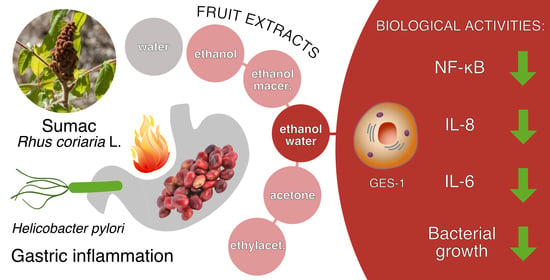
The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Method and Extracts Characterization
2.2. Total Phenol Content Assay
2.3. Cell Culture
2.4. Bacterial Culture
2.5. Cell Treatment
2.6. Cytotoxicity Assay
2.7. Measurement of IL-8 and IL-6 Release
2.8. NF-κB Activation
2.8.1. Western Blotting Analysis
2.8.2. Immunofluorescence
2.9. Minimum Inhibitory Concentration (MIC)
2.10. Urease Activity
2.11. In Vitro Simulated Gastric Digestion
2.12. Statistical Analysis
3. Results
3.1. Cytotoxicity of the Extracts in Human Gastric GES-1 Cells
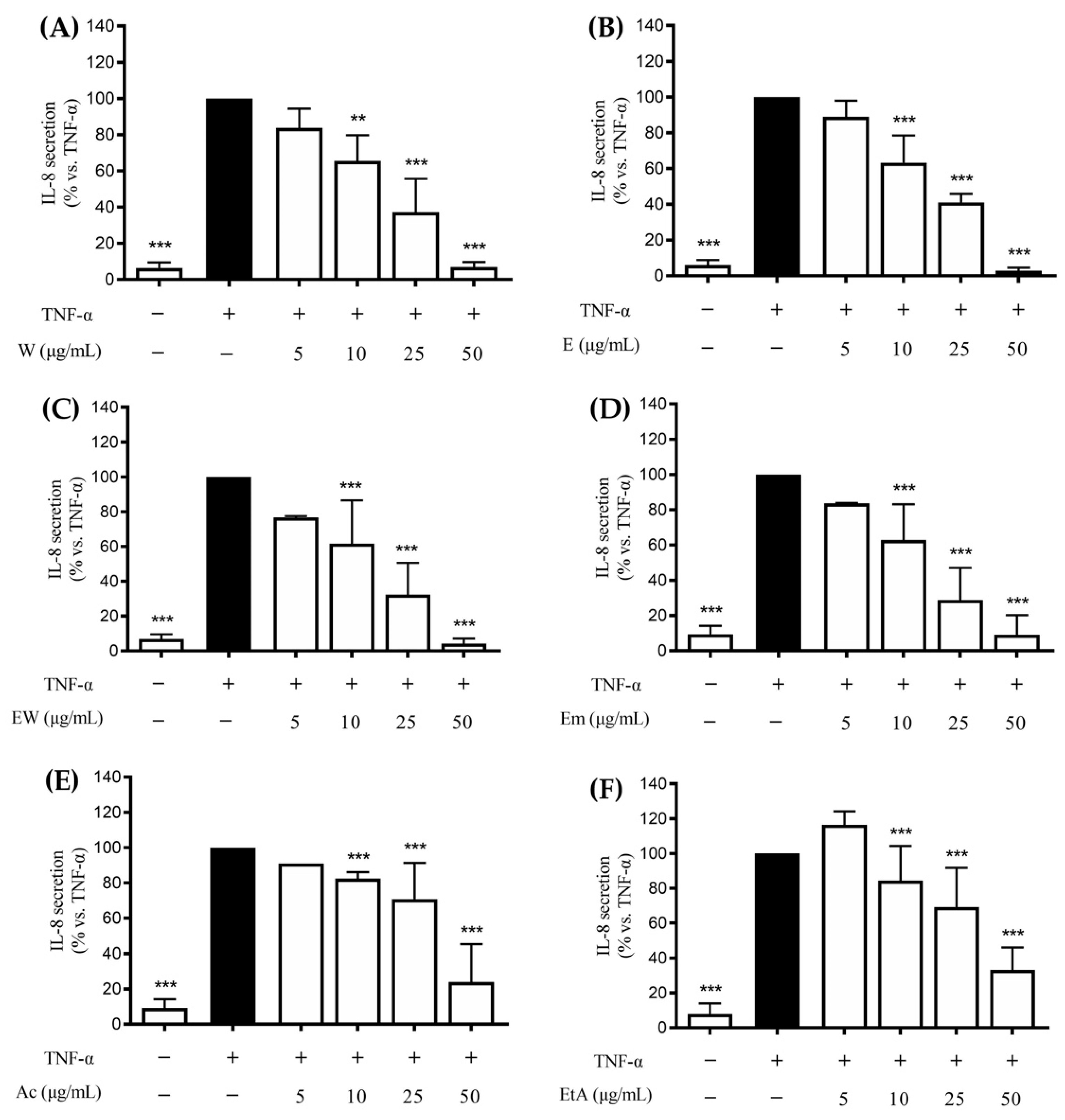
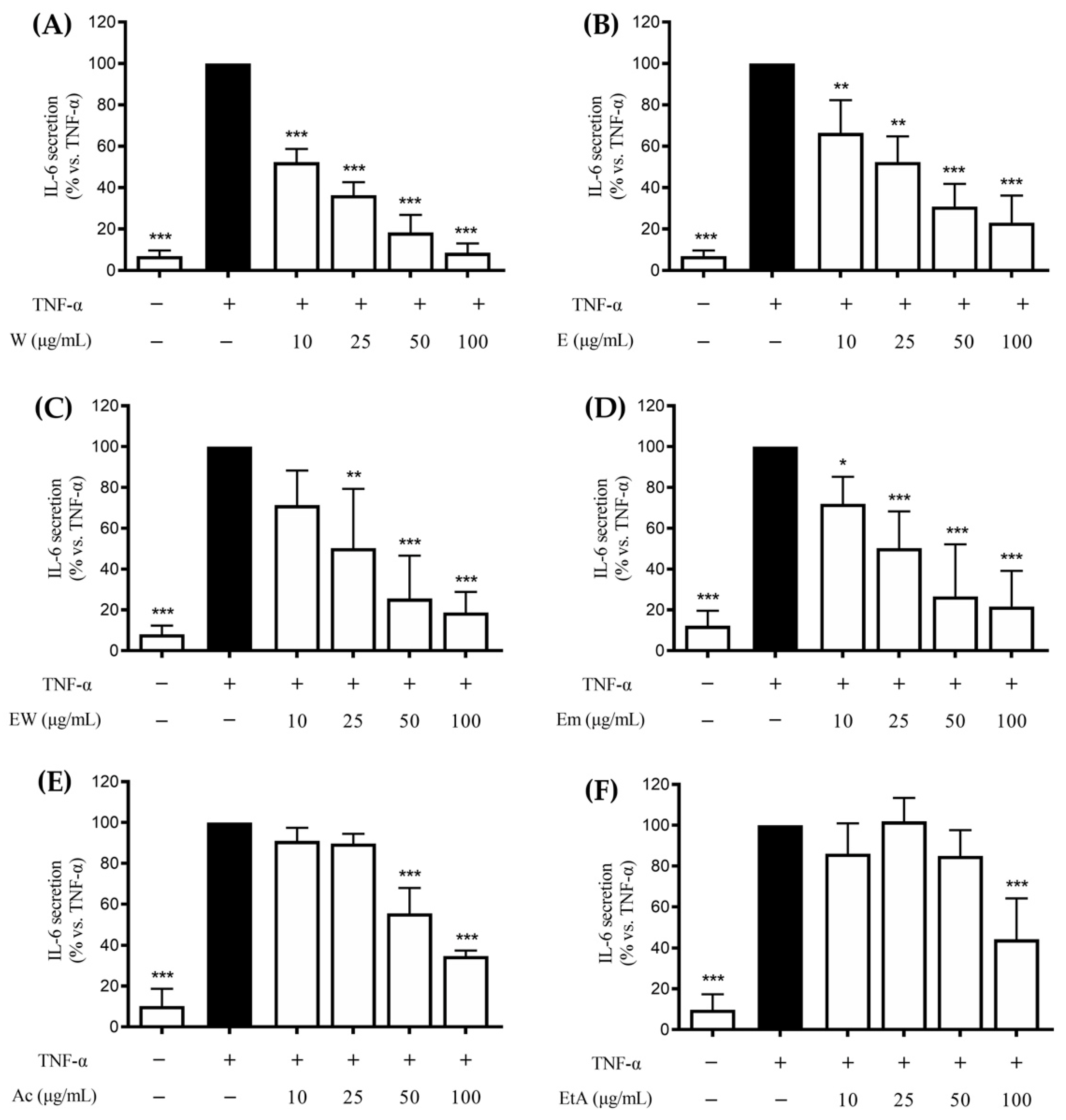
3.2. Effect of the Extracts on the TNF-α-Induced IL-8 and IL-6 Release in GES-1 Cells
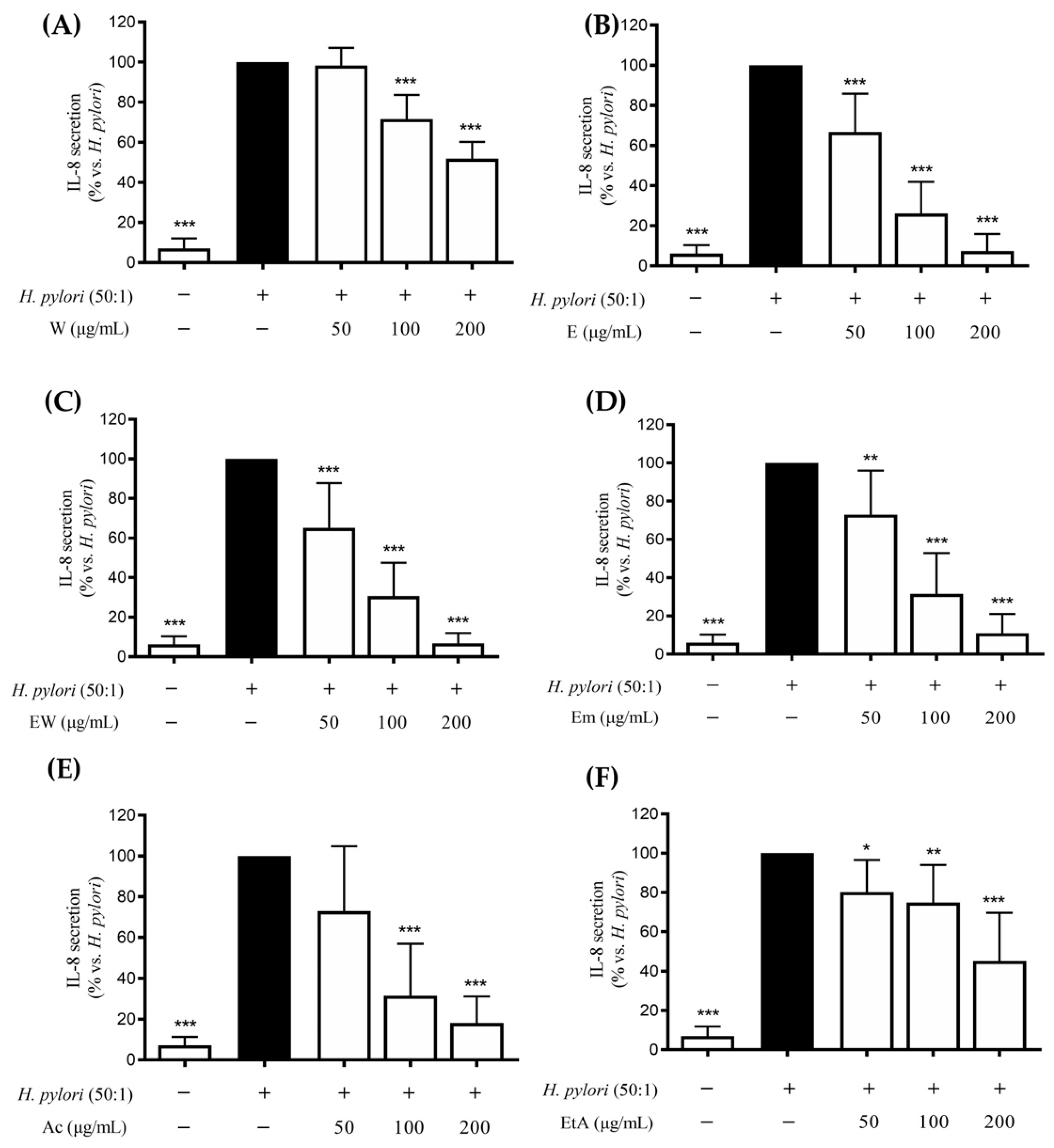
3.3. Effect of the Extracts on the H. pylori-Induced IL-8 and IL-6 Release in GES-1 Cells
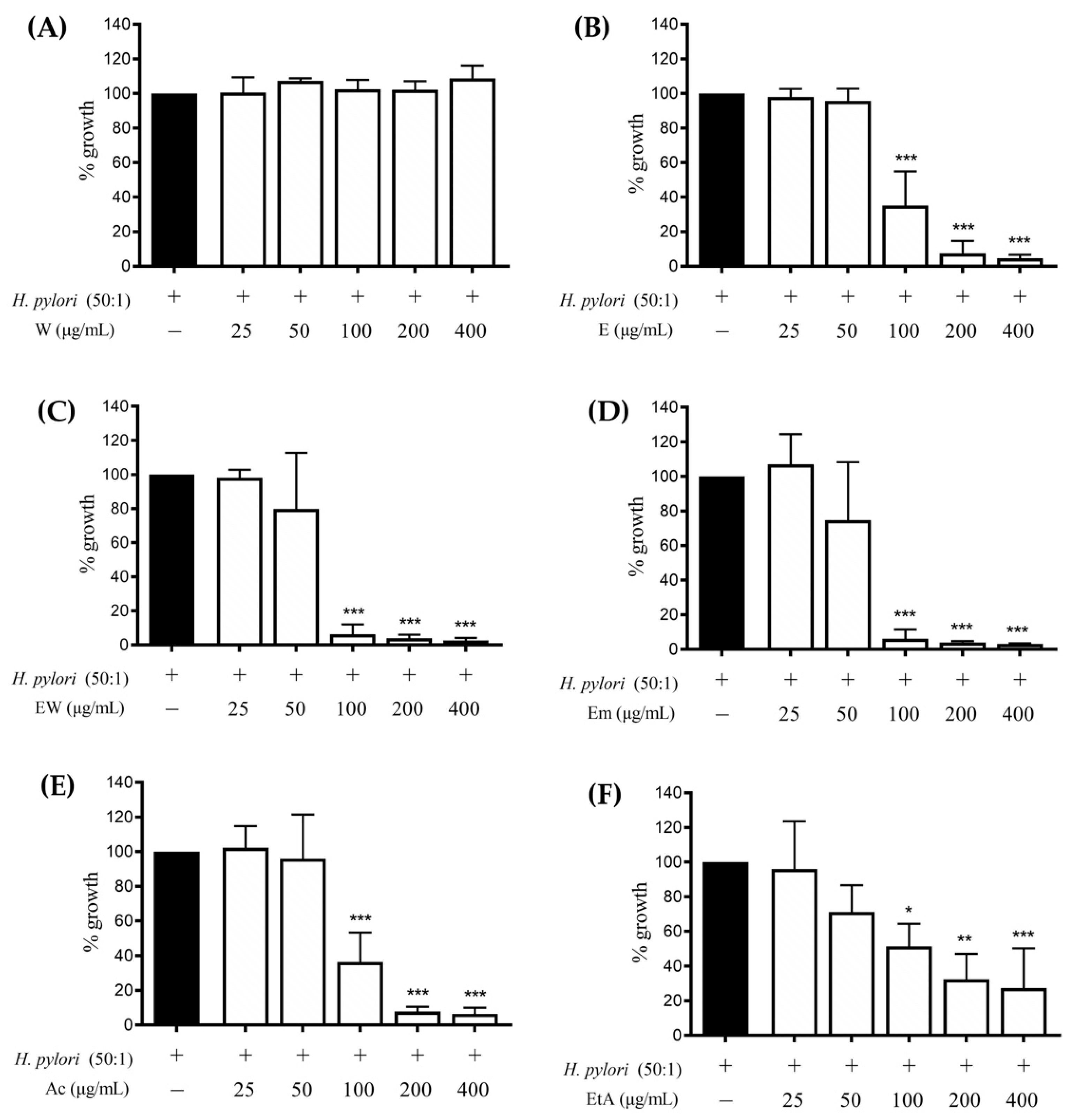
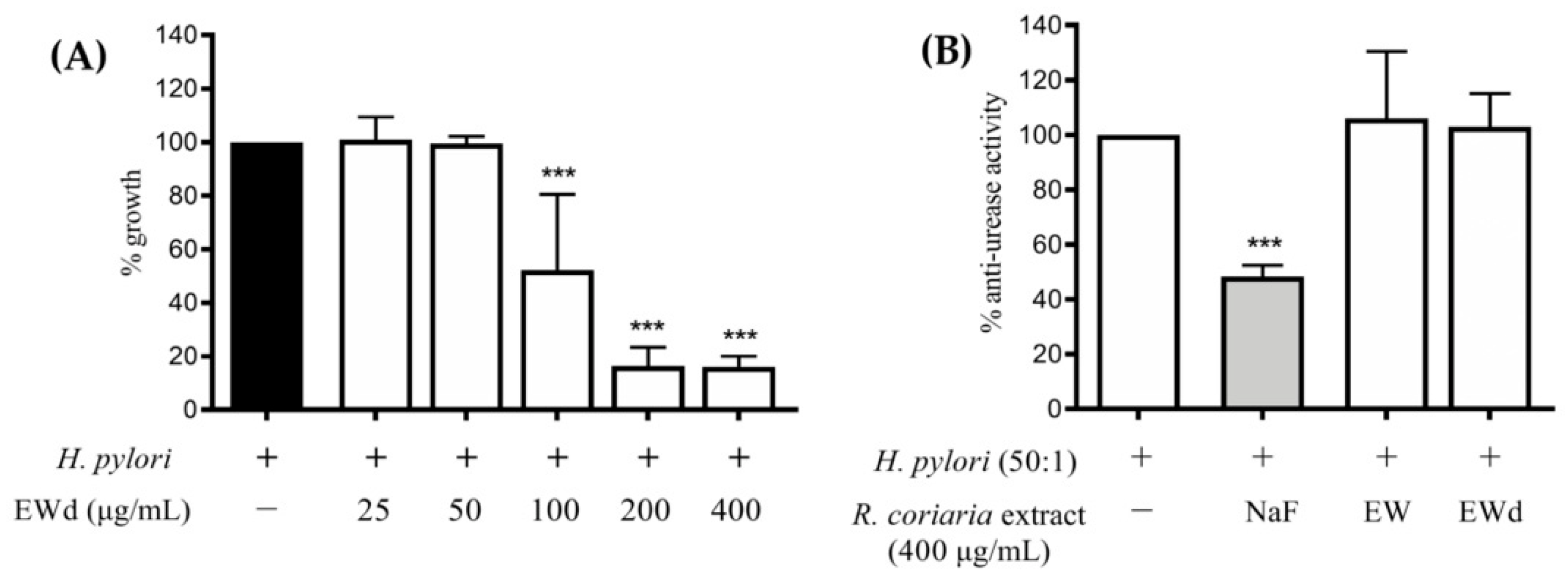
3.4. Effect of the Extracts on the H. pylori Growth
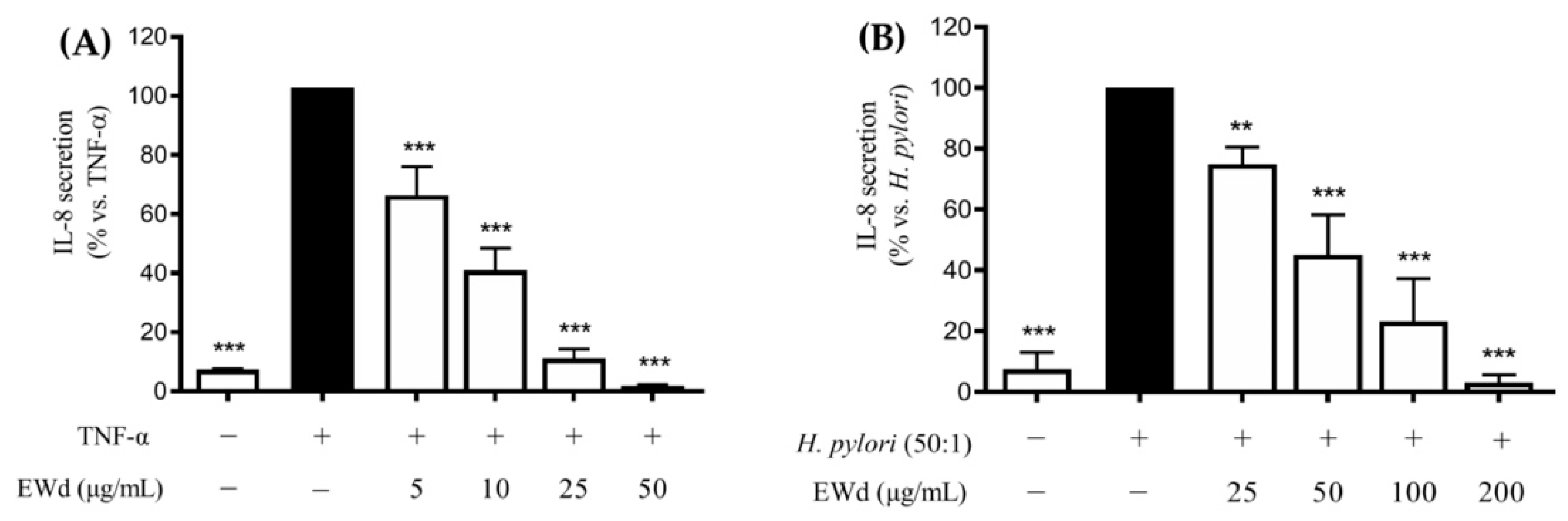
3.5. Effect of the In Vitro Digested Hydroethanolic Extract (EWd) on the TNF-α- or H. pylori-induced IL-8 Release in GES-1 Cells
