Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Data Collection
2.2. Anthropometric Measurements
2.3. Collection of Blood Samples and Laboratory Analysis
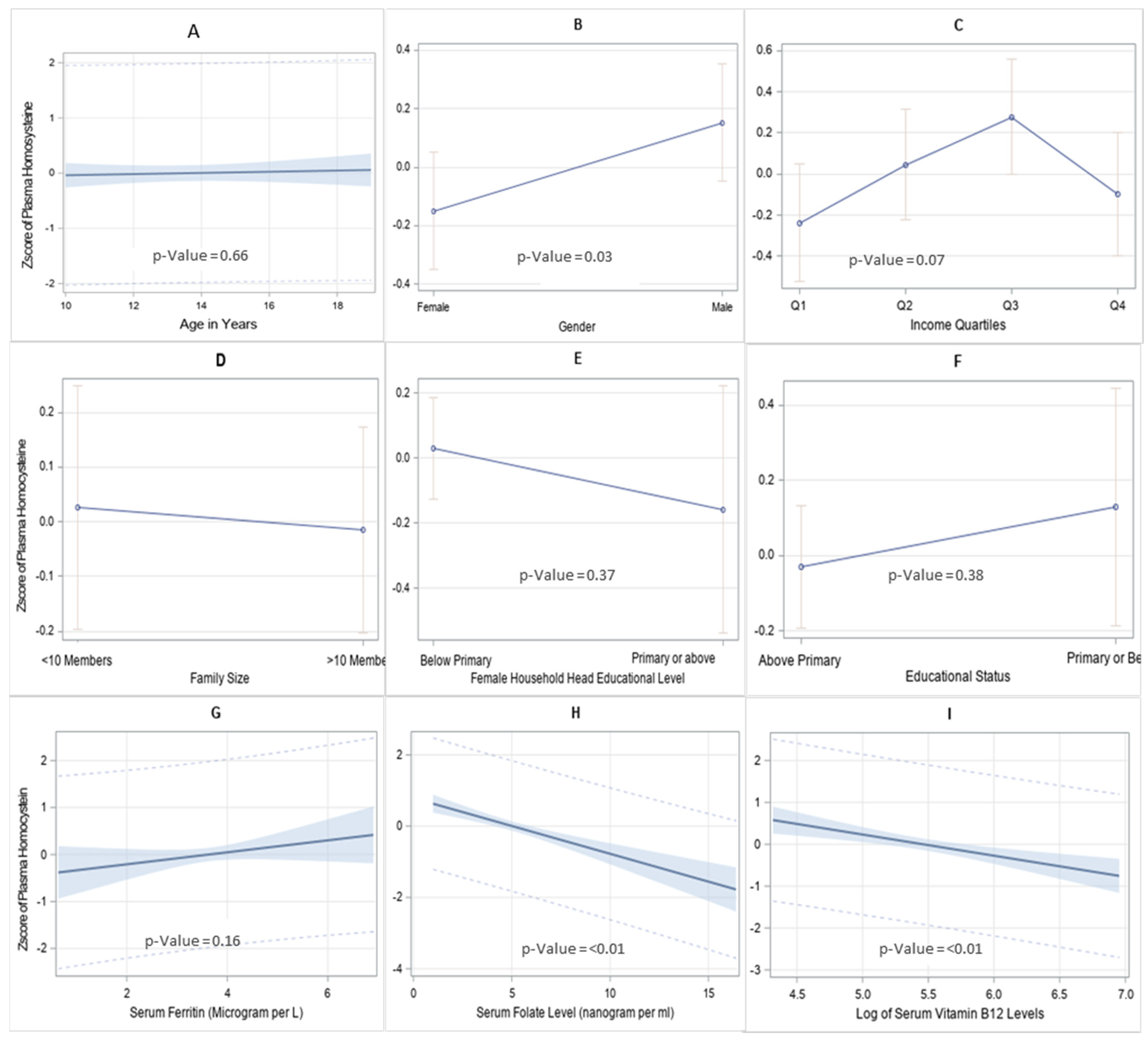
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations High Commissioner for UNHCR—Refugee Statistics. UNHCR. 2021. Available online: https://www.unhcr.org/refugee-statistics/ (accessed on 12 January 2022).
