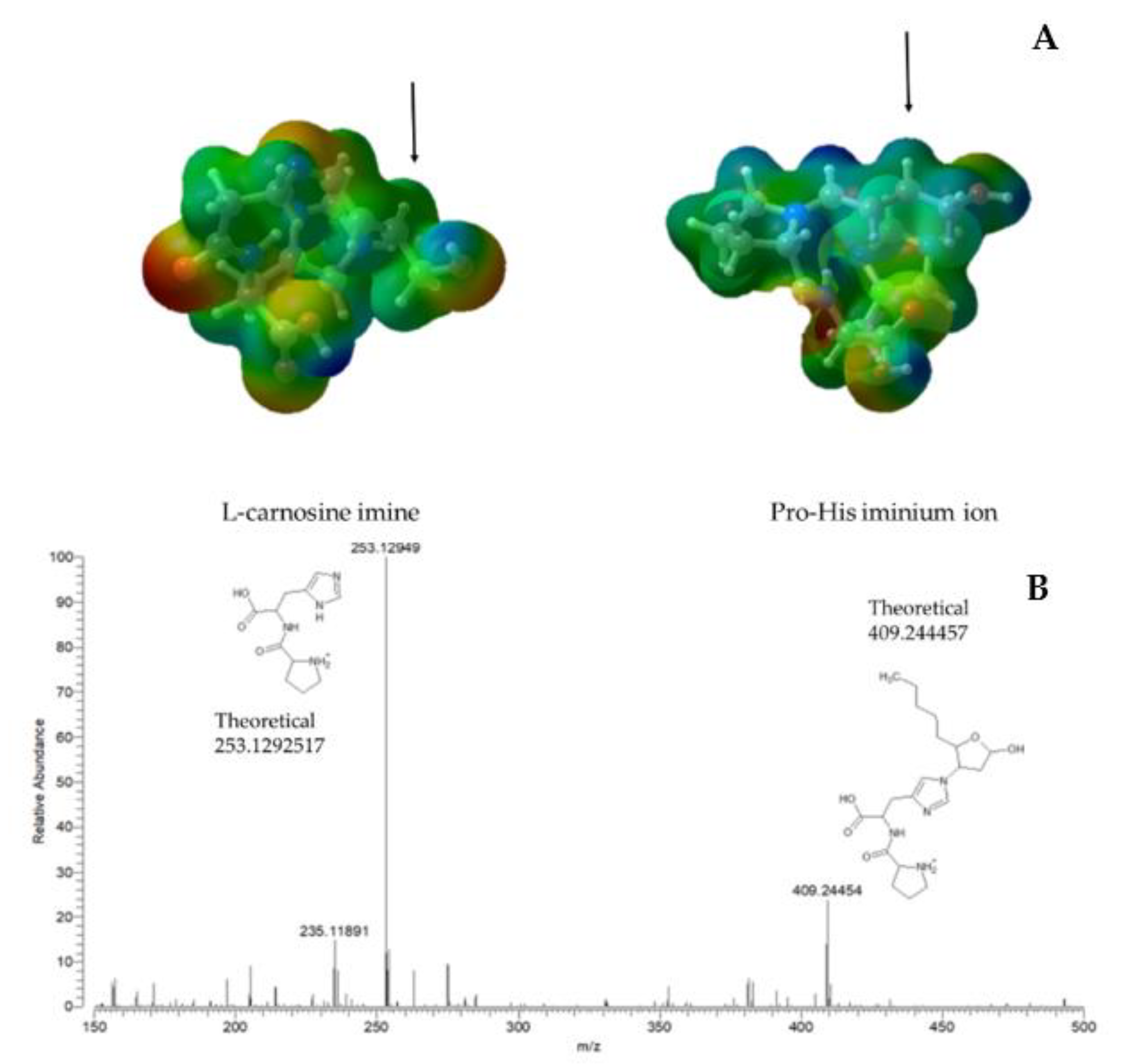
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilton, C.W.; Prasad, C.; Svec, F.; Vo, P.; Reddy, S. Cyclo(His-Pro) in Nutritional Supplements. Lancet 1990, 336, 1455. [Google Scholar] [CrossRef]
- Hilton, C.W.; Prasad, C.; Vo, P.; Mouton, C. Food Contains the Bioactive Peptide, Cyclo(His-Pro). J. Clin. Endocrinol. Metab. 1992, 75, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Imrhan, V.; Juma, S.; Maziarz, M.; Prasad, A.; Tiernan, C.; Vijayagopal, P. Bioactive Plant Metabolites in the Management of Non-Communicable Metabolic Diseases: Looking at Opportunities beyond the Horizon. Metabolites 2015, 5, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.K.; Rosenthal, M.J.; Song, A.M.; Yang, H.; Ao, Y.; Yamaguchi, D.T. Raw Vegetable Food Containing High Cyclo (His-pro) Improved Insulin Sensitivity and Body Weight Control. Metab. Clin. Exp. 2005, 54, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Lee, H.-S.; Choi, J.W.; Ra, K.S.; Kim, M.-R.; Suh, H.J. Glucose Tolerance and Antioxidant Activity of Spent Brewer’s Yeast Hydrolysate with a High Content of Cyclo-His-Pro (CHP). J. Food Sci. 2011, 76, C272–C278. [Google Scholar] [CrossRef]
- Lee, H.J.; Son, H.S.; Park, C.; Suh, H.J. Preparation of Yeast Hydrolysate Enriched in Cyclo-His-Pro (CHP) by Enzymatic Hydrolysis and Evaluation of Its Functionality. Prev. Nutr. Food Sci. 2015, 20, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-J.; Wang, Z.-Y.; Liu, X.-F.; Guo, X.-N.; He, X.-P.; Wensel, P.C.; Zhang, B.-R. Construction of an Industrial Brewing Yeast Strain to Manufacture Beer with Low Caloric Content and Improved Flavor. J. Microbiol. Biotechnol. 2010, 20, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Hilton, C.W.; Mizuma, H.; Svec, F.; Prasad, C. Relationship between Plasma Cyclo (His-Pro), a Neuropeptide Common to Processed Protein-Rich Food, and C-Peptide/Insulin Molar Ratio in Obese Women. Nutr. Neurosci. 2001, 4, 469–474. [Google Scholar] [CrossRef]
- Prasad, C.; Hilton, C.W.; Svec, F.; Onaivi, E.S.; Vo, P. Could Dietary Proteins Serve as Cyclo(His-Pro) Precursors? Neuropeptides 1991, 19, 17–21. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Grottelli, S.; Galli, F. Focus on Cyclo(His-Pro): History and Perspectives as Antioxidant Peptide. Amino Acids 2008, 35, 283–289. [Google Scholar] [CrossRef]
