The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection
Abstract
:1. Introduction
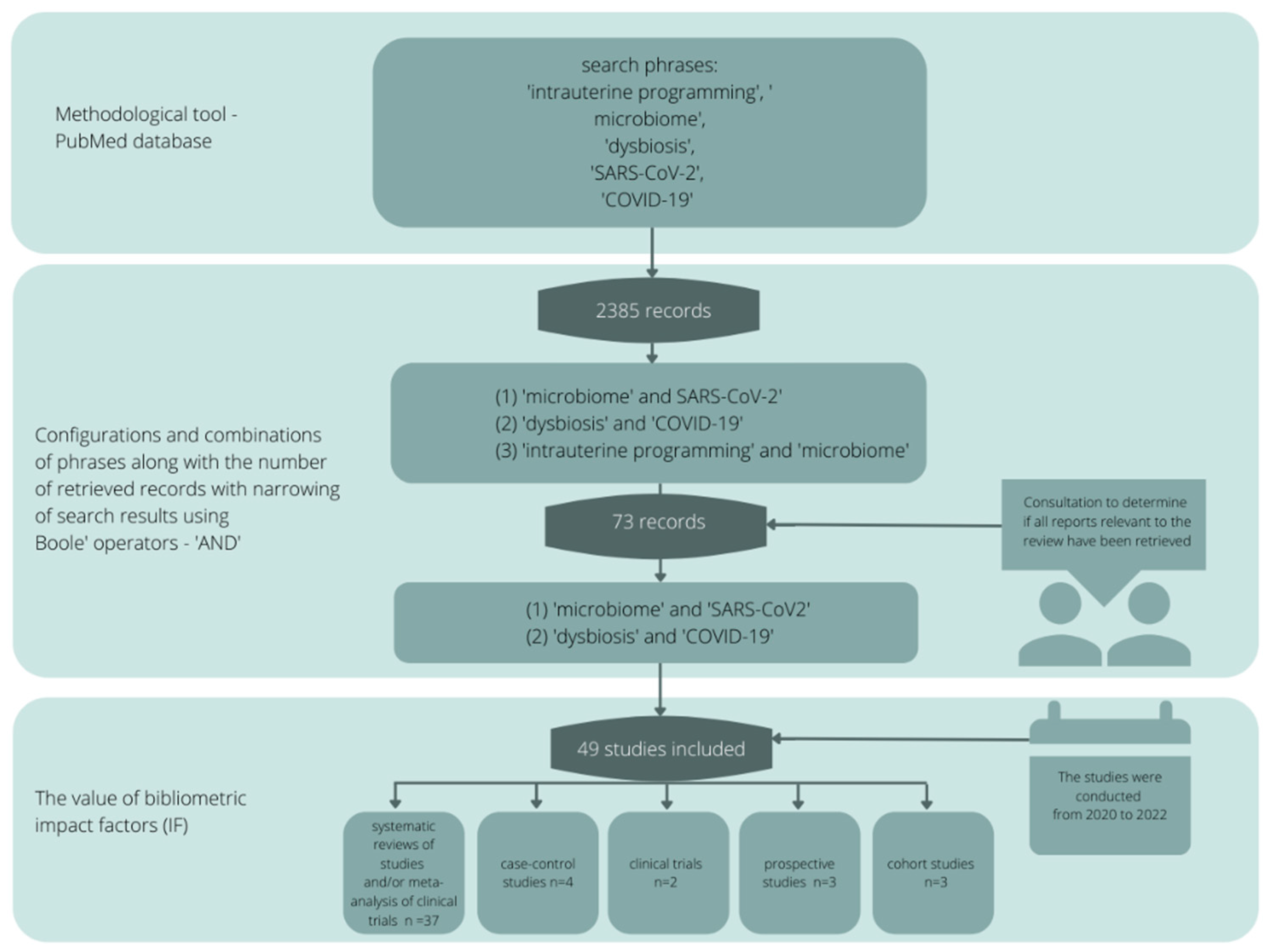
2. Review Methodology
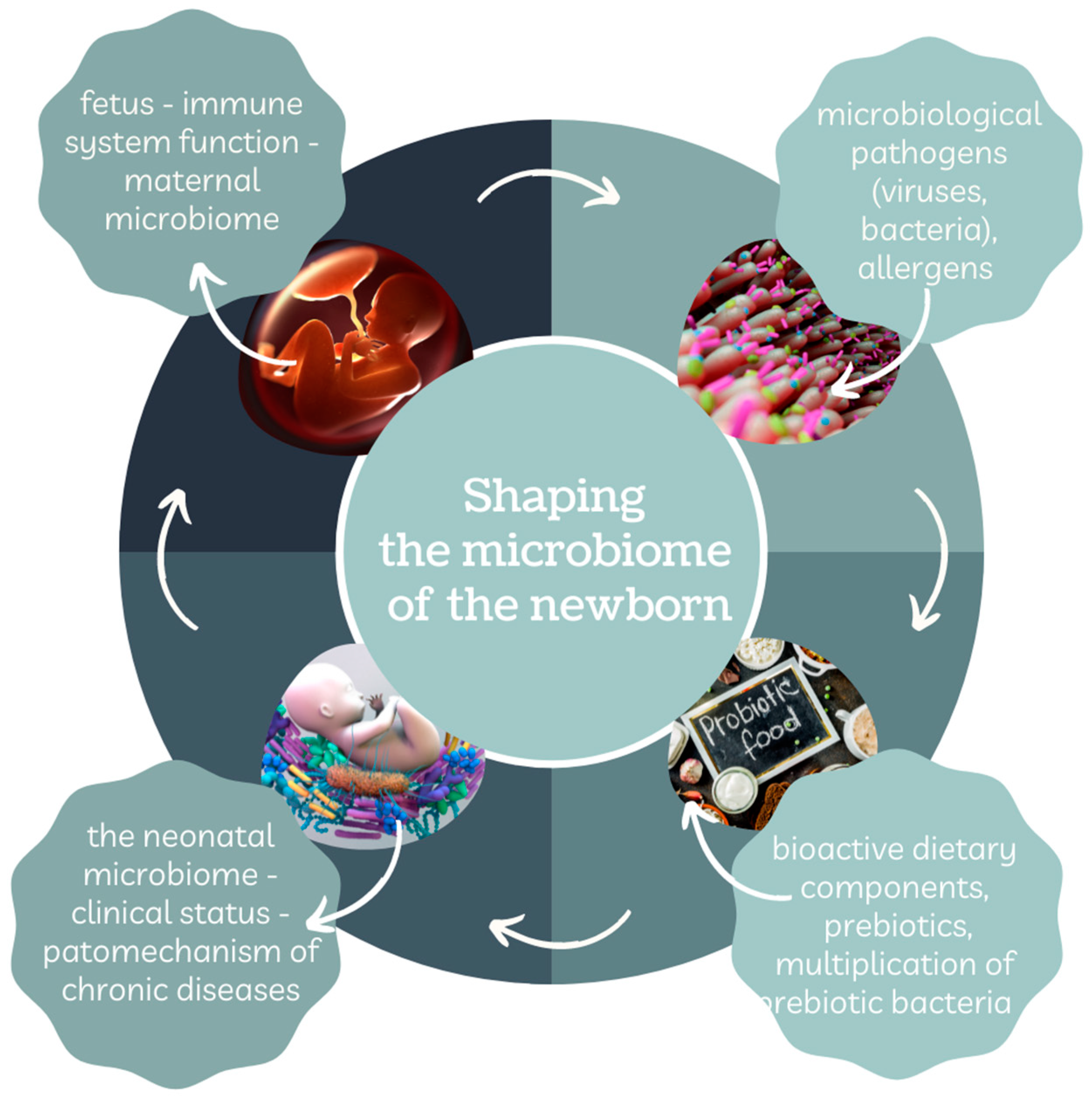
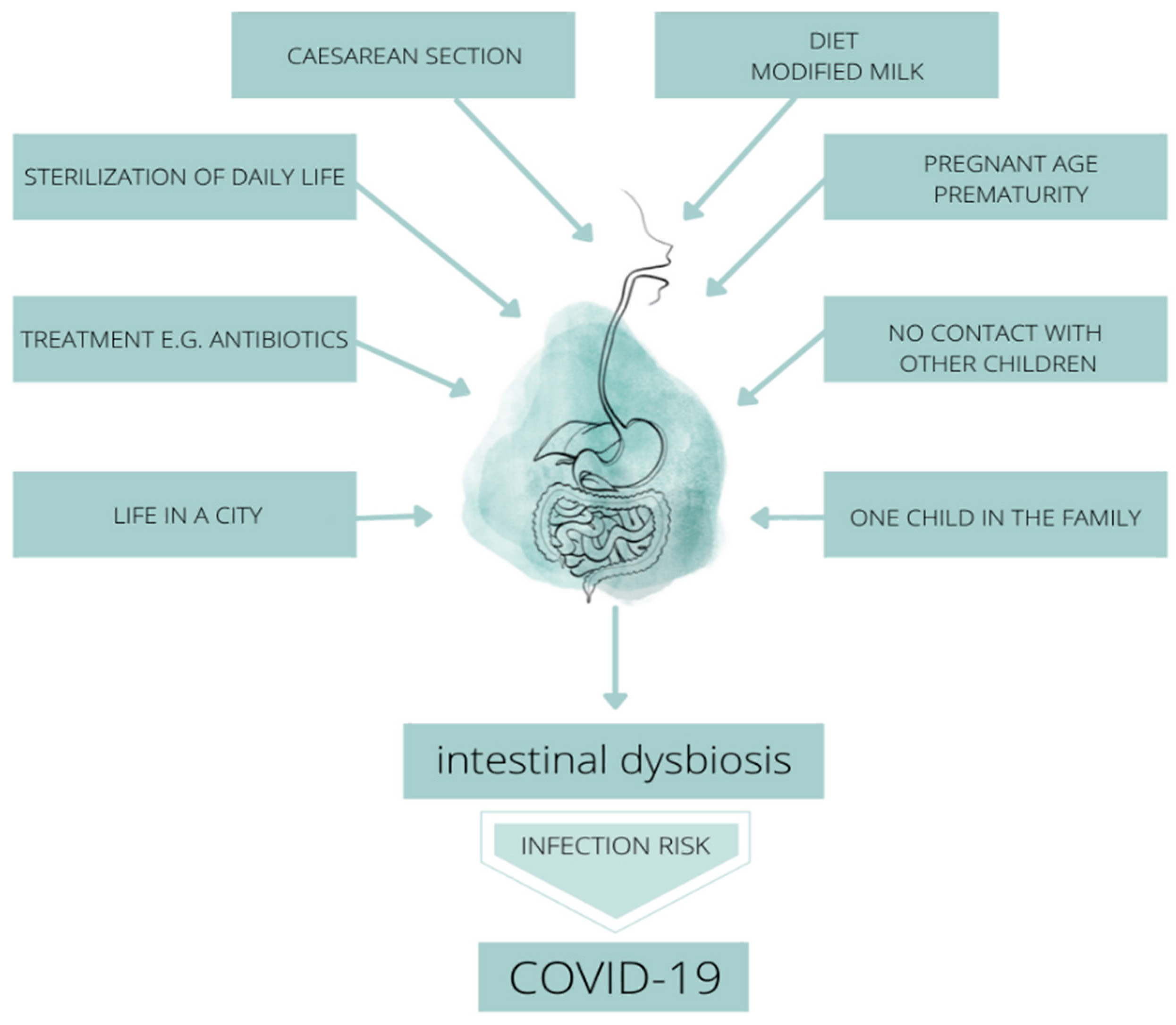
3. Formation and Importance of the Neonatal Microbiome
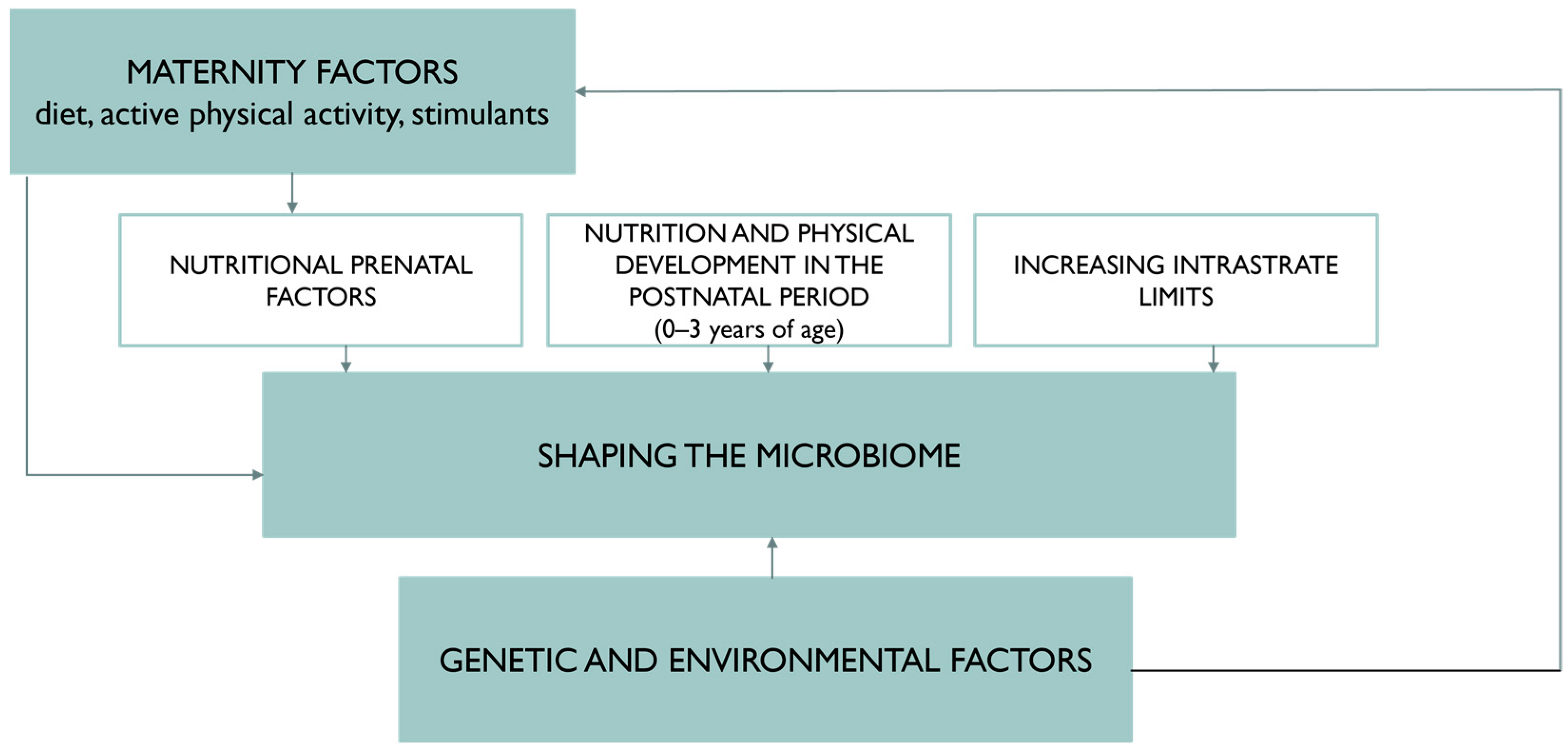
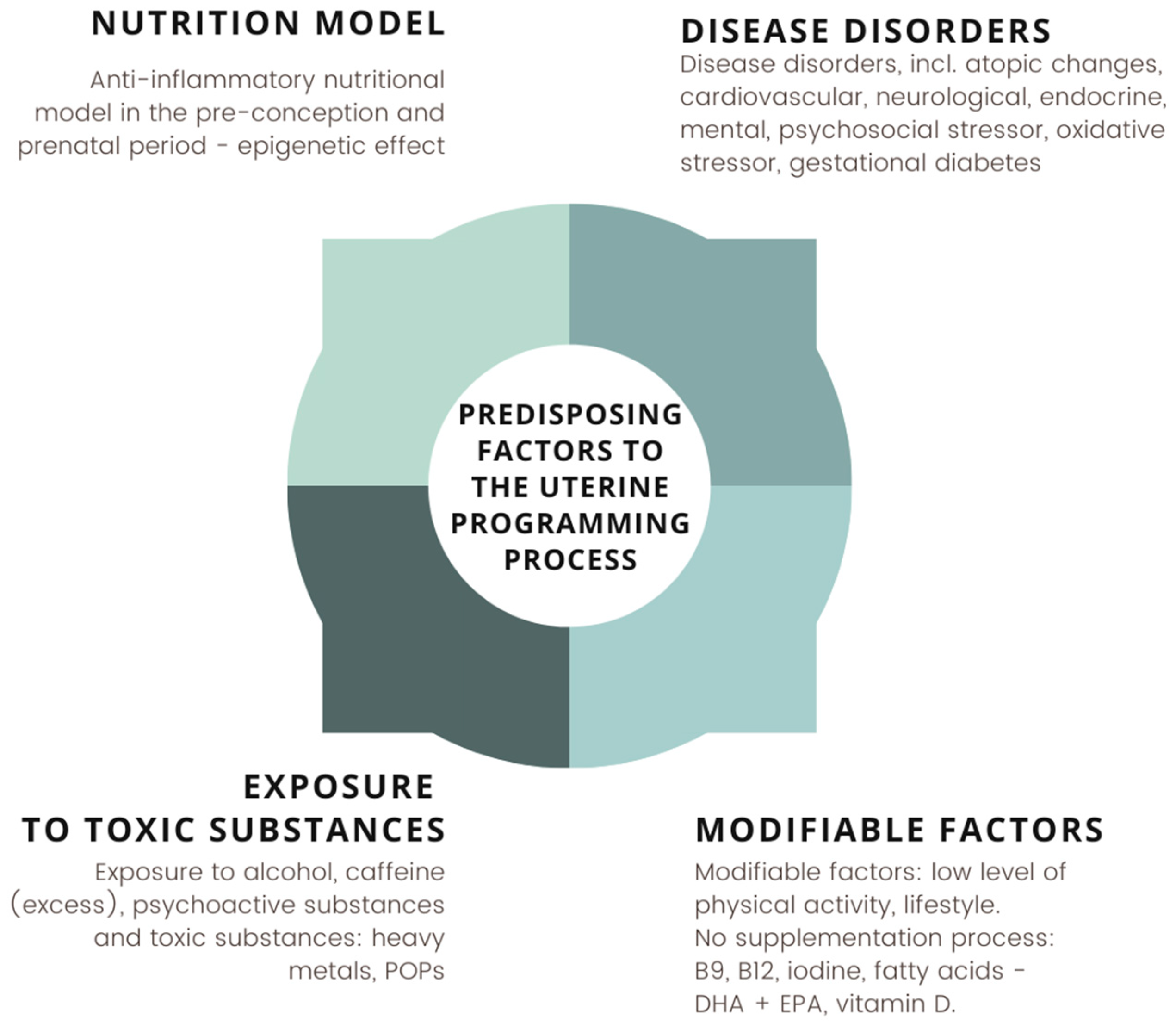
4. Nutritional Status and Diet of the Pregnant Woman Protectively Affect the Gut Microbiota of the Newborn
5. The Importance of the Microbiome in Terms of the Prevention of SARS-CoV-2 Virus Infection
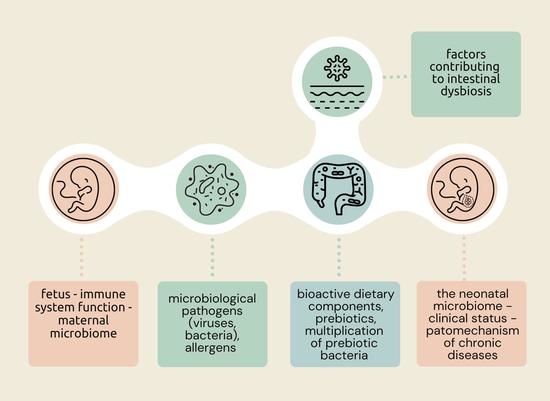
- Therapeutic effect, neutralizing quantitative and qualitative inflammatory changes due to the progression of intestinal dysbiosis, saturated bowel syndrome also reducing the duration of viral and bacterial infections.
- Preventive potential against the development of a leaky intestinal barrier and complete multiplication of a qualitatively and quantitatively pathogenic microbiome, especially in children.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gałęcka, M.; Bartnicka, A.; Szewc, M.; Mazela, J. Formation of the gut microbiota in infants a prerequisite for health maintenance. Med. Stand. Pediatr. 2016, 13, 359–367. [Google Scholar]
- Abrahamsson, T.R.; Wu, R.Y.; Jenmalm, M.C. Gut microbiota and allergy: The importance of the pregnancy period. Pediatr. Res. 2015, 77, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimber-Trojnar, Ż.; Marciniak, A.; Patro-Małysza, J.; Marciniak, B.; Mielnik-Niedzielska, G.; Leszczyńska-Gorzelak, B. Fetal programming. Gynecol. Pract. Perinatol. 2018, 2, 58–63. [Google Scholar]
- Gregorczyk-Maślanka, K.; Kurzawa, R. The human body microbiota and its impact on immune homeostasis—Part II. Allergy Asthma Immunol. 2016, 3, 151–155. [Google Scholar]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, E.D.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Trigo, N.F.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [Green Version]
- Seremak-Mrozikiewicz, A. Mechanisms of epigenetic modulation in intrauterine programming. Gynecol. Pract. Perinatol. 2016, 2, 66–72. [Google Scholar]
- Kinsner, M.; Kazimierska, A. Programowanie metaboliczne. Postępy Nauk. O Zdrowiu 2018, 2, 5–18. [Google Scholar]