- Abou-Saleh, M.T.; Christodoulou, G.N. Mental health of refugees: Global perspectives. BJPsych Int. 2016, 13, 79–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malik, M.S.; Afzal, M.; Farid, A.; Khan, F.U.; Mirza, B.; Waheed, M.T. Disease Status of Afghan Refugees and Migrants in Pakistan. Front. Public Health 2019, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. Epidemiological Features of Cardiovascular Disease in Asia. JACC Asia 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- McCully, K. The biomedical significance of homocysteine. J. Sci. Explor. 2001, 15, 5–20. [Google Scholar]
- Fonseca, V.; Guba, S.C.; Fink, L.M. Hyperhomocysteinemia and the endocrine system: Implications for atherosclerosis and thrombosis. Endocr. Rev. 1999, 20, 738–759. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharmacal Res. 2018, 41, 372–383. [Google Scholar] [CrossRef]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar]
- Graham, I.M.; Daly, L.E.; Refsum, H.M.; Robinson, K.; Brattström, L.E.; Ueland, P.M.; Palma-Reis, R.J.; Boers, G.H.; Sheahan, R.G.; Israelsson, B.; et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA 1997, 277, 1775–1781. [Google Scholar] [CrossRef]
- Cui, R.; Moriyama, Y.; Koike, K.A.; Date, C.; Kikuchi, S.; Tamakoshi, A.; Iso, H. Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: The JACC study. Atherosclerosis 2008, 198, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Hartwig, F.P.; Oliveira, I.O.; Horta, B.L. Is there a causal role for homocysteine concentration in blood pressure? A Mendelian randomization study. Am. J. Clin. Nutr. 2016, 103, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Huang, L.; Hong, J.; Zhao, X.; Chen, Y.; Hu, J.; Cong, X.; Xie, Y.; Pu, J. Elevated homocysteine levels in patients with heart failure: A systematic review and meta-analysis. Medicine 2021, 100, e26875. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, S.; Yang, F.; Wang, Y.; Zhang, K.; Fu, G.; Zhang, W. The impact of homocysteine on the risk of coronary artery diseases in individuals with diabetes: A Mendelian randomization study. Acta Diabetol. 2021, 58, 301–307. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef]
- Saeedullah, A.; Khan, M.S.; Andrews, S.C.; Iqbal, K.; Ul-Haq, Z.; Qadir, S.A.; Khan, H.; Iddrisu, I.; Shahzad, M. Nutritional Status of Adolescent Afghan Refugees Living in Peshawar, Pakistan. Nutrients 2021, 13, 3072. [Google Scholar] [CrossRef]
- Lahiri, K.D.; Datta, H.; Das, H.N. Reference Interval Determination of Total Plasma Homocysteine in an Indian Population. Indian J. Clin. Biochem. 2014, 29, 74–78. [Google Scholar] [CrossRef][Green Version]
- Chung, K.-H.; Chiou, H.Y.; Chen, Y.-H. Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Sci. Rep. 2017, 7, 8330. [Google Scholar] [CrossRef]
- Araújo, H.C.; Ramos, R.; Florindo, C.; Rivera, I.; Castro, R.; De Almeida, I.T. Homocysteine Metabolism in Children and Adolescents: Influence of Age on Plasma Biomarkers and Correspondent Genotype Interactions. Nutrients 2019, 11, 646. [Google Scholar] [CrossRef]