- Møss, J.; Bundgaard, H. Kinetics and Mechanism of the Facile Cyclization of Histidyl-Prolineamide to Cyclo (His-Pro) in Aqueous Solution and the Competitive Influence of Human Plasma. J. Pharm. Pharmacol. 1990, 42, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Peirce, M.J.; Minelli, A. Cyclic Dipeptides: From Bugs to Brain. Trends Mol. Med. 2014, 20, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Grimberg, G.; Stenzel, W.; Schömig, E. Identification of the Endogenous Key Substrates of the Human Organic Cation Transporter OCT2 and Their Implication in Function of Dopaminergic Neurons. PLoS ONE 2007, 2, e385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minelli, A.; Grottelli, S.; Mierla, A.; Pinnen, F.; Cacciatore, I.; Bellezza, I. Cyclo(His-Pro) Exerts Anti-Inflammatory Effects by Modulating NF-ΚB and Nrf2 Signalling. Int. J. Biochem. Cell Biol. 2012, 44, 525–535. [Google Scholar] [CrossRef]
- Jung, E.Y.; Hong, Y.H.; Park, C.; Suh, H.J. Effects of Cyclo-His-Pro-Enriched Yeast Hydrolysate on Blood Glucose Levels and Lipid Metabolism in Obese Diabetic Ob/Ob Mice. Nutr. Res. Pract. 2016, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.A.; Yun, J.W.; Park, H.S.; Choi, J.W. Hypoglycemic Dipeptide Cyclo (His-Pro) Significantly Altered Plasma Proteome in Streptozocin-Induced Diabetic Rats and Genetically-Diabetic (Ob/Ob) Mice. Mol. Biol. Rep. 2013, 40, 1753–1765. [Google Scholar] [CrossRef]
- Grottelli, S.; Ferrari, I.; Pietrini, G.; Peirce, M.; Minelli, A.; Bellezza, I. The Role of Cyclo(His-Pro) in Neurodegeneration. Int. J. Mol. Sci. 2016, 17, 1332. [Google Scholar] [CrossRef] [Green Version]
- Imamura, M.; Prasad, C. Cyclo (His-Pro) Potentiates GABA/Ethanol-Mediated Chloride Uptake by Neurosynaptosomes. Peptides 2003, 24, 445–448. [Google Scholar] [CrossRef]
- Ikegami, H.; Prasad, C. Neuropeptide-Dopamine Interactions. V. Cyclo(His-Pro) Regulation of Striatal Dopamine Transporter Complex. Peptides 1990, 11, 145–148. [Google Scholar] [CrossRef]
- Minelli, A.; Conte, C.; Grottelli, S.; Bellezza, M.; Cacciatore, I.; Bolaños, J.P. Cyclo(His-Pro) Promotes Cytoprotection by Activating Nrf2-Mediated up-Regulation of Antioxidant Defence. J. Cell. Mol. Med. 2009, 13, 1149–1161. [Google Scholar] [CrossRef] [Green Version]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and Non-Enzymatic Detoxification of 4-Hydroxynonenal: Methodological Aspects and Biological Consequences. Free. Radic. Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Bischoff, D.S.; Song, A.M.; Uyemura, K.; Yamaguchi, D.T. Metabolic Relationship between Diabetes and Alzheimer’s Disease Affected by Cyclo(His-Pro) plus Zinc Treatment. BBA Clin. 2017, 7, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Straniero, V.; Pedretti, A.; Fumagalli, L.; Bolchi, C.; Pallavicini, M.; Valoti, E.; Testa, B. Predicting the Physicochemical Profile of Diastereoisomeric Histidine-Containing Dipeptides by Property Space Analysis. Chirality 2012, 24, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Aldini, G.; Fumagalli, L.; Dallanoce, C.; Angeli, A.; Supuran, C.T. Activation Effects of Carnosine- and Histidine-Containing Dipeptides on Human Carbonic Anhydrases: A Comprehensive Study. Int. J. Mol. Sci. 2020, 21, 1761. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’Amato, A.; Vistoli, G.; Carini, M. Understanding the Antioxidant and Carbonyl Sequestering Activity of Carnosine: Direct and Indirect Mechanisms. Free. Radic. Res. 2021, 55, 321–330. [Google Scholar] [CrossRef]
- Vistoli, G.; Colzani, M.; Mazzolari, A.; De Maddis, D.; Grazioso, G.; Pedretti, A.; Carini, M.; Aldini, G. Computational Approaches in the Rational Design of Improved Carbonyl Quenchers: Focus on Histidine Containing Dipeptides. Future Med. Chem. 2016, 8, 1721–1737. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Lynch Miller, J.; Neuhaus, F.C.; Stammer, C.H.; Michalsky, J.; Ctvrtink, J.; Horakova, A.; Bydzovsky, V.; Hidy, P.H.; Hodge, E.B.; Young, V.V.; et al. Reactions of 3,6-Bis(Aminoxymethyl)-2,5-Piperazinedione with Acid and Alkali. Kinetic Study. J. Org. Chem. 2002, 33, 3908–3913. [Google Scholar] [CrossRef]
- Campbell, T.D.; Hart, C.A.; Febrian, R.; Cheneler, M.L.; Bracher, P.J. The Opposite Effect of K+ and Na+ on the Hydrolysis of Linear and Cyclic Dipeptides. Tetrahedron Lett. 2018, 59, 2264–2267. [Google Scholar] [CrossRef]
- Vistoli, G.; Colzani, M.; Mazzolari, A.; Gilardoni, E.; Rivaletto, C.; Carini, M.; Aldini, G. Quenching Activity of Carnosine Derivatives towards Reactive Carbonyl Species: Focus on A−(Methylglyoxal) and Β−(Malondialdehyde) Dicarbonyls. Biochem. Biophys. Res. Commun. 2017, 492, 487–492. [Google Scholar] [CrossRef]
- Fukizawa, S.; Watanabe, H.; Abe, K. Composition That Contains Ring-Shaped Dipeptide and Inhibits Serum Carnosinase. WO2017010537A1, 19 January 2017. [Google Scholar]
- Schjøth-Eskesen, C.; Jensen, H.H. Efficient Arndt-Eistert Synthesis of Selective 5-HT7 Receptor Antagonist SB-269970. Synth. Commun. 2009, 39, 3243–3253. [Google Scholar] [CrossRef]
- Gilardoni, E.; Gervasoni, S.; Maspero, M.; Dallanoce, C.; Vistoli, G.; Carini, M.; Aldini, G.; Regazzoni, L. Development of a Direct LC-ESI-MS Method for the Measurement of Human Serum Carnosinase Activity. J. Pharm. Biomed. Anal. 2020, 189, 113440. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA Suite of Programs: An Versatile Platform for Cheminformatics and Drug Design Projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C. Molecular Recognition of Receptor Sites Using a Genetic Algorithm with a Description of Desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Orioli, M.; Aldini, G.; Beretta, G.; Facino, R.M.; Carini, M. LC–ESI-MS/MS Determination of 4-Hydroxy-Trans-2-Nonenal Michael Adducts with Cysteine and Histidine-Containing Peptides as Early Markers of Oxidative Stress in Excitable Tissues. J. Chromatogr. B 2005, 827, 109–118. [Google Scholar] [CrossRef] [PubMed]