- Rodríguez-Rodríguez, P.; Cortijo, D.R.; Reyes-Hernández, C.G.; De Pablo, A.L.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2019, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Warrington, N.M.; Beaumont, R.N.; Horikoshi, M.; Day, F.R.; Laurin, C.; Bacelis, J.; Peng, S.; Hao, K.; Feenstra, B.; Wood, A.R.; et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 2019, 5, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Muñóz, A.M.; Gómez-Cantarino, S.; De Dios, M.D.L.M.; Abellán, M.V.; López, B.G.; Gallego, B.M.; Pascual, J.L.G.; Palencia, N.M.A. Nutritional habits and levels of physical activity during pregnancy, birth and the postpartum period of women in Toledo (Spain): Study protocol for a two-year prospective cohort study (the PrePaN study). BMJ Open 2019, 9, e029487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, I.; Maldonado-Contreras, A.; Eisele, C.; Frisard, C.; Simpson, S.; Nair, N.; Rendon, A.; Hawkins, K.; Cawley, C.; Debebe, A.; et al. A Dietary intervention to improve the microbiome composition of pregnant women with Crohn’s disease and their offspring: The MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial design. Contemp. Clin. Trials Commun. 2020, 18, 100573. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 6, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 4, e0175675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 10, 3516–3524. [Google Scholar] [CrossRef] [Green Version]
- Krupa-Kotara, K. Pediatric Dietetics, 1st ed.; Medical University of Silesia: Katowice, Poland, 2021; pp. 17–55. [Google Scholar]
- Imhoff-Kunsch, B.; Stein, A.D.; Martorell, R.; Parra-Cabrera, S.; Romieu, I.; Ramakrishnan, U. Prenatal docosahexaenoic acid supplementation and infant morbidity: Randomized controlled trial. Pediatrics 2011, 3, 505–512. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Costacurta, M.; Merra, G.; Gualtieri, P.; Cioccoloni, G.; Marchetti, M.; Varvaras, D.; Docimo, R.; Di Renzo, L. Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. J. Transl. Med. 2017, 15, 135. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Lingrand, L.; Maillard, M.; Feuz, B.; Tompkins, T.A. The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110142. [Google Scholar] [CrossRef]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Kadosh, K.C.; Muhardi, L.; Parikh, P.; Basso, M.; Mohamed, H.J.J.; Prawitasari, T.; Samuel, F.; Ma, G.; Geurts, J.M. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children-An Update and Novel Insights. Nutrients 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Axelrad, J.E.; Roelstraete, B.; Olén, O.; D’Amato, M.; Ludvigsson, J.F. Gastrointestinal Infection and Risk of Microscopic Colitis: A Nationwide Case-Control Study in Sweden. Gastroenterology 2021, 160, 1599–1607. [Google Scholar] [CrossRef]