- Kerr, M.A.; Livingstone, B.; Bates, C.J.; Bradbury, I.; Scott, J.M.; Ward, M.; Pentieva, K.; Mansoor, M.A.; McNulty, H. Folate, Related B Vitamins, and Homocysteine in Childhood and Adolescence: Potential Implications for Disease Risk in Later Life. Pediatrics 2009, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Stanger, M.O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. Clinical use and rational management of homocysteine, folic acid, and B vitamins in cardiovascular and thrombotic diseases. Z. Für Kardiol. 2004, 93, 439–453. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Wautrecht, J.C.; Brasseur, D.; Dramaix, M.; Boeynaems, J.M.; Decuyper, J.; Kahn, A. Plasma homocysteine concentration in a Belgian school-age population. Am. J. Clin. Nutr. 1999, 69, 968–972. [Google Scholar] [CrossRef] [PubMed]
- van Beynum, I.M.; den Heijer, M.; Thomas, C.M.G.; Afman, L.; Oppenraay-van Emmerzaal, D.; Blom, H.J. Total homocysteine and its predictors in Dutch children. Am. J. Clin. Nutr. 2005, 81, 1110–1116. [Google Scholar] [CrossRef]
- Osganian, S.K.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.; Cutler, J.A.; Feldman, H.A.; Montgomery, D.H.; Webber, L.S.; Lytle, L.A.; Bausserman, L.; et al. Distribution of and Factors Associated With Serum Homocysteine Levels in ChildrenChild and Adolescent Trial for Cardiovascular Health. JAMA 1999, 281, 1189–1196. [Google Scholar] [CrossRef]
- Ganji, V.; Kafai, M.R. Population References for Plasma Total Homocysteine Concentrations for U.S. Children and Adolescents in the Post-Folic Acid Fortification Era. J. Nutr. 2005, 135, 2253–2256. [Google Scholar] [CrossRef]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef]
- Benson, J.; Maldari, T.; Turnbull, T. Vitamin B12 deficiency—Why refugee patients are at high risk. Aust. Fam. Physician 2010, 39, 215–217. [Google Scholar]
- Yang, Y.; Zeng, Y.; Yuan, S.; Xie, M.; Dong, Y.; Li, J.; He, Q.; Ye, X.; Lv, Y.; Hocher, C.F.; et al. Prevalence and risk factors for hyperhomocysteinemia: A population-based cross-sectional study from Hunan, China. BMJ Open 2021, 11, e048575. [Google Scholar]
- Wang, Y.; Li, X.; Qin, X.; Cai, Y.; He, M.; Sun, L.; Li, J.; Zhang, Y.; Tang, G.; Wang, B.; et al. Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45–75 years. Br. J. Nutr. 2013, 109, 1284–1293. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Prudova, A.; Stabler, S.; Dayal, S.; Lentz, S.R.; Banerjee, R. Testosterone regulation of renal cystathionine beta-synthase: Implications for sex-dependent differences in plasma homocysteine levels. Am. J. Physiol. Renal. Physiol. 2007, 293, F594–F600. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, N.K.; Martin, J.M.; Wurthmann, A.; Prue, A.H.; Ebenstein, D.; O’Rourke, B. Sex-related differences in methionine metabolism and plasma homocysteine concentrations. Am. J. Clin. Nutr. 2000, 72, 22–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacques, P.F.; Bostom, A.G.; Wilson, P.W.; Rich, S.; Rosenberg, I.H.; Selhub, J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am. J. Clin. Nutr. 2001, 73, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kafai, M.R. Serum total homocysteine concentration determinants in non-Hispanic White, non-Hispanic Black, and Mexican-American populations of the United States. Ethn. Dis. 2004, 14, 476–482. [Google Scholar]
- Amarasinghe, G.; Jayasinghe, I.; Hettiarachchi, A.; Koralegedara, I.; Kappagoda, C.; Mendis, W.; Agampodi, T.; Agampodi, S. Can Homocysteine Be Used to Identify Vitamin B12 or Folate Deficiencies During Pregnancy in Low Resource Settings? Curr. Dev. Nutr. 2021, 5 (Suppl. S2), 707. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.A.M.; De Vries, J.H.M.; De Bree, A.; Van Der Put, N.; Van Staveren, W.A.; De Groot, L.C.P.G.M. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef]