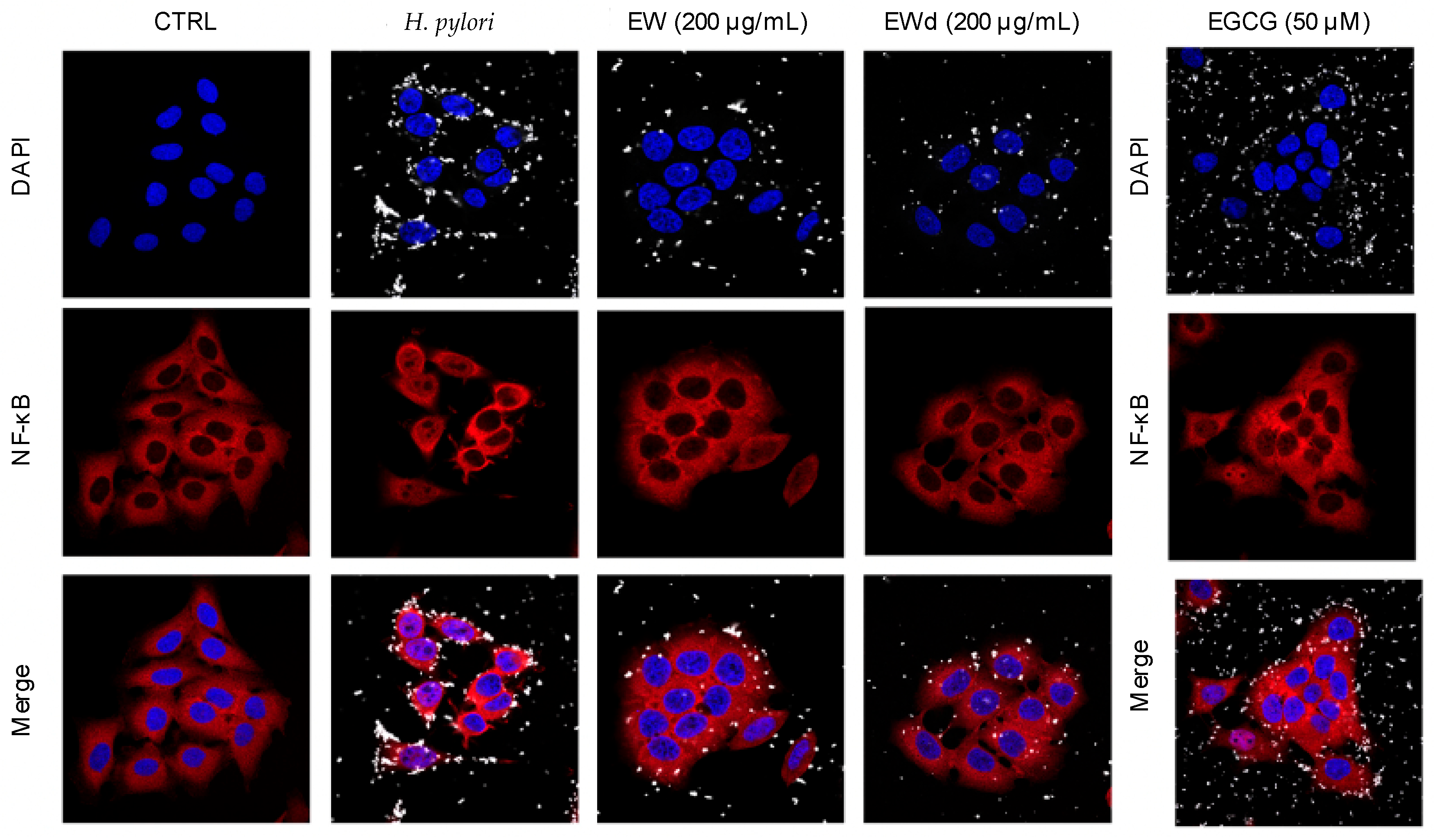
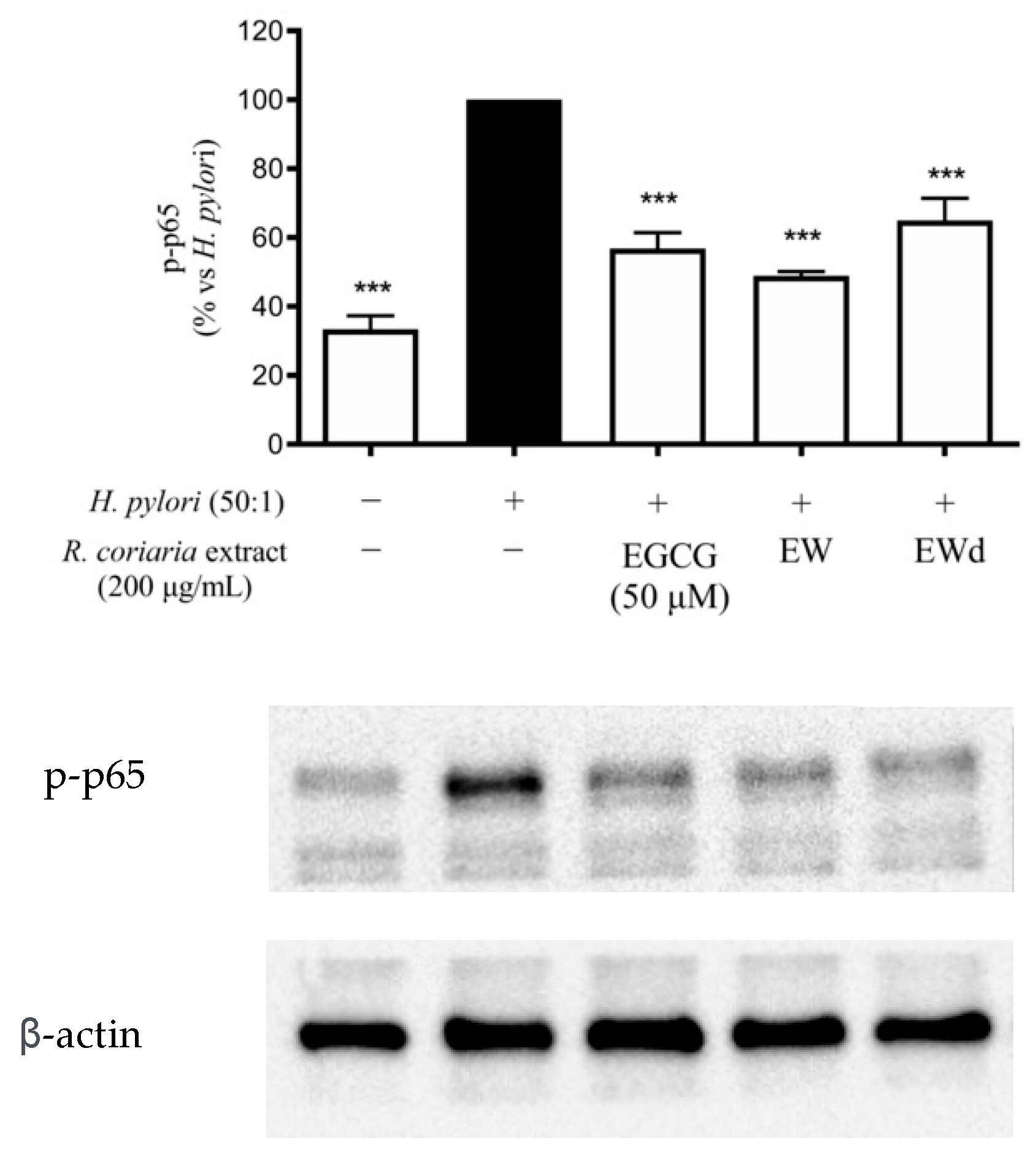
3.6. EW and EWd Extracts Impair the NF-κB Pathway in GES-1 Cells
3.7. Effect of EWd on H. pylori Growth and Urease Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elagbar, Z.A.; Shakya, A.K.; Barhoumi, L.M.; Al-Jaber, H.I. Phytochemical Diversity and Pharmacological Properties of Rhus coriaria. Chem. Biodivers. 2020, 17, e1900561. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Bozan, B.; Temelli, F.; Baser, K. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007, 103, 952–959. [Google Scholar]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Elid, H.A.; Iratni, R. Pharmacological and Antioxidant Activities of Rhus coriaria L. (Sumac). Antioxidants 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Nozza, E.; Melzi, G.; Marabini, L.; Marinovich, M.; Piazza, S.; Khalipour, S.; Dell’Agli, M.; Sangiovanni, E. Rhus coriaria L. fruit extract prevents UV-A-induced genotoxicity and oxidative injury in human microvascular endothelial cells. Antioxidants 2020, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef]