- van der Helm, M.P.; Klemm, B.; Eelkema, R. Organocatalysis in Aqueous Media. Nat. Rev. Chem. 2019, 3, 491–508. [Google Scholar] [CrossRef]
- Vistoli, G.; Carini, M.; Aldini, G. Transforming Dietary Peptides in Promising Leaad Compounds: The Case of Bioavailable Carnosine Analogs. Amino Acids 2012, 43, 111–126. [Google Scholar] [CrossRef]
- Vachan, B.S.; Karuppasamy, M.; Vinoth, P.; Vivek Kumar, S.; Perumal, S.; Sridharan, V.; Menéndez, J.C. Proline and Its Derivatives as Organocatalysts for Multi- Component Reactions in Aqueous Media: Synergic Pathways to the Green Synthesis of Heterocycles. Adv. Synth. Catal. 2020, 362, 87–110. [Google Scholar] [CrossRef]
- Wilk, P.; Wątor, E.; Weiss, M.S. Prolidase—A Protein with Many Faces. Biochimie 2021, 183, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.; Wolf, C.F.; Keck, F.S.; Rosenthal, J. Thyrotropin-Releasing Hormone: Pharmacokinetic and Pharmacodynamic Properties in Chronic Renal Failure. Clin. Nephrol. 1992, 38, 214–218. [Google Scholar] [PubMed]
- Mori, M.; Pegues, J.; Prasad, C.; Wilber, J.; Peterson, J.; Githens, S. Histidyl-Proline Diketopiperazine Cyclo (His-Pro): Identification and Characterization in Rat Pancreatic Islets. Biochem. Biophys. Res. Commun. 1983, 115, 281–286. [Google Scholar] [CrossRef]
- Song, M.K.; Hwang, I.K.; Rosenthal, M.J.; Harris, D.M.; Yamaguchi, D.T.; Yip, I.; Go, V.L.W. Anti-Hyperglycemic Activity of Zinc plus Cyclo (His-pro) in Genetically Diabetic Goto-Kakizaki and Aged Rats. Exp. Biol. Med. 2003, 228, 1338–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.B. Protective Effect of Cyclo(His-Pro) on Streptozotocin-Induced Cytotoxicity and Apoptosis In Vitro. J. Microbiol. Biotechnol. 2011, 21, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Daimon, C.; Chirdon, P.; Maudsley, S.; Martin, B. The Role of Thyrotropin Releasing Hormone in Aging and Neurodegenerative Diseases. Am. J. Alzheimer’s Dis. 2013, 1, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Blake Monroe, T.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D.; et al. A Carnosine Analog Mitigates Metabolic Disorders of Obesity by Reducing Carbonyl Stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef]
- Barbas, C.F. Organocatalysis Lost: Modern Chemistry, Ancient Chemistry, and an Unseen Biosynthetic Apparatus. Angew. Chem. Int. Ed. Engl. 2008, 47, 42–47. [Google Scholar] [CrossRef] [PubMed]