- Sirdah, M.M.; Yassin, M.M.; El Shekhi, S.; Lubbad, A.M. Homocysteine and vitamin B12 status and iron deficiency anemia in female university students from Gaza Strip, Palestine. Rev. Bras. Hematol. Hemoter. 2014, 36, 208–212. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Berenson, G.S. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease: The Bogalusa Heart Study. Am. J. Cardiol. 2002, 90, L3–L7. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef]
- Huemer, M.; Vonblon, K.; Födinger, M.; Krumpholz, R.; Hubmann, M.; Ulmer, H.; Simma, B. Total homocysteine, folate, and cobalamin, and their relation to genetic polymorphisms, lifestyle and body mass index in healthy children and adolescents. Pediatr. Res. 2006, 60, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Prengler, M.; Sturt, N.; Krywawych, S.; Surtees, R.; Liesner, R.; Kirkham, F. Homozygous thermolabile variant of the methylenetetrahydrofolate reductase gene: A potential risk factor for hyperhomocysteinaemia, CVD, and stroke in childhood. Dev. Med. Child. Neurol. 2001, 43, 220–225. [Google Scholar] [CrossRef] [PubMed]
| Variables | Categories | Male (n = 94) | Female (n = 96) | Total (n = 190) | p-Value |
|---|---|---|---|---|---|
| Age | |||||
| Age (years) 3 | 12.5 (11.0, 15.0) | 13.5 (10.5, 16.0) | 13.0 (11.0, 16.0) | 0.52 1 | |
| 10–14 age group | 66 (70.2%) | 55 (57.3%) | 121 (63.7%) | 0.06 2 | |
| 15–19 age group | 28 (29.8%) | 41 (42.7%) | 69 (36.3%) | ||
| Anthropometry | |||||
| Weight (kg) 3 | 34.8 (27.4, 50.0) | 40.5 (30.0, 46.0) | 38.0 (28.5, 48.0) | 0.75 1 | |
| Height (cm) 3 | 141.0 (131.0, 160.0) | 145.0 (130.0, 151.8) | 143.3 (130.2, 155.0) | 0.31 1 | |
| BMI (kg/m2) | 18.0 (15.8, 20.1) | 18.6 (17.3, 20.1) | 18.4 (16.4, 20.1) | 0.07 1 | |
| Education level of the participants | |||||
| No formal education | 9 (9.6%) | 27 (28.1%) | 36 (18.9%) | <0.01 2 | |
| Primary | 60 (63.8%) | 54 (56.3%) | 114 (60.0%) | ||
| High school | 22 (23.4%) | 15 (15.6%) | 37 (19.5%) | ||
| College and university | 3 (3.2%) | 0 (0.0%) | 3 (1.6%) | ||
| Education level of the female head of the household | |||||
| No formal education | 75 (79.8%) | 69 (71.9%) | 144 (75.8%) | 0.03 2 | |
| Primary | 12 (12.8%) | 7 (7.3%) | 19 (10.0%) | ||
| High school | 7 (7.4%) | 17 (17.7%) | 24 (12.6%) | ||
| College and university | 0 (0.0%) | 3 (3.1%) | 3 (1.6%) | ||
| Total number of household members (family size) | |||||
| 1–4 | 5 (5.3%) | 1 (1.0%) | 6 (3.2%) | 0.17 2 | |
| 5–9 | 40 (42.6%) | 33 (34.4%) | 73 (38.4%) | ||
| 10–19 | 42 (44.7%) | 51 (53.1%) | 93 (48.9%) | ||
| 20 or more | 7 (7.4%) | 11 (11.5%) | 18 (9.5%) | ||
| Income status | |||||
| 1st Quartile | 19 (20.2%) | 28 (29.2%) | 47 (24.7%) | 0.24 2 | |
| 2nd Quartile | 25 (26.6%) | 28 (29.2%) | 53 (27.9%) | ||
| 3rd Quartile | 24 (25.5%) | 24 (25.0%) | 48 (25.3%) | ||
| 4th Quartile | 26 (27.7%) | 16 (16.7%) | 42 (22.1%) | ||
| Characteristics | Geometric Mean (95% CI) | Minimum | Percentile | Maximum | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | 99th | ||||
| All | 12.9 (12.1, 13.7) | 4.5 | 6.8 | 9.9 | 12.2 | 15.9 | 28.6 | 36.9 | 37.3 |
| Sex | |||||||||
| Boys | 14.1 (13.0, 15.1) | 6.6 | 8.6 | 10.6 | 13.7 | 18.1 | 27.7 | 37.3 | 37.3 |
| Girls | 11.8 (10.8, 12.9) | 4.5 | 6.1 | 9.1 | 11.4 | 14.8 | 30.1 | 36.3 | 36.3 |
| p-Value | 0.004 | ||||||||
| Age Categories (years) | |||||||||