- Khalilpour, S.; Behnammanesh, G.; Suede, F.; Ezzat, M.O.; Muniandy, J.; Tabana, Y.; Ahamed, M.K.; Tamayol, A.; Shah Majid, A.K.; Sangiovanni, E.; et al. Neuroprotective and anti-inflammatory effects of Rhus coriaria extract in a mouse model of ischemic optic neuropathy. Biomedicines 2018, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Alsarayreh, A.Z.; Oran, S.A.; Shakhanbeh, M.J. Effect of Rhus coriaria L. methanolic fruit extract on wound healing in diabetic and non-diabetic rats. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef]
- Hashem-Dabaghian, F.; Ghods, R.; Shojaii, A.; Abdi, L.; Campos-Toimil, M.; Yousefsani, B.S. Rhus coriaria L., a new candidate for controlling metabolic syn-drome: A systematic review. J. Pharm. Pharmacol. 2022, 74, 1–12. [Google Scholar]
- Ranjbar, R.; Behzadi, P.; Farshad, S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol. Immunol. Hung. 2017, 64, 273–292. [Google Scholar]
- Sharma, S.A.; Tummuru, M.K.; Blaser, M.J.; Kerr, L.D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 1998, 160, 2401–2407. [Google Scholar]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar]
- Caruso, R.; Fina, D.; Paoluzi, O.A.; Blanco, G.D.V.; Stolfi, C.; Rizzo, A.; Caprioli, F.; Sarra, M.; Andrei, F.; Fantini, M.C.; et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immunol. 2008, 38, 470–478. [Google Scholar]