- Mathew, S.; Smatti, M.K.; Al Ansari, K.; Nasrallah, G.K.; Al Thani, A.A.; Yassine, H.M. Mixed Viral-Bacterial Infections and Their Effects on Gut Microbiota and Clinical Illnesses in Children. Sci. Rep. 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Zhang, G.; Wang, X.; Guo, M.; Zeng, W.; Xu, Z.; Cao, D.; Pan, A.; Wang, Y.; Zhang, K.; et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med. Microecol. 2020, 5, 100023. [Google Scholar] [CrossRef] [PubMed]
- Moya-Alvarez, V.; Sansonetti, P.J. Understanding the pathways leading to gut dysbiosis and enteric environmental dysfunction in infants: The influence of maternal dysbiosis and other microbiota determinants during early life. FEMS Microbiol. Rev. 2022, 28, 1–13. [Google Scholar] [CrossRef]
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.D.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut microbiota, inflammation, and molecular signatures of host response to infection. J. Genet. Genom. 2021, 48, 792–802. [Google Scholar] [CrossRef]
- Gwela, A.; Mupere, E.; Berkley, J.A.; Lancioni, C. Undernutrition, Host Immunity and Vulnerability to Infection Among Young Children. Pediatr. Infect. Dis. J. 2019, 38, 175–177. [Google Scholar] [CrossRef]
- Tang, L.; Gu, S.; Gong, Y.; Li, B.; Lu, H.; Li, Q.; Zhang, R.; Gao, X.; Wu, Z.; Zhang, J.; et al. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering 2020, 6, 1178–1184. [Google Scholar] [CrossRef]
- Li, F.; Lu, H.; Li, X.; Wang, X.; Zhang, Q.; Mi, L. The impact of COVID-19 on intestinal flora: A protocol for systematic review and meta analysis. Medicine 2020, 99, e22273. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.H.; Zhou, H.W.; Wu, W.K.; Fu, T.; Yan, M.; He, Z.; Sun, S.W.; Ji, Z.H.; Shao, Z.J. Alterations in the Composition of Intestinal DNA Virome in Patients With COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuang, D.; Li, D.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Cao, H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022, 63, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Agarwal, S.; Banerjee, R.; Paul, M.K.; Purbey, P.K. COVID-19 and gut immunomodulation. World J. Gastroenterol. 2021, 27, 7925–7942. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, S.; Chen, Y.; Lu, H.; Shi, D.; Guo, J.; Wu, W.R.; Yang, Y.; Li, Y.; Xu, K.J.; et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut 2022, 1, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, X.; Jiang, G.; Tang, H.; Ming, S.; Tang, L.; Lu, J.; Guo, C.; Shan, H.; Huang, X. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. NPJ Biofilms Microbiomes 2021, 7, 61. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L.; Henson, J.D.; Hall, S. The intestinal microbiota and improving the efficacy of COVID-19 vaccinations. J. Funct. Foods 2021, 87, 104850. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Zhang, J.; Sun, J. COVID-19 and the neonatal microbiome: Will the pandemic cost infants their microbes? Gut Microbes 2021, 1, 1912562. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 3, 544–552. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Lopes, A.L.F.; Pacheco, G.; Nolêto, I.R.D.S.G.; Nicolau, L.A.D.; Medeiros, J.V.R. Premises among SARS-CoV-2, dysbiosis and diarrhea: Walking through the ACE2/mTOR/autophagy route. Med. Hypotheses 2020, 144, 110243. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z.; Wang, Y.; Zhou, F.; Li, S.; Li, C.; Li, L.; Zhang, W.; Li, X. Recent advance of ACE2 and microbiota dysfunction in COVID-19 pathogenesis. Heliyon 2021, 7, e07548. [Google Scholar] [CrossRef] [PubMed]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Nashed, L.; Mani, J.; Hazrati, S.; Stern, D.B.; Subramanian, P.; Mattei, L.; Bittinger, K.; Hu, W.; Levy, S.; Maxwell, G.L.; et al. Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection. Gut 2022. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C. Wie wirkt sich COVID-19 auf die intestinale Mikrobiota aus? [How does COVID-19 affect intestinal microbiota?]. MMW-Fortschr. Der Med. 2021, 163, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Lagier, J.C.; Raoult, D. New Insights Into the Physiopathology of COVID-19: SARS-CoV-2-Associated Gastrointestinal Illness. Front. Med. 2021, 8, 99. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, K.Y.; Meng, T.Q.; Ye, Z.; Guo, S.M.; Li, Z.M.; Xiong, C.L.; Yin, Y.; Li, H.G.; Zhou, L.Q. Gut Microbiota May Not Be Fully Restored in Recovered COVID-19 Patients After 3-Month Recovery. Front. Nutr. 2021, 8, 638825. [Google Scholar] [CrossRef]
- Delgado-Gonzalez, P.; Gonzalez-Villarreal, C.A.; Roacho-Perez, J.A.; Quiroz-Reyes, A.G.; Islas, J.F.; Delgado-Gallegos, J.L.; Arellanos-Soto, D.; Galan-Huerta, K.A.; Garza-Treviño, E.N. Inflammatory effect on the gastrointestinal system associated with COVID-19. World J. Gastroenterol. 2021, 26, 4160–4171. [Google Scholar] [CrossRef]