| 10–14 | 12.8 (12.0, 13.7) | 6.2 | 7.9 | 9.6 | 12.5 | 15.5 | 25.4 | 30.1 | 35.5 |
| 15–19 | 12.9 (11.5, 14.6) | 4.5 | 5.9 | 10.1 | 11.8 | 16.2 | 32.6 | 37.3 | 37.27 |
| p-Value | 0.87 | ||||||||
| Variables | Categories | Homocysteine Levels (Freqeuncy/Median (IQR) | Uni-Variable Logistic Regression | Multivariable Logistic Regression | ||||
|---|---|---|---|---|---|---|---|---|
| <15 μmol/L | ≥15 μmol/L | p-Value | OR (95% CI) | p-Value | Adjusted | p-Value | ||
| Age categories (years) | 10–14 | 86 (64.2%) | 35 (62.5%) | 0.83 | 0.91 (0.48, 1.74) | 0.77 | 0.86 (0.3, 2.42) | 0.77 |
| 15–19 | 48 (35.8%) | 21 (37.5%) | Ref. | Ref. | ||||
| Gender | Male | 11.3 (9.6, 13.6) | 20.1 (16.7, 25.2) | <0.01 | 1.92 (1.03, 3.64) | 0.04 | 1.19 (0.43, 3.27) | 0.74 |
| Female | 10.3 (8.3, 11.9) | 20.3 (16.9, 28.6) | <0.01 | |||||
| BMI (kg/m2) | 18.3 (16.7, 20.1) | 18.4 (15.4, 20.2) | 0.4 | 0.93 (0.84, 1.03) | 0.94 (0.81, 1.08) | 0.38 | ||
| Educational Status of the participants | Below primary | 108 (80.6%) | 42 (75.0%) | 0.39 | 0.69 (0.33, 1.46) | 0.32 | 1.12 (0.41, 3.09) | 0.83 |
| Primary and above | 26 (19.4%) | 14 (25.0%) | Ref. | Ref. | ||||
| Education level of the female head of the household | Below primary | 116 (86.6%) | 47 (83.9%) | 0.64 | 0.82 (0.34, 1.95) | 0.62 | 0.58 (0.19, 1.82) | 0.35 |
| Primary and above | 18 (13.4%) | 9 (16.1%) | Ref. | Ref. | ||||
| Family size | <10 | 116 (86.6%) | 47 (83.9%) | 0.13 | 1.61 (0.86, 3.02) | 0.14 | 3.64 (1.56, 8.49) | <0.01 |
| ≥10 | 18 (13.4%) | 9 (16.1%) | Ref. | Ref. | ||||
| Income Quartiles | 1st Quartile | 38 (28.6%) | 9 (16.1%) | 0.32 | 0.54 (0.2, 1.395) | 0.21 | 0.57 (0.16, 2) | 0.38 |
| 2nd Quartile | 35 (26.3%) | 17 (30.4%) | 1.08 (0.46, 2.58) | 0.87 | 0.95 (0.32, 2.84) | 0.92 | ||
| 3rd Quartile | 31 (23.3%) | 17 (30.4%) | 1.21 (0.51, 2.93) | 0.67 | 1.32 (0.45, 3.88) | 0.61 | ||
| 4th Quartile | 29 (21.8%) | 13 (23.2%) | Ref. | Ref. | ||||
| Biomarkers status | Serum Vitamin D | 22.5 (15.7, 28.4) | 21.9 (17.8, 25.3) | 0.57 | 0.87 (0.41, 1.61) | 0.68 | 0.48 (0.11, 1.99) | 0.31 |
| Serum Ferritin | 37.2 (26.0, 58.3) | 42.0 (30.5, 63.7) | 0.17 | 1.37 (0.93, 2.1) | 0.12 | 1.55 (0.93, 2.57) | 0.09 | |
| Serum Vitamin B12 | 237.0 (179.0, 357.0) | 165.0 (119.5, 255.0) | <0.001 | 0.31 (0.15, 0.58) | <0.001 | 0.29 (0.14, 0.62) | <0.01 | |
| Serum Folate | 5.0 (3.9, 6.7) | 3.6 (2.7, 4.2) | <0.001 | 0.14 (0.06, 0.32) | <0.001 | 0.1 ( 0.03, 0.27) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Saeedullah, A.; Andrews, S.C.; Iqbal, K.; Qadir, S.A.; Shahzad, B.; Ahmed, Z.; Shahzad, M. Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life. Nutrients 2022, 14, 1751. https://doi.org/10.3390/nu14091751
Khan MS, Saeedullah A, Andrews SC, Iqbal K, Qadir SA, Shahzad B, Ahmed Z, Shahzad M. Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life. Nutrients. 2022; 14(9):1751. https://doi.org/10.3390/nu14091751
Chicago/Turabian StyleKhan, Muhammad Shabir, Anum Saeedullah, Simon C. Andrews, Khalid Iqbal, Syed Abdul Qadir, Babar Shahzad, Zahoor Ahmed, and Muhammad Shahzad. 2022. "Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life" Nutrients 14, no. 9: 1751. https://doi.org/10.3390/nu14091751
APA StyleKhan, M. S., Saeedullah, A., Andrews, S. C., Iqbal, K., Qadir, S. A., Shahzad, B., Ahmed, Z., & Shahzad, M. (2022). Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life. Nutrients, 14(9), 1751. https://doi.org/10.3390/nu14091751