- Bagheri, N.; Rahimian, G.; Salimzadeh, L.; Azadegan, F.; Rafieian-Kopaei, M.; Taghikhani, A.; Shirzad, H. Association of the virulence factors of Helicobacter pylori and gastric mucosal interleukin-17/23 mRNA expression in dyspeptic patients. EXCLI J. 2013, 12, 5–14. [Google Scholar]
- Rahimian, G.; Sanei, M.H.; Shirzad, H.; Azadegan-Dehkordi, F.; Taghikhani, A.; Salimzadeh, L.; Hashemzadeh-Chaleshtori, M.; Rafieian-Kopaei, M.; Bagheri, N. Virulence factors of Helicobacter pylori vacA increase markedly gastric mucosal TGF-beta1 mRNA expression in gastritis patients. Microb. Pathog. 2014, 67–68, 1–7. [Google Scholar]
- Crabtree, J.E.; Lindley, I.J. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 1994, 6 (Suppl. S1), S33–S38. [Google Scholar]
- Crabtree, J.E.; Wyatt, J.I.; Sobala, G.M.; Miller, G.; Tompkins, D.S.; Primrose, J.N.; Morgan, A.G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut 1993, 34, 1339–1343. [Google Scholar]
- Ghobadi, E.; Ghanbarimasir, Z.; Emami, S. A review on the structures and biological activities of anti-Helicobacter pylori agents. Eur. J. Med. Chem. 2021, 223, 113669. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar]