- Linares-García, L.; Cárdenas-Barragán, M.E.; Hernández-Ceballos, W.; Pérez-Solano, C.S.; Morales-Guzmán, A.S.; Miller, D.S.; Schmulson, M. Bacterial and Fungal Gut Dysbiosis and Clostridium difficile in COVID-19: A Review. J. Clin. Gastroenterol. 2022, 56, 285–298. [Google Scholar] [CrossRef]
- Hussain, I.; Cher, G.L.Y.; Abid, M.A.; Abid, M.B. Role of Gut Microbiome in COVID-19: An Insight Into Pathogenesis and Therapeutic Potential. Front. Immunol. 2021, 12, 4164. [Google Scholar] [CrossRef]
- Chen, J.; Hall, S.; Vitetta, L. Altered gut microbial metabolites could mediate the effects of risk factors in COVID-19. Rev. Med. Virol. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Ponziani, F.R.; Bibbò, S.; Segal, J.P.; Cammarota, G.; Gasbarrini, A. COVID-19 as a trigger of irritable bowel syndrome: A review of potential mechanisms. World J. Gastroenterol. 2021, 43, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Do an Altered Gut Microbiota and an Associated Leaky Gut Affect COVID-19 Severity? MBio 2021, 12, e03022-20. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.L.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Spagnolello, O.; Pinacchio, C.; Santinelli, L.; Vassalini, P.; Innocenti, G.P.; De Girolamo, G.; Fabris, S.; Giovanetti, M.; Angeletti, S.; Russo, A.; et al. Targeting Microbiome: An Alternative Strategy for Fighting SARS-CoV-2 Infection. Chemotherapy 2021, 66, 24–32. [Google Scholar] [CrossRef]
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef]
- Hirayama, M.; Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ueyama, J.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ohno, K. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS ONE 2021, 16, e0260451. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.; Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in COVID19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 201899. [Google Scholar] [CrossRef]