| Compound | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (V) |
|---|---|---|---|
| His-Pro & Pro-His | 253.2 | 138.1 | 15 |
| 253.2 | 110.2 | 30 | |
| Pro-His-NH2 | 252.1 | 155.1 | 20 |
| 252.1 | 110.2 | 35 | |
| TRH | 363.1 | 221.3 | 35 |
| 363.1 | 115.0 | 25 | |
| Ciclo(His-Pro) | 235.1 | 162.1 | 30 |
| 235.1 | 110.2 | 26 |
| Compound | HNE Quenching (%) | Estimated HNE t1/2 (min) | Serum Stability t1/2 (min) | ||
|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | |||
| blank | −0.83 ± 1.79 | −2.90 ± 3.49 | −1.56 ± 2.13 | - | - |
| l-carnosine | 17.04 ± 6.17 | 29.05 ± 4.33 | 40.40 ±3.73 | 240.8 | 1.17 |
| CHP | 2.42 ± 0.71 | 5.46 ± 0.78 | 8.09 ± 0.77 | 1465.0 | Stable |
| TRH | 2.31 ± 6.72 | 4.16 ± 6.16 | 6.17 ± 6.41 | 1973.7 | 24.35 |
| HisPro | −0.33 ± 0.45 | −0.26 ± 0.53 | 3.54 ± 1.66 | 3878.5 | 3.08 |
| ProHis | 20.39 ± 1.14 | 36.94 ± 0.80 | 49.87 ± 1.06 | 180.6 | 33.36 |
| ProHis-NH2 | 17.08 ± 1.11 | 27.66 ± 0.58 | 36.29 ± 1.08 | 272.3 | 127.68 |
| Pro + His | 3.72 ± 2.25 | 6.84 ± 0.65 | 9.82 ± 1.42 | 1210.9 | - |
| Peptide | Chirality | Michael Addition | Hydrolysis | Overall Reaction | |||
|---|---|---|---|---|---|---|---|
| Path A | Path B | Path A | Path B | Path A | Path B | ||
| l-carnosine | - | −8.05 | −0.69 | −16.71 | −8.46 | −24.77 | −9.15 |
| Pro-His | (R) | −6.83 | −1.90 | −17.91 | −10.15 | −24.74 | −12.05 |
| Pro-His | (S) | −3.30 | +3.35 | −21.66 | −19.43 | −24.95 | −16.08 |
| Pro-His-NH2 | (R) | −0.76 | −1.57 | −12.93 | −8.65 | −13.69 | −10.22 |
| Pro-His-NH2 | (S) | +6.37 | +2.25 | −20.36 | −17.04 | −13.99 | −14.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Gervasoni, S.; Gilardoni, E.; Mazzolari, A.; Aldini, G.; Vistoli, G. Cyclo(His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides. Nutrients 2022, 14, 1775. https://doi.org/10.3390/nu14091775
Regazzoni L, Fumagalli L, Artasensi A, Gervasoni S, Gilardoni E, Mazzolari A, Aldini G, Vistoli G. Cyclo(His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides. Nutrients. 2022; 14(9):1775. https://doi.org/10.3390/nu14091775
Chicago/Turabian StyleRegazzoni, Luca, Laura Fumagalli, Angelica Artasensi, Silvia Gervasoni, Ettore Gilardoni, Angelica Mazzolari, Giancarlo Aldini, and Giulio Vistoli. 2022. "Cyclo(His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides" Nutrients 14, no. 9: 1775. https://doi.org/10.3390/nu14091775
APA StyleRegazzoni, L., Fumagalli, L., Artasensi, A., Gervasoni, S., Gilardoni, E., Mazzolari, A., Aldini, G., & Vistoli, G. (2022). Cyclo(His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides. Nutrients, 14(9), 1775. https://doi.org/10.3390/nu14091775