- Nwakiban, A.P.A.; Fumagalli, M.; Piazza, S.; Magnavacca, A.; Martinelli, G.; Beretta, G.; Magni, P.; Tchamgoue, A.D.; Agbor, G.A.; Kuiaté, J.-R.; et al. Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells. Nutrients 2020, 12, 3787. [Google Scholar] [CrossRef]
- Weinstein, M.D.; Patel, J.B.; Burnham, C.A.; Campeau, S.; Conville, P.S.; Doern, C.; Eliopolous, G.M.; Galas, M.F.; Humphries, R.M.; Jenkins, S.G.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical And Laboratory Standards Intitute: Wayne, PA, USA, 2018. [Google Scholar]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [Green Version]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 2020, 10, 8503. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar]
- Yasumoto, K.; Okamoto, S.I.; Mukaida, N.; Murakami, S.; Mai, M.; Matsushima, K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J. Biol. Chem. 1992, 267, 22506–22511. [Google Scholar]
- Ahmad, H.; Wadud, A.; Jahan, N.; Khazir, M.; Sofi, G. Evaluation of Anti-ulcer activity of hydro alcoholic extract of Post Sumaq (Rhus coriaria Linn.) in Ethanol induced Gastric ulcer in experimental Rats. Int. Res. J. Med. Sci. 2013, 1, 7–12. [Google Scholar]