- Ngo, V.L.; Gewirtz, A.T. Microbiota as a potentially-modifiable factor influencing COVID-19. Curr. Opin. Virol. 2021, 49, 21–26. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. Int. J. Mol. Sci. 2021, 22, 4942. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Hansbro, P.M.; Sohal, S.S.; Dingle, P.; Eri, R.; Stanley, R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms 2020, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Cheng, L.; Zhang, R.; Liu, Y.; Wu, Z.; Zhang, X. Tea Polyphenols Prevent and Intervene in COVID-19 through Intestinal Microbiota. Foods 2022, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Suardi, C.; Cazzaniga, E.; Graci, S.; Dongo, D.; Palestini, P. Link between Viral Infections, Immune System, Inflammation and Diet. Int. J. Environ. Res. Public Health 2021, 18, 2455. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Diet, Probiotics and Their Impact on the Gut Microbiota during the COVID-19 Pandemic. Nutrients 2021, 13, 3172. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Benahmed, A.G.; Bjørklund, G. The microbiota-mediated dietary and nutritional interventions for COVID-19. Clin. Immunol. 2021, 226, 108725. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y.; Xu, H.; Deng, L. Herbal Medicine, Gut Microbiota, and COVID-19. Front. Pharmacol. 2021, 12, 1722. [Google Scholar] [CrossRef]
- Daoust, L.; Pilon, G.; Marette, A. Perspective: Nutritional Strategies Targeting the Gut Microbiome to Mitigate COVID-19 Outcomes. Adv. Nutr. 2021, 4, 1074–1086. [Google Scholar] [CrossRef]
- Wu, L.H.; Ye, Z.N.; Peng, P.; Xie, W.R.; Xu, J.; Zhang, X.Y.; Xia, H.H.; He, X.X. Efficacy and Safety of Washed Microbiota Transplantation to Treat Patients with Mild-to-Severe COVID-19 and Suspected of Having Gut Microbiota Dysbiosis: Study Protocol for a Randomized Controlled Trial. Curr. Med. Sci. 2021, 6, 1087–1095. [Google Scholar] [CrossRef]
- Ghani, R.; Mullish, B.H.; Davies, F.J.; Marchesi, J.R. How to adapt an intestinal microbiota transplantation programme to reduce the risk of invasive multidrug-resistant infection. Clin. Microbiol. Infect. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Fiechtner, L.; Vincenti, A.; Magenes, V.C.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G.V.; Calcaterra, V. COVID-19 Pandemic as Risk Factors for Excessive Weight Gain in Pediatrics: The Role of Changes in Nutrition Behavior. A Narrative Review. Nutrients 2021, 13, 4255. [Google Scholar] [CrossRef] [PubMed]
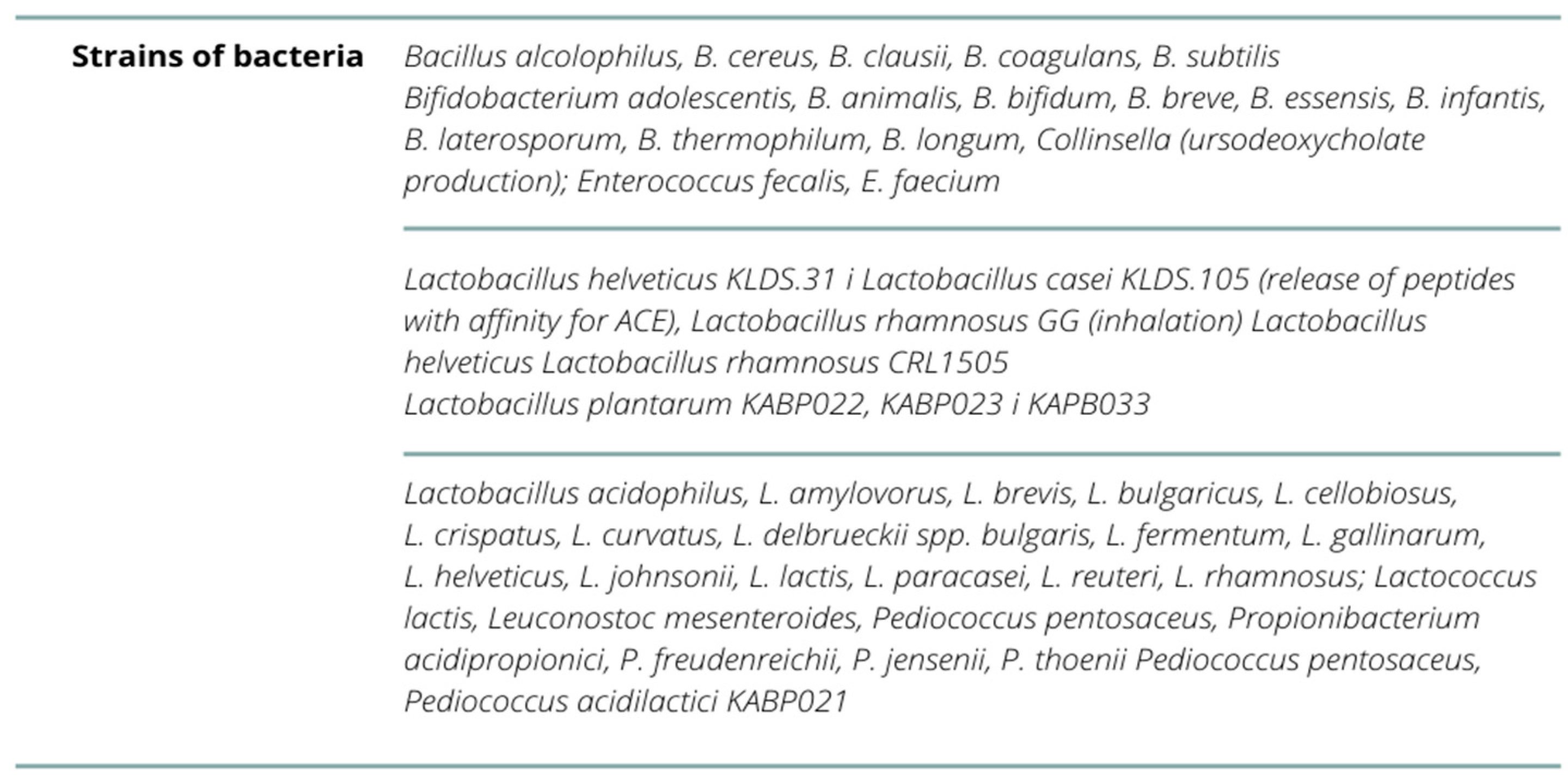
| Impact on Reducing the Risk of Infection-Strain | ||
|---|---|---|
| Authors | Increased (Probiotic Potential) | Reduced (Pathogenic Potential) |