- Haqeeq, A.; Abdul, W.; Nasreen, J.; Izharul, H.; Ahmad, H.; Wadud, A.; Jahan, N.; Hasan, I. Anti-Ulcer activity of Rhus coriaria in indomethacin and water immersion restraint induced gastric ulcer in experimental rats. Glob. J. Med. Res. B 2014, 14, 1–7. [Google Scholar]
- Motaharinia, Y. Comparison of in vitro antimicrobial effect of ethanol extracts of Satureja khuzestanica, Rhus coriaria, and Ocimum basilicum L. on Helicobacter pylori. J. Med. Plants Res. 2012, 6, 3749–3753. [Google Scholar]
- Kossah, R.; Zhang, H.; Chen, W. Antimicrobial and antioxidant activities of Chinese sumac (Rhus typhina L.) fruit extract. Food Control 2011, 22, 128–132. [Google Scholar]
- Fazeli, M.R.; Amin, G.; Attari, M.M.A.; Ashtiani, H.; Jamalifar, H.; Samadi, N. Antimicrobial activities of Iranian sumac and avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Control 2007, 18, 646–649. [Google Scholar]
- Mahernia, S.; Bagherzadeh, K.; Mojab, F.; Amanlou, M. Urease Inhibitory Activities of some Commonly Consumed Herbal Medicines. Iran J. Pharm. Res. 2015, 14, 943–947. [Google Scholar] [PubMed]
| Rhus coriaria L. Extracts | Total Polyphenols (mg Gallic Acid Equivalents/g extract ± S.D.) |
|---|---|
| Water (W) | 93.2 ± 14.7 |
| Ethanol (E) | 224.7 ± 27.3 |
| Ethanol-water (EW) | 258.5 ± 37.1 |
| Ethanol macerated (Em) | 296.0 ± 39.0 |
| Acetone (Ac) | 223.6 ± 29.7 |
| Ethylacetate (EtA) | 82.6 ± 13.4 |
| Rhus coriaria L. Extracts | IL-8 Release (μg/mL) | 95% C.I. | IL-6 Release (μg/mL) | 95% C.I. |
|---|---|---|---|---|
| Water (W) | 14.1 | 10.0 to 19.7 | 10.3 | 8.0 to 13.2 |
| Ethanol (E) | 14.7 | 11.0 to 19.7 | 19.3 | 13.1 to 28.5 |
| Ethanol-water (EW) | 12.7 | 8.8 to 18.5 | 19.3 | 11.8 to 31.6 |
| Macerated ethanol (Em) | 12.1 | 8.5 to 17.4 | 18.3 | 11.3 to 29.6 |
| Acetone (Ac) | 29.5 | 20.4 to 42.4 | 69.9 | 46.3 to 69.9 |
| Ethylacetate (EtA) | 32.5 | 21.8 to 48.4 | 85.4 | 66.6 to 109.5 |
| Rhus coriaria L. Extracts | IL-8 Release (μg/mL) | 95% C.I. | IL-6 Release (μg/mL) | 95% C.I. |
|---|---|---|---|---|
| Water (W) | 183.1 | 150.9 to 222.1 | >200 | – |
| Ethanol (E) | 62.0 | 52.2 to 73.7 | >200 | – |
| Ethanol-water (EW) | 62.4 | 51.4 to 75.6 | >200 | – |
| Macerated ethanol (Em) | 69.7 | 56.1 to 86.4 | >200 | – |
| Acetone (Ac) | 70.1 | 49.2 to 100.0 | >200 | – |
| Ethylacetate (EtA) | 166.2 | 109.3 to 252.8 | >200 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, G.; Angarano, M.; Piazza, S.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Khalilpour, S.; Dell’Agli, M.; Sangiovanni, E. The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori. Nutrients 2022, 14, 1757. https://doi.org/10.3390/nu14091757
Martinelli G, Angarano M, Piazza S, Fumagalli M, Magnavacca A, Pozzoli C, Khalilpour S, Dell’Agli M, Sangiovanni E. The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori. Nutrients. 2022; 14(9):1757. https://doi.org/10.3390/nu14091757
Chicago/Turabian StyleMartinelli, Giulia, Marco Angarano, Stefano Piazza, Marco Fumagalli, Andrea Magnavacca, Carola Pozzoli, Saba Khalilpour, Mario Dell’Agli, and Enrico Sangiovanni. 2022. "The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori" Nutrients 14, no. 9: 1757. https://doi.org/10.3390/nu14091757
APA StyleMartinelli, G., Angarano, M., Piazza, S., Fumagalli, M., Magnavacca, A., Pozzoli, C., Khalilpour, S., Dell’Agli, M., & Sangiovanni, E. (2022). The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori. Nutrients, 14(9), 1757. https://doi.org/10.3390/nu14091757