| Li F. et al. (2020) [31] | Firmicutes, Romboutsia, Faecalibacterium, Fusicatenibacter Eubacterium hallii, Faecalibacterium prausnitzii | Bacteroidetes, Streptococcus, Rothia, Veillonella, Erysipelatoclostridium, Actinomyces, Clostridium ramosum, Coprobacillus and Clostridium hathewayi |
| Hu J. et al. (2021) [55] | Faecalibacterium prausnitzii, Lachnospiraceae, Eubacterium rectale, Ruminococcus obeum and Dorea formicigenerans, Bacillus, Lactobacilli, Bifidobacteria, Lactococcus lactis | Clostridium hathewayi, Actinomyces viscous, Bacteroides nordii, Coprobacillus, Clostridium ramosum |
| Olaimat AN. et al. (2020) [56] | Lactobacillus acidophilus, L. amylovorus, L. brevis, L.bulgaricus, L. casei, L. cellobiosus, L. crispatus, L. curvatus, L.delbrueckii spp. bulgaris, L. fermentum, L. gallinarum, L.helveticus, L. johnsonii, L. lactis, L. paracasei, L. plantarum, L.reuteri, L. rhamnosus; Streptococcus thermophilus, Lactococcus lactis, Leuconostoc mesenteroides, Pediococcus pentosaceus, P. acidilactici, Bifidobacterium adolescentis, B.animalis, B. bifidum, B. breve, B. essensis, B. infantis, B.laterosporum, B. thermophilum, B. longum, Propionibacterium acidipropionici, P. freudenreichii, P.jensenii, P. thoenii, Enterococcus fecalis, E. faecium, B. alcolophilus, B.cereus, B. clausii, B. coagulans, B. subtilis, Escherichia coli, Sporolactobac, L. gasseri, L. delbrueckii ssp. yeast: Saccharomyces boulardii and yeast S. cerevisiae B. breve, L.pentosus, L. casei, L. plantarum, L. rhamnosus, L. delbrueckii ssp. bulgaricus, L. gasseri, L. reuteri, L. lactis i L. brevis—given intranasally or orally | - |
| Shahbazi R. et al. (2020) [58] | Lactobacillus, Bifidobacterium, Faecalibacterium prausnitzii, Lactobacillus helveticus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteni, Bifidobacterium bifidum and Streptococcus thermophilus, Candida kefyr, Bifidobacterium, Prevotella and Lactobacillus, Bifidobacterium longum subsp. infantis E4 and Bifidobacterium breve M2CF22M7, Lactobacillus mucosae NK41, Bifidobacterium longum NK46, Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 | L. rhamnosus GG, Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1, Anaeroplasma, Rikenellaceae and Clostridium, C. butyricum, Lactobacillus casei DG |
| Gutiérrez-Castrellón, P. et al. (2022) [60] | Lactiplantibacillus plantarum KABP022, KABP023 and KAPB033 and strain Pediococcus acidilactici KABP021 | - |
| Ailioaie, LM. et al. (2021) [63] | Lactobacillus, Bifidobacterium, Streptococcus, Pediococcus, Leuconostoc, Bacillus and Escherichia coli, Lactobacillus paracasei 28.4, L. reuteri-CFS, Lactobacillus casei CRL 431 and Bacillus coagulans GBI-30, Lactococcus, L. acidophilus, Streptococcus thermophilus | Clostridioides difficile, Lactobacillus rhamnosus GG (LGG) and Bifidobacterium animalis subsp. lactis BB-12, Shigella, Salmonella, E. coli, Yersinia enterocolitica, Campylobacter jejuni, C. auris, Clostridium butyricum, Leuconostoc cremoris, Faecalibacterium prausnitzii, Eubacterium rectale, Bifidobacterium |
| Shinde T. et al. (2020) [64] | L. rhamnosus. B. lactis HN019, Bacillus coagulans BC30 PB, L.acidophilus DDS-1, Anaerostipes hadrus | B. infantis R0033, B. bifidum R0071 and L.helveticus—poorly proven beneficial effects |
| Jabczyk M. et al. (2021) [67] | Roseburia, Lachnospira, Bificobacteria i Collinsella, Actinobacteria, Faecalibacterium prausnitzi, Bifidobacterium bifidum, Eubacterium ventriosum, Lachnospiraceae, Lactobacillus, Akkermansia, Firmicutes/Bacteroidetes, Lactobacillus rhamnosus GG, Bacillus subtilis, Enterococcus faecalis, Lactobacillus plantarum, Lactobacillus reuteri, Lactococcus lactis, Bifidobacterium infantis, Bifidobacterium animalis | Proteobacteria, Akkermansia muciniphila, Bacteroides dorei, Bacteroides nordii, Clostridium hathewayi and Actinomyces viscosus, Staphylococcus, Escherichia, Streptococcus, Lactobacillus acidophilus, Bacillus clausii, Fusobacterium |
| Daoust L. et al. (2021) [70] | Aecalibacterium prausnitzii, Lactobacillus rhamnosus GG kombinacje: Bacillus subtilis and Enterococcus faecalis | Listeria monocytogenes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grot, M.; Krupa-Kotara, K.; Wypych-Ślusarska, A.; Grajek, M.; Białek-Dratwa, A. The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection. Nutrients 2022, 14, 1702. https://doi.org/10.3390/nu14091702
Grot M, Krupa-Kotara K, Wypych-Ślusarska A, Grajek M, Białek-Dratwa A. The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection. Nutrients. 2022; 14(9):1702. https://doi.org/10.3390/nu14091702
Chicago/Turabian StyleGrot, Martina, Karolina Krupa-Kotara, Agata Wypych-Ślusarska, Mateusz Grajek, and Agnieszka Białek-Dratwa. 2022. "The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection" Nutrients 14, no. 9: 1702. https://doi.org/10.3390/nu14091702
APA StyleGrot, M., Krupa-Kotara, K., Wypych-Ślusarska, A., Grajek, M., & Białek-Dratwa, A. (2022). The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection. Nutrients, 14(9), 1702. https://doi.org/10.3390/nu14091702